-
PDF
- Split View
-
Views
-
Cite
Cite
Eloise Williams, Paul Kinsella, Jordan Kahn, Adam Testro, Elizabeth Jones, Louise Jackett, Jason Trubiano, A Cryptic Clot, Clinical Infectious Diseases, Volume 73, Issue 10, 15 November 2021, Pages 940–943, https://doi.org/10.1093/cid/ciab138
Close - Share Icon Share
QUESTION
A 28-year-old man with a past medical history of a multivisceral transplant (liver, pancreas, intestine, and stomach) 3 years prior, end-stage kidney disease, adrenal insufficiency, and recently diagnosed influenza-associated pulmonary aspergillosis (wild-type Aspergillus fumigatus treated with voriconazole and reduction in immunosuppressive therapy) presented to the hospital with presyncopal symptoms. He described several days of fevers and sweats. Blood pressure was 78/40; however, he was afebrile and other vital signs were within normal limits. He had a permacath in situ in his left internal jugular vein and a peripherally inserted central cannula in his right upper limb without signs of local inflammation. Physical examination was otherwise unremarkable. Initial investigations revealed leukopenia (white cell count, 2.9 × 109/L; reference range [RR], 4.0–12.0 × 109/L) with an absolute neutrophil count of 1.4 × 109/L (RR, 2.0–8.0 × 109/L), absolute lymphocyte count of 0.7 × 109/L (RR, 1.5–3.0 × 109/L), and C-reactive protein of 27.5 mg/L (RR, <5 mg/L). Three sets of blood cultures remained negative after 5 days’ incubation. The patient was admitted to the intensive care unit, but his hypotension remained refractory despite broad-spectrum antimicrobials and stress-dose corticosteroids. Computed tomography (CT) of his chest, abdomen, and pelvis performed 1 week after presentation demonstrated moderate resolution of the previously seen extensive pulmonary opacities (Figure 1) and no other pathology was identified. A transesophageal echocardiogram was performed on day 10, which identified a 1.4- × 1.2-cm pedunculated mass arising from the left ventricular outflow tract, below a congenitally abnormal bicuspid aortic valve (Figure 2). Ceftriaxone and vancomycin were commenced empirically for management of culture-negative endocarditis. Voriconazole 300 mg twice daily was continued. The following day, the patient developed acute right lower limb ischemia, with CT demonstrating a 1-cm filling defect in the right common femoral artery consistent with an embolus. A right common femoral embolectomy and left ventricular outflow tract vegetectomy were performed. Samples were sent for histopathology, microscopy, and culture. Gomori methanamine silver stain of the common femoral embolus and left ventricular outflow tract mass are demonstrated in Figure 3A and 3B, respectively.
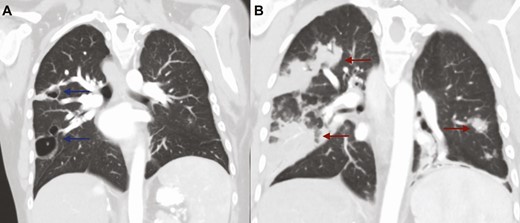
Coronal CT scan of the chest (A) demonstrating moderate resolution of previously seen extensive opacities, with 2 remaining cavities in the right middle and upper lobes with associated peripheral nodularity (blue arrows); (B) coronal CT scan of the chest on initial presentation with influenza-related pulmonary aspergillosis demonstrating extensive bilateral opacities (red arrows). Abbreviation: CT, computed tomography.
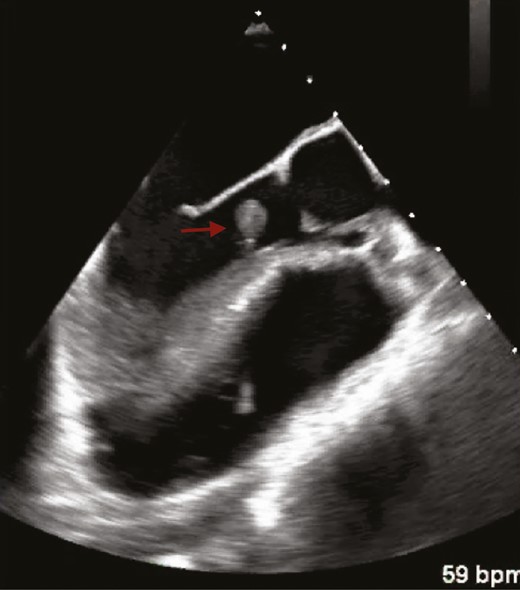
Transesophageal echocardiogram demonstrating a 1.4- × 1.2-cm pedunculated mass (red arrow) arising from the left ventricular septum below the aortic valve apparatus consistent with mural endocarditis.
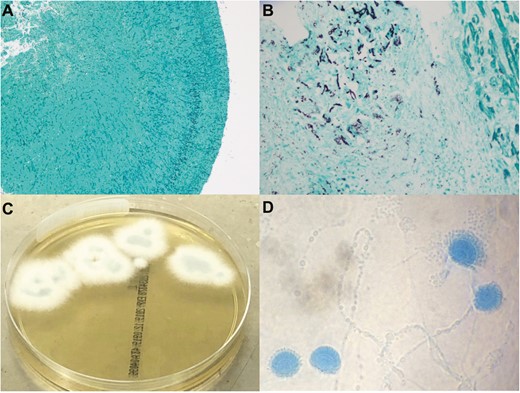
Gomori methenamine silver stain of the common femoral artery embolism (A) (original magnification, ×100) and the left ventricular outflow tract vegetation (B) demonstrating septate narrow-angle branching hyphae in endocardium adjacent to myocardium (original magnification, ×400). Left ventricular outflow tract mass culture demonstrating Aspergillus lentulus on Sabouraud dextrose agar (C) and scotch tape preparation with Lactophenol cotton blue staining (D).
What is the diagnosis?
Diagnosis: Aspergillus endocarditis caused by Aspergillus lentulus
Aspergillus lentulus was recovered on culture of the common femoral embolus and left ventricular outflow tract mass obtained at the time of surgery (Figure 3C and 3D) as well as subsequent respiratory cultures, with identification confirmed by genetic sequencing of the B-tubulin gene. Despite management with intravenous liposomal amphotericin B (3 mg/kg daily) and delayed-release posaconazole tablets (300 mg daily), the patient deteriorated and died due to sepsis-related multiorgan failure several days later. Antifungal susceptibility testing was performed on the Aspergillus lentulus isolate using Clinical Laboratory Standards Institute (CLSI) methodology. The minimum inhibitory concentration (MIC) of voriconazole was 2 μg/mL, confirming the Aspergillus lentulus isolate as a non–wild-type strain according to the CLSI Aspergillus fumigatus complex epidemiological cutoff value. The MIC for posaconazole was 0.12 μg/mL, itraconazole was 0.12 μg/mL (wild-type), amphotericin was 2 μg/mL (wild-type), and anidulafungin was 0.015 μg/mL.
Aspergillus endocarditis (AE) is a rare but life-threatening disease, representing 20–30% of all fungal endocarditis cases [1, 2]. The mortality rate of AE ranges between 42% and 87% despite cardiac surgery and antifungal therapy [1–4]. Delayed diagnosis likely contributes to the high mortality rate, due to a lack of specific clinical and noninvasive laboratory criteria, with blood cultures almost always remaining negative [3]. Diagnosis has previously relied upon the isolation of Aspergillus from cardiac or sterile site samples, with up to one-third of cases diagnosed postmortem [3]. However, the recent availability of nonculture diagnostic tests for Aspergillus spp., such as galactomannan antigen and molecular tests for fungal nucleic acid, may improve timely AE diagnosis [5]. Risk factors for AE include an underlying cardiac abnormality, prosthetic valve, other noncardiac surgery, recent healthcare association, central venous catheterization, solid-organ transplantation, and hematopoietic stem cell transplantation [1–5]. Embolic phenomena are common, with acute limb ischemia at presentation significantly associated with AE in a recent review [4]. Due to the rarity of the disease and heterogeneity of reported cases, management guidelines for AE are based on expert opinion. There is a strong consensus for surgical management and long-term suppressive antifungal therapy in the American Heart Association (AHA) [6] and European Society of Cardiology (ESC) [7] infective endocarditis guidelines. Antifungal agent recommendations vary, with amphotericin B recommended by the AHA and voriconazole recommended by the ESC. Combination therapy involving amphotericin B, voriconazole, or an echinocandin may also be considered. The evidence for superiority of either amphotericin B, voriconazole, or combination therapy in the context of AE is weak [3].
Aspergillus lentulus was first described in 2004 by Balajee et al [8] and assigned to a new species within the Aspergillus section Fumigati on the basis of phylogenetic analyses. Aspergillus lentulus can be differentiated morphologically from Aspergillus fumigatus by its poor sporulation, smaller conidial heads, and inability to grow at temperatures above 48°C [9]. Aspergillus lentulus appears to represent less than 3% of clinical Aspergillus spp. of the section Fumigati [9]; however, it may account for up to 30% of azole-resistant isolates [10]. Decreased susceptibility to all 3 antifungal classes has been reported in clinical isolates [11–13]. In particular, there appears to be intrinsic resistance to voriconazole mediated by cyp51A [14]. Aspergillus lentulus has been described in a number of cases of invasive pulmonary aspergillosis infections and has been associated with a high mortality rate (71%) in the reported literature [15]. To our knowledge, this is the first reported case of Aspergillus lentulus infective endocarditis.
Note
Potential conflicts of interests. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- endocarditis
- transesophageal echocardiography
- bacterial endocarditis
- influenza
- computed tomography
- amphotericin b
- antifungal agents
- bronchopulmonary aspergillosis
- aspergillus
- aspergillus fumigatus
- surgical procedures, operative
- diagnosis
- mortality
- silver
- chest
- transplantation
- embolism
- voriconazole
- blood culture
- left ventricular outflow tract




