-
PDF
- Split View
-
Views
-
Cite
Cite
Windy D Tanner, Molly K Leecaster, Yue Zhang, Kristina M Stratford, Jeanmarie Mayer, Lindsay D Visnovsky, Heba Alhmidi, Jennifer L Cadnum, Annette L Jencson, Sreelatha Koganti, Christina P Bennett, Curtis J Donskey, Judith Noble-Wang, Sujan C Reddy, Laura J Rose, Lauren Watson, Emma Ide, Tyler Wipperfurth, Nasia Safdar, Maria Arasim, Colleen Macke, Patti Roman, Sarah L Krein, Catherine Loc-Carrillo, Matthew H Samore, Environmental Contamination of Contact Precaution and Non-Contact Precaution Patient Rooms in Six Acute Care Facilities, Clinical Infectious Diseases, Volume 72, Issue Supplement_1, 15 January 2021, Pages S8–S16, https://doi.org/10.1093/cid/ciaa1602
Close - Share Icon Share
Abstract
Environmental contamination is an important source of hospital multidrug-resistant organism (MDRO) transmission. Factors such as patient MDRO contact precautions (CP) status, patient proximity to surfaces, and unit type likely influence MDRO contamination and bacterial bioburden levels on patient room surfaces. Identifying factors associated with environmental contamination in patient rooms and on shared unit surfaces could help identify important environmental MDRO transmission routes.
Surfaces were sampled from MDRO CP and non-CP rooms, nursing stations, and mobile equipment in acute care, intensive care, and transplant units within 6 acute care hospitals using a convenience sampling approach blinded to cleaning events. Precaution rooms had patients with clinical or surveillance tests positive for methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, carbapenem-resistant Enterobacteriaceae or Acinetobacter within the previous 6 months, or Clostridioides difficile toxin within the past 30 days. Rooms not meeting this definition were considered non-CP rooms. Samples were cultured for the above MDROs and total bioburden.
Overall, an estimated 13% of rooms were contaminated with at least 1 MDRO. MDROs were detected more frequently in CP rooms (32% of 209 room-sample events) than non-CP rooms (12% of 234 room-sample events). Surface bioburden did not differ significantly between CP and non-CP rooms or MDRO-positive and MDRO-negative rooms.
CP room surfaces are contaminated more frequently than non-CP room surfaces; however, contamination of non-CP room surfaces is not uncommon and may be an important reservoir for ongoing MDRO transmission. MDRO contamination of non-CP rooms may indicate asymptomatic patient MDRO carriage, inadequate terminal cleaning, or cross-contamination of room surfaces via healthcare personnel hands.
In 2015, there were an estimated 633 000 patients with a hospital-onset healthcare-associated infection (HAI) and 687 200 HAIs in US hospitals [1]. In hospitals, multidrug-resistant organisms (MDROs) are responsible for approximately 14% of HAIs [2]. Contamination of the healthcare environment contributes to transmission of pathogens, including MDROs [3, 4], and these organisms can survive on healthcare surfaces for days, weeks, or even months [5, 6]. Reports of MDRO detection on patient room surfaces, shared medical equipment, and shared nursing workstation areas are common [3, 7], and these sites have been implicated in nosocomial MDRO outbreaks [8, 9].
Contamination of healthcare environmental surfaces may contribute to transmission of MDROs via healthcare personnel (HCP) hands. Detection of MDROs on environmental surfaces is strongly associated with HCP contamination with MDROs [10–12], and the likelihood of HCP hand contamination from contact with surfaces in a colonized patient’s room is comparable to contact with skin of colonized patients [13, 14]. Contamination of patient room surfaces typically varies based on patient proximity to the surfaces and can be influenced by patient characteristics [15, 16]. Room surface contamination with MDROs has been significantly correlated with occupancy by patients with wounds, diarrhea, and urinary tract infection or colonization [8, 17, 18].
Likelihood of environmental contamination also varies depending on the room occupant’s contact precaution status [19–22]. Contact precaution status determines what barrier precautions, cleaning agents, and other infection control practices are necessary to reduce the risk of MDRO transmission. Asymptomatic patients are unlikely to be placed on MDRO contact precautions if their MDRO carriage is unknown, but they may still shed these organisms into the surrounding environment. In some cases, such as with toxigenic C. difficile spores, unknown patient carriage could lead to inadequate terminal room cleaning. Studies have shown that prior room occupancy with an MDRO-positive patient is a risk factor for acquisition of an MDRO by the subsequent room occupant [23, 24].
Surface contamination also likely varies by facility and unit type [15], making it difficult to draw conclusions about surface contamination results from single ward or facility studies. Characterization of the patterns and variability of environmental surface contamination in acute care facilities with respect to facility, unit type, and patient precaution status can provide important information on exposure risks and the potential role of the environment in HAI transmission. To better estimate the extent and variation of MDRO contamination of room and common area environmental surfaces by facility, unit, patient proximity to surfaces, and patient room precaution status, we performed a multifacility environmental sampling study in 6 US acute care facilities.
METHODS
Environmental surface sampling was conducted in 6 acute care facilities, including 2 tertiary care medical centers and 4 Veterans Affairs (VA) Medical Centers from April 2016 to June 2017. Differences in infection control policies and procedures existed between facilities, particularly with respect to personal protective equipment (PPE) use requirements in contact precautions rooms, cleaning practices, and the implementation of surveillance testing for identifying MRSA contact precautions patients. Within each facility, acute care, intensive care, and transplant units were designated for participation prior to study start. For the first sampling cycle, 2 hospital units caring for at least 1 patient meeting the MDRO contact precaution (CP) study definition were selected for sampling. In subsequent cycles, units not sampled in the previous cycle were prioritized for selection to ensure balanced participation among units within each facility. For each facility and cycle, a third unit was selected only if the 2 selected units had only 1 MDRO patient each. Sampling of 1–3 units occurred at each facility every 2 weeks for 8–11 cycles, resulting in 98 unit-sample events.
Room Selection
For study purposes, MDRO CP rooms were defined as rooms of patients who were on contact precautions and had a positive clinical test for methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), carbapenem-resistant Enterobacteriaceae (CRE), or carbapenem-resistant Acinetobacter (CRA) in the previous 6 months or were on contact precautions and had a positive Clostridioides difficile toxin test in the previous 30 days. VA Medical Centers perform MRSA surveillance tests and were used in addition to the clinical tests for all listed MDROs to determine precaution status. Patient rooms were designated as CP rooms for the duration of occupancy by the patient on contact precautions. Following patient transfer or discharge and terminal cleaning, CP rooms would be considered control rooms unless the new occupant was on contact precautions as defined for this study.
The MDRO CP rooms were identified for sampling from each facility using the daily MDRO precaution lists obtained from the facility’s infection prevention and control group. Up to 4 MDRO CP rooms were sampled during a unit sample event. Random selection of MDRO CP rooms occurred if there were more than 4 MDRO patients on a unit for a sampling cycle. Control rooms, defined as rooms of patients not meeting the MDRO CP room criteria listed above, were selected from the same unit using a random room number generator. One control room was sampled for each CP room sampled. In units where only 1 CP room was present at the time of sampling, 2 control rooms were sampled within the unit. Both single occupancy and double occupancy rooms were sampled.
Sample Collection
A composite surface sampling approach was used to collect samples from multiple surfaces on each premoistened cellulose sponge-wipe (3MTMSponge-Stick with neutralizing buffer, 3M, Sparks, MD, USA), as previously described [25]. Sampling was performed randomly, utilizing a convenience sampling approach that was blinded to daily cleaning times, activities, and patient length-of-stay. Briefly, composite surface samples were collected using a sponge-wipe rotated to an unused side after each individual surface was sampled. Within each room, up to 3 surface composite samples were collected from: (1) patient touch areas (“near patient”); (2) HCP touch areas (“far from patient”); and if present (3) “toileting areas.” Surfaces and surface area sampled were standardized for all facilities. Video of the standardized sampling procedures were used to train samplers at each facility and disposable measuring tapes were used to ensure accurate surface area measurements. If one of the predetermined surfaces was not present in the facility, a specified alternate surface was sampled. The near-patient composite included the patient bed rail, patient over bed table, room phone, and hand-held call button or TV remote. The far from patient composite included the room inner door handle, patient room charting bar code scanner and keyboard, and the outer bed control panel. The bathroom composite included the toilet grab bar, toilet flush handle, toilet rinse spout handle, and inner bathroom door handle. Alternatively, if a hopper was present in the room, a sample was collected from the hopper handle and the inner hopper closet handle. If the room occupant was using a commode, the grab handles and commode seat were sampled. Sampling was conducted at the time of patient occupancy of the room and not following discharge or transfer.
Standardized common surfaces from nursing stations and shared equipment were also sampled in each unit. Unit nursing station common surface samples were a composite of 4 surfaces: the medication dispensing cabinet’s touchpad, a portable computer keyboard, a nursing station phone, and either a portable communication device used by nursing staff or desktop mouse. The shared unit equipment sample was a composite of surfaces from a glucometer, bladder scanner probe handle, and Doppler ultrasound or portable vital signs monitor. If present, samples were also collected from shared unit hoppers. Equipment sampling was standardized between facilities by selecting equipment items that are commonly present in all healthcare facilities and units. In cases where a piece of equipment was not present in the unit, an alternate predetermined piece of equipment was sampled instead. The surface area sampled on each common area surface or piece of equipment was standardized between facilities.
Sponges were shipped on ice to a single central laboratory for testing within 24 hours of collection.
Microbiological Testing
Sponges were processed as previously described [26], using 45 mL sterile phosphate buffered saline with 0.02% Tween 80 (PBST). Bacterial cells in the PBST sponge eluate were concentrated by centrifugation and resuspended in 3 mL eluate. Two hundred microliter aliquots of the concentrated PBST eluate were plated to C. difficile Brucella Agar and CDBB-TC broth for C. difficile, CHROMagar Staph aureus + 6 µg/mL cefoxitin and mannitol salt broth with 6 µg/mL cefoxitin for MRSA, Enterococcosel agar and broth + 20 µg/mL vancomycin for VRE, and MacConkey agar and broth with 1 µg/mL meropenem for CRE/CRA. Isolate identification was confirmed by MALDI-TOF (Bruker Biotyper CA System, Bellerica, MA, USA). A 1000-µL aliquot was also plated on nonselective blood agar to determine the overall bacterial bioburden of the composite surfaces. Carbapenem resistance to meropenem was confirmed on suspected CRE and CRA isolates using the disk diffusion method and interpreted using the 2016 Clinical Laboratory Standards Institute definition for meropenem resistance of Enterobacteriaceae or Acinetobacter isolates [27]. Isolates identified as C. difficile were tested for toxins A and B production using the Alere C. difficile Tox A/B II ELISA (TECHLAB, Blacksburg, VA, USA). Sample processing and testing procedures were consistent throughout the study for all samples.
Data Analysis
Microbiological results for patient room samples were summarized by facility, unit-type, patient room status, and microorganism. The 2-stage stratified sample design was used to ensure inclusion of MDRO rooms, although balancing the number of control rooms and selection of units resulted in unequal probability of inclusion among rooms within each facility. CP rooms were more likely to be included because there were fewer of them. The inclusion probabilities for each facility cycle were calculated as the probability of the unit being selected multiplied by the probability of the room being selected [28]. If only 2 units had CP rooms and each unit had 4 or fewer MDRO rooms, then the inclusion probabilities were 1. If random selection of units was necessary, then the probability was 2 (or 3) divided by the number of units with MDRO rooms. For random selection of control [or CP] rooms within each unit, the probability was the number of control [or CP] rooms to select divided by the number of control [or CP] rooms occupied. The inclusion probabilities differed by cycle, and although a room could be sampled more than once, they were considered independent because of the time between samples.
Estimates incorporated sample weights (the inverse of inclusion probabilities) to ensure unbiased estimation of unit and facility contamination. Unweighted estimates would be overly influenced by results from MDRO rooms, which were more likely to be sampled. Instead of equal weighting, 1 divided by the sample size (1/n), each result was weighted using its sample weight divided by the sum of sample weights for the facility cycle in estimates of mean and variance.
Presence of target MDROs, defined as any detected quantity, and total bioburden were summarized. A patient room was considered MDRO-positive if 1 or more of the composite samples tested positive for one of the study MDROs. The extent of contamination in the units sampled was estimated, adjusting for the unequal inclusion probabilities, facility, unit type, and room status. Weighted estimates and 95% confidence intervals were calculated for both the proportion of rooms with contamination for MRSA, VRE, and any MDRO and for the mean log10 bacterial bioburden normalized to colony-forming unit (CFU) counts per 100 square centimeters sampled. Weighted estimates were not calculated for CRE, CRA, and C. difficile due to the small number of positive samples. Unweighted estimates of sample mean and median MDRO CFU/100 cm2 for samples with detectable quantities were calculated for MRSA, VRE, and C. difficile.
RESULTS
A total of 1532 environmental composite surface samples were collected during 98 unit-sample events; 622 samples representing 209 MDRO CP room-sample events, 692 samples representing 234 control room-sample events, and 218 samples from common areas (Table 1 and Supplementary Tables S1 and S2). Each of the 23 units from the 6 facilities was sampled at least twice, and all but one unit was sampled at least 3 times. Of the 511 rooms eligible for sampling in the 6 facilities, 63% were sampled at least once.
Summary of Positive MDRO Culture Results and Estimated Contamination with 95% Confidence Intervals (CI). Rooms Meeting Study Criteria for MDRO Contact Precautions Rooms are Indicated as CP Rooms
| Facility . | Control Rooms With Any MDRO . | . | CP Rooms With Any MDRO . | . | Estimated Contamination Weighted Percent of Patient Rooms With Any MDRO . | . |
|---|---|---|---|---|---|---|
| . | n . | (%) . | n . | (%) . | % . | (95% CI) . |
| 1 | 41 | (14.6) | 36 | (27.8) | 16.3 | (6–26) |
| 2 | 34 | (5.9) | 22 | (27.3) | 8.6 | (0–20) |
| 3 | 39 | (12.8) | 37 | (37.8) | 11.6 | (0–23) |
| 4 | 47 | (8.5) | 44 | (38.6) | 9.6 | (0–20) |
| 5 | 30 | (13.3) | 25 | (56.0) | 24.5 | (11–38) |
| 6 | 43 | (16.3) | 45 | (11.1) | 9.4 | (2–16) |
| All | 234 | (12.0) | 209 | (31.6) | 12.7 | (9–17) |
| Facility . | Control Rooms With Any MDRO . | . | CP Rooms With Any MDRO . | . | Estimated Contamination Weighted Percent of Patient Rooms With Any MDRO . | . |
|---|---|---|---|---|---|---|
| . | n . | (%) . | n . | (%) . | % . | (95% CI) . |
| 1 | 41 | (14.6) | 36 | (27.8) | 16.3 | (6–26) |
| 2 | 34 | (5.9) | 22 | (27.3) | 8.6 | (0–20) |
| 3 | 39 | (12.8) | 37 | (37.8) | 11.6 | (0–23) |
| 4 | 47 | (8.5) | 44 | (38.6) | 9.6 | (0–20) |
| 5 | 30 | (13.3) | 25 | (56.0) | 24.5 | (11–38) |
| 6 | 43 | (16.3) | 45 | (11.1) | 9.4 | (2–16) |
| All | 234 | (12.0) | 209 | (31.6) | 12.7 | (9–17) |
Abbreviations: CI, confidence interval; CP, contact precautions.
Summary of Positive MDRO Culture Results and Estimated Contamination with 95% Confidence Intervals (CI). Rooms Meeting Study Criteria for MDRO Contact Precautions Rooms are Indicated as CP Rooms
| Facility . | Control Rooms With Any MDRO . | . | CP Rooms With Any MDRO . | . | Estimated Contamination Weighted Percent of Patient Rooms With Any MDRO . | . |
|---|---|---|---|---|---|---|
| . | n . | (%) . | n . | (%) . | % . | (95% CI) . |
| 1 | 41 | (14.6) | 36 | (27.8) | 16.3 | (6–26) |
| 2 | 34 | (5.9) | 22 | (27.3) | 8.6 | (0–20) |
| 3 | 39 | (12.8) | 37 | (37.8) | 11.6 | (0–23) |
| 4 | 47 | (8.5) | 44 | (38.6) | 9.6 | (0–20) |
| 5 | 30 | (13.3) | 25 | (56.0) | 24.5 | (11–38) |
| 6 | 43 | (16.3) | 45 | (11.1) | 9.4 | (2–16) |
| All | 234 | (12.0) | 209 | (31.6) | 12.7 | (9–17) |
| Facility . | Control Rooms With Any MDRO . | . | CP Rooms With Any MDRO . | . | Estimated Contamination Weighted Percent of Patient Rooms With Any MDRO . | . |
|---|---|---|---|---|---|---|
| . | n . | (%) . | n . | (%) . | % . | (95% CI) . |
| 1 | 41 | (14.6) | 36 | (27.8) | 16.3 | (6–26) |
| 2 | 34 | (5.9) | 22 | (27.3) | 8.6 | (0–20) |
| 3 | 39 | (12.8) | 37 | (37.8) | 11.6 | (0–23) |
| 4 | 47 | (8.5) | 44 | (38.6) | 9.6 | (0–20) |
| 5 | 30 | (13.3) | 25 | (56.0) | 24.5 | (11–38) |
| 6 | 43 | (16.3) | 45 | (11.1) | 9.4 | (2–16) |
| All | 234 | (12.0) | 209 | (31.6) | 12.7 | (9–17) |
Abbreviations: CI, confidence interval; CP, contact precautions.
MDRO Detection
Unweighted Results
Rooms were considered positive if at least 1 of the 3 zone composite samples (near-patient, far-patient, and toileting area) was culture-positive for a target MDRO. Of the 443 room sample events, 21.2% (n = 94) (range among facilities 13.6%–32.7%) were culture-positive for at least 1 target MDRO, including 31.6% (n = 66) of the CP rooms and 12.0% (n = 28) of those from control rooms. MDRO contamination among facilities ranged from 11.1% to 56.0% of CP rooms and from 5.9% to 16.3% of nonprecaution rooms (Table 1). Nine (10%) of the 94 room sample events that were culture-positive had 2 target MDROs recovered, and no room-sample events had more than 2. Of 218 common area samples collected, 2 nursing areas and 3 nursing equipment surface samples were positive from 3 facilities (Supplementary Table S2). MDROs were not detected in any shared unit bathroom or hopper samples.
VRE was the most common MDRO isolated (72 samples, 50 rooms), followed by MRSA (67 samples, 44 rooms), C. difficile (9 samples, 8 rooms), CRE (1 sample, 1 room), and no CRA. VRE contamination was frequently detected in MDRO CP rooms; 31% of VRE CP rooms and 14% of CP rooms for MDROs other than VRE. VRE was less frequently detected in nonprecaution rooms (n = 16, 6.8% of control rooms) (Table 2). When detected, the VRE plate count ranged from <1.3 to 4254 CFU per 100 cm2. The means and medians were similar in CP and control rooms but samples from bathrooms were higher than near or far-patient zones (Table 3). MRSA contamination was primarily detected in MRSA CP rooms (23%), with infrequent detection in non-MRSA CP rooms (4%) and control rooms (4%). For MRSA CP rooms, the occupant’s MRSA status was determined by either surveillance or clinical testing, but environmental contamination results were combined because the distribution across contamination was similar for both test types. MRSA contamination in MRSA CP patient rooms due to MRSA clinical tests was 2% higher than CP rooms due to MRSA surveillance tests. When detected, MRSA plate count ranged from <1.3 to 229 CFU per 100 cm2. The mean and median CFU counts were higher in CP rooms than control rooms and higher in bathroom samples than near or far-patient zones (Table 3). C. difficile was detected in only 8 room samples; the plate count ranged from <1.3 to 30 CFU per 100 cm2 with higher mean and median CFU counts in CP rooms than in control rooms and higher in bathroom versus near-patient samples (Table 3). Overall, 14.8% (n = 31) of the 209 MDRO CP rooms sampled were culture-positive for a target MDRO other than the MDRO(s) the occupying patient was known to have (Table 2, Supplementary Table S3).
| Room Type . | MDRO Detected . | . | . | . | . | . | Number of Rooms, n . |
|---|---|---|---|---|---|---|---|
| . | None (%) . | C. difficile (%) . | MRSA (%) . | VRE (%) . | CRE (%) . | CRA (%) . | . |
| Control | 88.0 | 1.3 | 4.3 | 6.8 | 0 | 0 | 234 |
| CP C. difficile | 78.7 | 4.3 | 4.3 | 12.8 | 0 | 0 | 47 |
| CP MRSAa | 64.5 | 2.2 | 23.2 | 15.2 | 0.7 | 0 | 138 |
| CP VRE | 69.0 | 0 | 3.4 | 31.0 | 0 | 0 | 29 |
| CP CRE | 66.7 | 0 | 0 | 33.3 | 0 | 0 | 3 |
| Any CP | 68.4 | 2.4 | 16.3 | 16.3 | 0.5 | 0 | 209 |
| Number of rooms, n | 348 | 8 | 44 | 50 | 1 | 0 | 443 |
| Room Type . | MDRO Detected . | . | . | . | . | . | Number of Rooms, n . |
|---|---|---|---|---|---|---|---|
| . | None (%) . | C. difficile (%) . | MRSA (%) . | VRE (%) . | CRE (%) . | CRA (%) . | . |
| Control | 88.0 | 1.3 | 4.3 | 6.8 | 0 | 0 | 234 |
| CP C. difficile | 78.7 | 4.3 | 4.3 | 12.8 | 0 | 0 | 47 |
| CP MRSAa | 64.5 | 2.2 | 23.2 | 15.2 | 0.7 | 0 | 138 |
| CP VRE | 69.0 | 0 | 3.4 | 31.0 | 0 | 0 | 29 |
| CP CRE | 66.7 | 0 | 0 | 33.3 | 0 | 0 | 3 |
| Any CP | 68.4 | 2.4 | 16.3 | 16.3 | 0.5 | 0 | 209 |
| Number of rooms, n | 348 | 8 | 44 | 50 | 1 | 0 | 443 |
MDRO CP rooms (n = 10 rooms) or control rooms with MDRO contamination (n = 9 rooms) based on more than 1 microorganism are represented in the table multiple times.
Abbreviations: CP, contact precautions; CRA, carbapenem-resistant Acinetobacter; CRE, carbapenem-resistant Enterobacteriaceae; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci.
a. Room type MRSA includes patients with previous positive MRSA surveillance and MRSA clinical tests combined because the distribution across contamination was similar.
| Room Type . | MDRO Detected . | . | . | . | . | . | Number of Rooms, n . |
|---|---|---|---|---|---|---|---|
| . | None (%) . | C. difficile (%) . | MRSA (%) . | VRE (%) . | CRE (%) . | CRA (%) . | . |
| Control | 88.0 | 1.3 | 4.3 | 6.8 | 0 | 0 | 234 |
| CP C. difficile | 78.7 | 4.3 | 4.3 | 12.8 | 0 | 0 | 47 |
| CP MRSAa | 64.5 | 2.2 | 23.2 | 15.2 | 0.7 | 0 | 138 |
| CP VRE | 69.0 | 0 | 3.4 | 31.0 | 0 | 0 | 29 |
| CP CRE | 66.7 | 0 | 0 | 33.3 | 0 | 0 | 3 |
| Any CP | 68.4 | 2.4 | 16.3 | 16.3 | 0.5 | 0 | 209 |
| Number of rooms, n | 348 | 8 | 44 | 50 | 1 | 0 | 443 |
| Room Type . | MDRO Detected . | . | . | . | . | . | Number of Rooms, n . |
|---|---|---|---|---|---|---|---|
| . | None (%) . | C. difficile (%) . | MRSA (%) . | VRE (%) . | CRE (%) . | CRA (%) . | . |
| Control | 88.0 | 1.3 | 4.3 | 6.8 | 0 | 0 | 234 |
| CP C. difficile | 78.7 | 4.3 | 4.3 | 12.8 | 0 | 0 | 47 |
| CP MRSAa | 64.5 | 2.2 | 23.2 | 15.2 | 0.7 | 0 | 138 |
| CP VRE | 69.0 | 0 | 3.4 | 31.0 | 0 | 0 | 29 |
| CP CRE | 66.7 | 0 | 0 | 33.3 | 0 | 0 | 3 |
| Any CP | 68.4 | 2.4 | 16.3 | 16.3 | 0.5 | 0 | 209 |
| Number of rooms, n | 348 | 8 | 44 | 50 | 1 | 0 | 443 |
MDRO CP rooms (n = 10 rooms) or control rooms with MDRO contamination (n = 9 rooms) based on more than 1 microorganism are represented in the table multiple times.
Abbreviations: CP, contact precautions; CRA, carbapenem-resistant Acinetobacter; CRE, carbapenem-resistant Enterobacteriaceae; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci.
a. Room type MRSA includes patients with previous positive MRSA surveillance and MRSA clinical tests combined because the distribution across contamination was similar.
Unweighted Sample MDRO Concentration (CFU/100cm2) Mean and Median Overall and for Sets of Samples From CP and Control Rooms and by Zone
| Samples Included . | MRSA . | . | VRE . | . | C. difficile . | . |
|---|---|---|---|---|---|---|
| . | n . | Mean / Median . | n . | Mean / Median . | n . | Mean / Median . |
| All | 67 | 25.5 / 4.1 | 72 | 221.9 / 4.3 | 9 | 7.4 / 4.2 |
| CP rooms | 54 | 28.4 / 5.0 | 51 | 225.1 / 4.2 | 5 | 9.4 / 4.2 |
| Control rooms | 13 | 13.5 / 3.8 | 21 | 214.3 / 7.1 | 4 | 2.3 / 2.3 |
| Near-patient | 32 | 25.2 / 2.9 | 37 | 117.1 / 6.4 | 3 | 2.4 / 2.6 |
| Far-patient | 16 | 10.4 / 4.1 | 20 | 130.3 / 4.1 | 1 | NA |
| Bathroom | 19 | 38.7 / 8.5 | 15 | 589.4 / 8.5 | 5 | 11.2 / 5.3 |
| Samples Included . | MRSA . | . | VRE . | . | C. difficile . | . |
|---|---|---|---|---|---|---|
| . | n . | Mean / Median . | n . | Mean / Median . | n . | Mean / Median . |
| All | 67 | 25.5 / 4.1 | 72 | 221.9 / 4.3 | 9 | 7.4 / 4.2 |
| CP rooms | 54 | 28.4 / 5.0 | 51 | 225.1 / 4.2 | 5 | 9.4 / 4.2 |
| Control rooms | 13 | 13.5 / 3.8 | 21 | 214.3 / 7.1 | 4 | 2.3 / 2.3 |
| Near-patient | 32 | 25.2 / 2.9 | 37 | 117.1 / 6.4 | 3 | 2.4 / 2.6 |
| Far-patient | 16 | 10.4 / 4.1 | 20 | 130.3 / 4.1 | 1 | NA |
| Bathroom | 19 | 38.7 / 8.5 | 15 | 589.4 / 8.5 | 5 | 11.2 / 5.3 |
Abbreviations: CP, contact precautions; MRSA, methicillin-resistant Staphylococcus aureus; NA, not applicable; VRE, vancomycin-resistant enterococci.
Unweighted Sample MDRO Concentration (CFU/100cm2) Mean and Median Overall and for Sets of Samples From CP and Control Rooms and by Zone
| Samples Included . | MRSA . | . | VRE . | . | C. difficile . | . |
|---|---|---|---|---|---|---|
| . | n . | Mean / Median . | n . | Mean / Median . | n . | Mean / Median . |
| All | 67 | 25.5 / 4.1 | 72 | 221.9 / 4.3 | 9 | 7.4 / 4.2 |
| CP rooms | 54 | 28.4 / 5.0 | 51 | 225.1 / 4.2 | 5 | 9.4 / 4.2 |
| Control rooms | 13 | 13.5 / 3.8 | 21 | 214.3 / 7.1 | 4 | 2.3 / 2.3 |
| Near-patient | 32 | 25.2 / 2.9 | 37 | 117.1 / 6.4 | 3 | 2.4 / 2.6 |
| Far-patient | 16 | 10.4 / 4.1 | 20 | 130.3 / 4.1 | 1 | NA |
| Bathroom | 19 | 38.7 / 8.5 | 15 | 589.4 / 8.5 | 5 | 11.2 / 5.3 |
| Samples Included . | MRSA . | . | VRE . | . | C. difficile . | . |
|---|---|---|---|---|---|---|
| . | n . | Mean / Median . | n . | Mean / Median . | n . | Mean / Median . |
| All | 67 | 25.5 / 4.1 | 72 | 221.9 / 4.3 | 9 | 7.4 / 4.2 |
| CP rooms | 54 | 28.4 / 5.0 | 51 | 225.1 / 4.2 | 5 | 9.4 / 4.2 |
| Control rooms | 13 | 13.5 / 3.8 | 21 | 214.3 / 7.1 | 4 | 2.3 / 2.3 |
| Near-patient | 32 | 25.2 / 2.9 | 37 | 117.1 / 6.4 | 3 | 2.4 / 2.6 |
| Far-patient | 16 | 10.4 / 4.1 | 20 | 130.3 / 4.1 | 1 | NA |
| Bathroom | 19 | 38.7 / 8.5 | 15 | 589.4 / 8.5 | 5 | 11.2 / 5.3 |
Abbreviations: CP, contact precautions; MRSA, methicillin-resistant Staphylococcus aureus; NA, not applicable; VRE, vancomycin-resistant enterococci.
MDRO CP rooms were more likely than nonprecaution rooms to have more than 1 sample composite positive for an MDRO. For the 66 CP rooms that were positive for an MDRO, just over half (58%) had a single MDRO-positive sample from 1 of the room surface composites, 29% had MDRO-positive samples from 2 of the room surface composites, and 14% had MDRO-positive samples from all 3 room surface composites (Table 4, Supplementary Table S4). In contrast, 75%, 18%, and 7% of the 28 control rooms that were culture positive for an MDRO had 1, 2, or 3 positive room surface composite samples, respectively. The near-patient composite was positive more frequently (72%) than the far-patient (38%) or toileting area (38%) composites from rooms positive for an MDRO. This pattern differed by room type, with 74%, 41%, and 41% of CP rooms positive for an MDRO in near-patient, far-patient, and toileting area, respectively, and 68%, 32%, and 32% of control rooms positive for an MDRO (Supplementary Table S4).
| . | MDRO CP Room n = 209 . | . | . | Control n = 234 . | . | . |
|---|---|---|---|---|---|---|
| . | Number of Rooms . | Percent of Rooms . | Percent of MDRO-Positive CP Rooms . | Number of Rooms . | Percent of Rooms . | Percent of MDRO-Positive Control Rooms . |
| None + | 143 | 68 | … | 206 | 88 | … |
| 1 zone + | 38 | 18 | 58 | 21 | 9 | 75 |
| 2 zone + | 19 | 9 | 29 | 5 | 2 | 18 |
| 3 zone + | 9 | 4 | 14 | 2 | 1 | 7 |
| . | MDRO CP Room n = 209 . | . | . | Control n = 234 . | . | . |
|---|---|---|---|---|---|---|
| . | Number of Rooms . | Percent of Rooms . | Percent of MDRO-Positive CP Rooms . | Number of Rooms . | Percent of Rooms . | Percent of MDRO-Positive Control Rooms . |
| None + | 143 | 68 | … | 206 | 88 | … |
| 1 zone + | 38 | 18 | 58 | 21 | 9 | 75 |
| 2 zone + | 19 | 9 | 29 | 5 | 2 | 18 |
| 3 zone + | 9 | 4 | 14 | 2 | 1 | 7 |
Abbreviation: CP, contact precautions.
| . | MDRO CP Room n = 209 . | . | . | Control n = 234 . | . | . |
|---|---|---|---|---|---|---|
| . | Number of Rooms . | Percent of Rooms . | Percent of MDRO-Positive CP Rooms . | Number of Rooms . | Percent of Rooms . | Percent of MDRO-Positive Control Rooms . |
| None + | 143 | 68 | … | 206 | 88 | … |
| 1 zone + | 38 | 18 | 58 | 21 | 9 | 75 |
| 2 zone + | 19 | 9 | 29 | 5 | 2 | 18 |
| 3 zone + | 9 | 4 | 14 | 2 | 1 | 7 |
| . | MDRO CP Room n = 209 . | . | . | Control n = 234 . | . | . |
|---|---|---|---|---|---|---|
| . | Number of Rooms . | Percent of Rooms . | Percent of MDRO-Positive CP Rooms . | Number of Rooms . | Percent of Rooms . | Percent of MDRO-Positive Control Rooms . |
| None + | 143 | 68 | … | 206 | 88 | … |
| 1 zone + | 38 | 18 | 58 | 21 | 9 | 75 |
| 2 zone + | 19 | 9 | 29 | 5 | 2 | 18 |
| 3 zone + | 9 | 4 | 14 | 2 | 1 | 7 |
Abbreviation: CP, contact precautions.
Weighted Results
Estimated facility contamination, adjusted by sample weights, ranged from 8.6% to 24.5%, with overlapping confidence intervals but overall did not differ significantly among facilities (Figure 1A, Table 1). Estimated facility contamination with individual target MDROs was lower and varied by facility (Table 5). MRSA and VRE were detected in rooms at all 6 facilities, with the proportions of MRSA- or VRE-positive rooms varying little among facility and unit type (Figure 1B and 1C). C. difficile, CRE, and CRA were detected in 5, 1, and 0 facilities, respectively (Table 5).
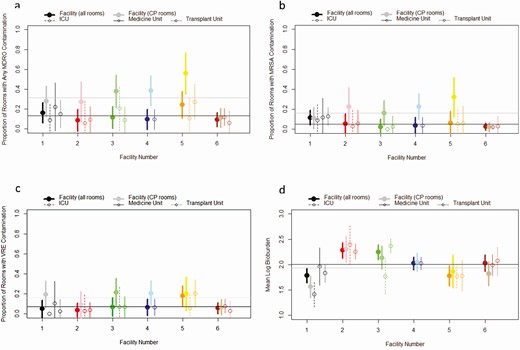
Facility and unit-level estimates of contamination: (A) any MDRO, (B) MRSA, (C) VRE, and (D) weighted mean of median room-level log10 total CFU/100 cm2. Facility level estimates for CP rooms are not weighted. Unit type estimates incorporate weights based on the sample design and are aggregated to the unit type and facility. The95% confidence intervals incorporate variance among sampling events. Overall mean for all rooms (black) and CP rooms (gray) are represented by horizontal lines. Abbreviations: CFU, colony-forming unit; CP, contact precautions; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci.
Estimates of Weighted Percent of Rooms With Specific MDRO Detection and 95% Confidence Interval (CI) by Facility
| Facility . | MRSA, % (95% CI) . | VRE, % (95% CI) . | C. difficile, % (95% CI) . | CRE, % (95% CI) . | CRA, % (95% CI) . | Any MDRO, % (95% CI) . |
|---|---|---|---|---|---|---|
| 1 | 11.6 (4–19) | 4.9 (0–14) | .3 (0–4) | 0 NA | 0 NA | 16.3 (6–26) |
| 2 | 5.4 (0–16) | 3.8 (0–11) | .5 (0–5) | 0 NA | 0 NA | 8.6 (0–20) |
| 3 | 2.2 (0–10) | 6.9 (0–16) | 2.9 (0–6) | 0 NA | 0 NA | 11.6 (0–23) |
| 4 | 3.6 (0–12) | 6.5 (0–15) | 0 NA | 0 NA | 0 NA | 9.6 (0–20) |
| 5 | 6.4 (0–18) | 17.9 (8–28) | 4.1 (0–9) | 0.5 (0–5) | 0 NA | 24.5 (11–38) |
| 6 | 2.6 (0–6) | 5.7 (0–11) | 1.4 (0–4) | 0 NA | 0 NA | 9.4 (2–16) |
| All | 5.2 (2–8) | 7.1 (4–10) | 1.2 (0–3) | 0.1 (0–1) | 0 NA | 12.7 (9–17) |
| Facility . | MRSA, % (95% CI) . | VRE, % (95% CI) . | C. difficile, % (95% CI) . | CRE, % (95% CI) . | CRA, % (95% CI) . | Any MDRO, % (95% CI) . |
|---|---|---|---|---|---|---|
| 1 | 11.6 (4–19) | 4.9 (0–14) | .3 (0–4) | 0 NA | 0 NA | 16.3 (6–26) |
| 2 | 5.4 (0–16) | 3.8 (0–11) | .5 (0–5) | 0 NA | 0 NA | 8.6 (0–20) |
| 3 | 2.2 (0–10) | 6.9 (0–16) | 2.9 (0–6) | 0 NA | 0 NA | 11.6 (0–23) |
| 4 | 3.6 (0–12) | 6.5 (0–15) | 0 NA | 0 NA | 0 NA | 9.6 (0–20) |
| 5 | 6.4 (0–18) | 17.9 (8–28) | 4.1 (0–9) | 0.5 (0–5) | 0 NA | 24.5 (11–38) |
| 6 | 2.6 (0–6) | 5.7 (0–11) | 1.4 (0–4) | 0 NA | 0 NA | 9.4 (2–16) |
| All | 5.2 (2–8) | 7.1 (4–10) | 1.2 (0–3) | 0.1 (0–1) | 0 NA | 12.7 (9–17) |
Abbreviations: CP, contact precautions; CRE, carbapenem-resistant Enterobacteriaceae; MRSA, methicillin-resistant Staphylococcus aureus; NA, not applicable; VRE, vancomycin-resistant enterococci.
Estimates of Weighted Percent of Rooms With Specific MDRO Detection and 95% Confidence Interval (CI) by Facility
| Facility . | MRSA, % (95% CI) . | VRE, % (95% CI) . | C. difficile, % (95% CI) . | CRE, % (95% CI) . | CRA, % (95% CI) . | Any MDRO, % (95% CI) . |
|---|---|---|---|---|---|---|
| 1 | 11.6 (4–19) | 4.9 (0–14) | .3 (0–4) | 0 NA | 0 NA | 16.3 (6–26) |
| 2 | 5.4 (0–16) | 3.8 (0–11) | .5 (0–5) | 0 NA | 0 NA | 8.6 (0–20) |
| 3 | 2.2 (0–10) | 6.9 (0–16) | 2.9 (0–6) | 0 NA | 0 NA | 11.6 (0–23) |
| 4 | 3.6 (0–12) | 6.5 (0–15) | 0 NA | 0 NA | 0 NA | 9.6 (0–20) |
| 5 | 6.4 (0–18) | 17.9 (8–28) | 4.1 (0–9) | 0.5 (0–5) | 0 NA | 24.5 (11–38) |
| 6 | 2.6 (0–6) | 5.7 (0–11) | 1.4 (0–4) | 0 NA | 0 NA | 9.4 (2–16) |
| All | 5.2 (2–8) | 7.1 (4–10) | 1.2 (0–3) | 0.1 (0–1) | 0 NA | 12.7 (9–17) |
| Facility . | MRSA, % (95% CI) . | VRE, % (95% CI) . | C. difficile, % (95% CI) . | CRE, % (95% CI) . | CRA, % (95% CI) . | Any MDRO, % (95% CI) . |
|---|---|---|---|---|---|---|
| 1 | 11.6 (4–19) | 4.9 (0–14) | .3 (0–4) | 0 NA | 0 NA | 16.3 (6–26) |
| 2 | 5.4 (0–16) | 3.8 (0–11) | .5 (0–5) | 0 NA | 0 NA | 8.6 (0–20) |
| 3 | 2.2 (0–10) | 6.9 (0–16) | 2.9 (0–6) | 0 NA | 0 NA | 11.6 (0–23) |
| 4 | 3.6 (0–12) | 6.5 (0–15) | 0 NA | 0 NA | 0 NA | 9.6 (0–20) |
| 5 | 6.4 (0–18) | 17.9 (8–28) | 4.1 (0–9) | 0.5 (0–5) | 0 NA | 24.5 (11–38) |
| 6 | 2.6 (0–6) | 5.7 (0–11) | 1.4 (0–4) | 0 NA | 0 NA | 9.4 (2–16) |
| All | 5.2 (2–8) | 7.1 (4–10) | 1.2 (0–3) | 0.1 (0–1) | 0 NA | 12.7 (9–17) |
Abbreviations: CP, contact precautions; CRE, carbapenem-resistant Enterobacteriaceae; MRSA, methicillin-resistant Staphylococcus aureus; NA, not applicable; VRE, vancomycin-resistant enterococci.
Clostridioides difficile Toxin Testing
All culture isolates identified as C. difficile by agar plate or broth culture were tested for C. difficile toxins A and B. C. difficile isolates from 11 samples were tested for toxins (9 patient rooms samples and 2 shared unit surfaces). Nine of the 11 samples were toxin positive. One toxin-negative isolate was isolated from shared nursing equipment and 1 was isolated from a patient room.
Surface Bacterial Bioburden
The sample bacterial bioburden plate count per 100 cm2 ranged from <2.6 to >5391 CFU, with room-level median values ranging from <1.4 to >4119 CFU. Bioburden varied among facilities and among units within facilities, but nonoverlapping confidence intervals demonstrated no statistically significant differences (Figure 1D). Patterns of bioburden magnitude by room type varied by facility; CP room bioburden was higher than control rooms in some facilities and lower in others (Figure 2A). A similar, but not consistent, pattern was seen in rooms with and without MDRO contamination (Figure 2B).

Weighted mean of median room-level log10 total CFU/100 cm2 by facility and separated for (A) “Control” rooms (C, green) and CP “MDRO” rooms (M, yellow), and (B) rooms with no MDRO contamination (0, green) and rooms with any detectable MDRO contamination (1, yellow). Numbers along the top are the number of room-sample events included in the boxplot. Abbreviations: CFU, colony-forming units; CP, contact precautions; MDRO, multidrug-resistant organism.
The bioburden on the composite surface samples revealed patterns by composite type with near-patient zone bioburden always highest and far-patient zone bioburden usually lowest (Supplementary Figure S1). Bioburden of shared areas ranged from <2.6 to >5391 CFU per 100 cm2 and was higher on shared nursing surfaces (median = 181 CFU) than the shared nursing equipment (median = 27 CFU).
DISCUSSION
Using a composite environmental sampling approach, we conducted a multifacility assessment of MDRO surface contamination in patient rooms and shared areas in 6 acute care facilities in the United States. We found that environmental surface contamination of CP rooms was common and frequently associated with the pathogen for which the patient was on contact precautions. However, discordant contamination in CP rooms (detection of an MDRO the patient was not known to have), as well as contamination of control rooms, was also detected at all participating facilities. Discordant MDRO contamination of CP rooms was slightly more frequent than MDRO contamination of control rooms. This may be due to higher antibiotic use, decreased immunity, or underlying medical issues of precaution patients, making them more prone to additional HAI acquisition, including MDROs [29, 30]. Discordant MDRO contamination of CP rooms and MDRO contamination of non-CP rooms and shared nursing areas suggests that colonized patients may be going undetected, cleaning is not removing contamination from prior MDRO patient rooms or shared area surfaces, or poor hand hygiene practices are allowing MDROs to be transferred from infected or colonized patients to other surfaces within the unit.
Detection frequency of C. difficile on C. difficile CP room surfaces (4.3%) was substantially lower than the detection of MRSA on MRSA CP room surfaces (23.2%) or VRE on VRE CP room surfaces (31.0%) and generally lower than what has been observed in other studies on C. difficile environmental contamination [31]. The lower C. difficile detection frequency may be attributable to improved cleaning and disinfection in C. difficile CP rooms during patient occupancy, or due to the changing epidemiology of C. difficile [32]. The definition used for C. difficile CP rooms (up to 30 days since positive toxin test) may have also influenced environmental detection due to rapid decline in C. difficile shedding following treatment for C. difficile disease [33].
Detection of MDROs on room surfaces could not be directly linked to the timing or adequacy of cleaning, as these samples were collected at various times throughout the day while patients were occupying and shedding within the room. We also did not determine the length of time in which the patient had occupied the room prior to the room surface sampling event. Additionally, infection control practices such as MDRO surveillance testing, PPE use, and institutional cleaning policies often vary, and may differ in their designation of cleaning tasks for nursing and environmental services staff [34]. We did not observe any correlation between bioburden levels and MDRO presence or absence (Supplementary Figure S2), which conflicts with previous reports [25, 35, 36]. It is possible that differences between our results and previous reports may be attributable to timing of when surface sampling was done in relation to cleaning, non-inclusion of postterminal cleaning room samples, size of the sampling area, or differences in testing methods.
Although environmental contamination was significantly more frequent in CP rooms compared to non-CP rooms, the frequency of MDRO detection on non-CP room surfaces was not negligible and suggests that non-CP rooms may also serve as an important reservoir for ongoing MDRO transmission. Consequently, interventions that focus solely on improved terminal cleaning of CP rooms are insufficient. Evaluating MDRO presence in patient rooms may provide insight into MDRO transmission by detecting unrecognized MDRO carriage or identifying opportunities for patient acquisition when the patient is a noncarrier. Culture-based testing results may take several days to obtain, thus future research could explore rapid or molecular-based environmental testing methods to improve environmental sample turnaround times. Further research could better characterize the sources of environmental contamination of healthcare surfaces. Targeted interventions to reduce environmental contamination in shared unit areas, on mobile equipment, and in CP and non-CP patient rooms could include identifying asymptomatic colonizers, creating improved cleaning protocols for shared medical and other high touch surfaces, such as floors, and identifying other clinical areas within acute care facilities that may still play an important role in MDRO transmission.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We would like to acknowledge funding for this supplement from the Informatics, Decision-Enhancement and Analytic Sciences (IDEAS) Center and the VA Health Services Research and Development (HSR&D) Service.
Disclaimer. Its contents are the authors’ sole responsibility and do not necessarily represent official National Institutes of Health (NIH) views.
Financial support. This work was supported by the United States Centers for Disease Control and Prevention (contract 200-2011-42039 2015-002 to M. S.) and the United States Department of Veterans Affairs Health Services Research and Development (grant CIN-13-414 to M. S.).
The contents are solely the responsibility of the authors and do not necessarily represent the official position or policy of the Centers for Disease Control and Prevention, the Department of Health and Human Services, the US Department of Veterans Affairs, or the United States government. REDCap electronic data capture software for this project was supported by NIH/NCRR Colorado CTSI grant UL1 RR025780.
Supplement sponsorship. This supplement is sponsored by the Informatics, Decision-Enhancement and Analytic Sciences (IDEAS) Center.
Potential conflicts of interest. C. D. reports grants from PDI and Clorox, outside the submitted work. W. T. reports grants from the U.S. Centers for Disease Control and Prevention and Bill and Melinda Gates Foundation, outside the submitted work. L. V. reports nonfinancial support from the Society for Healthcare Epidemiology of America. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Present affiliation: Department of Epidemiology of Microbial Diseases, Yale School of Public Health, New Haven, Connecticut, USA




