-
PDF
- Split View
-
Views
-
Cite
Cite
Matthew P Cheng, Alexander Lawandi, Guillaume Butler-Laporte, Samuel De l’Étoile-Morel, Katryn Paquette, Todd C Lee, Adjunctive Daptomycin in the Treatment of Methicillin-susceptible Staphylococcus aureus Bacteremia: A Randomized, Controlled Trial, Clinical Infectious Diseases, Volume 72, Issue 9, 1 May 2021, Pages e196–e203, https://doi.org/10.1093/cid/ciaa1000
Close - Share Icon Share
Abstract
Bloodstream infections (BSIs) with methicillin-susceptible Staphylococcus aureus (MSSA) are associated with significant morbidity and mortality. Our objective in this study was to determine the efficacy of synergistic treatment with daptomycin when given with either cefazolin or cloxacillin for the treatment of MSSA BSI.
A randomized, double-blind, placebo-controlled trial was performed at 2 academic hospitals in Montreal, Canada. Patients aged ≥18 years with MSSA BSI receiving either cefazolin or cloxacillin monotherapy were considered for inclusion. In addition to the standard-of-care treatment, participants received a 5-day course of adjunctive daptomycin or placebo. The primary outcome was the duration of MSSA BSI in days.
Of 318 participants screened, 115 were enrolled and 104 were included in the intention-to-treat analysis (median age, 67 years; 34.5% female). The median duration of bacteremia was 2.04 days among patients who received daptomycin vs 1.65 days in those who received placebo (absolute difference, 0.39 days; P = .40). In a modified intention-to-treat analysis that involved participants who remained bacteremic at the time of enrollment, we found a median duration of bacteremia of 3.06 days among patients who received daptomycin vs 3.0 days in those who received placebo (absolute difference, 0.06 days; P = .77). Ninety-day mortality in the daptomycin arm was 18.9% vs 17.7% in the placebo arm (P = 1.0).
Among patients with MSSA BSIs, the administration of adjunctive daptomycin therapy to standard-of-care treatment did not shorten the duration of bacteremia and should not be routinely considered.
NCT02972983.
(See the Editorial Commentary by Tong and Davis on pages e204–5.)
Staphylococcus aureus bacteremia remains among the most common and serious bacterial infections worldwide due to its proclivity for causing metastatic infections, severe manifestations of sepsis, and death [1–7]. While progress in reducing S. aureus bacteremia-related complications has been made through the development of standard bundles of care [8, 9], there are still more than 100 000 episodes and approximately 20 000 deaths each year in the United States alone [10].
Over the past several years, there has been an increase in the number of randomized, controlled trials involving patients with S. aureus bloodstream infections (BSIs) [9, 11–13], including the use of adjunctive rifampicin for S. aureus bacteremia and combination treatment trials for methicillin-resistant BSIs. Unfortunately, none have been able to improve overall patient outcomes, with mortality rates approximating 15%–20% [5, 14–17]. The cornerstone of management for patients with methicillin-susceptible S. aureus (MSSA) bacteremia continues to be early administration of an anti-staphylococcal beta-lactam (ASBL), source control, and supportive care [18–20]. Due to the potential severity of MSSA BSI, there remains active interest in novel treatment strategies to improve clinical outcomes.
Daptomycin is a lipopeptide antibiotic that is active against S. aureus and is approved by both the US Food and Drug Administration and Health Canada for the treatment of S. aureus BSI [21]. Daptomycin functions by inserting itself into the bacterial cell membrane, resulting in depolarization, extravasation of cellular contents, and microbial death [22, 23]. It has demonstrated synergistic activity with beta-lactam antibiotics against S. aureus through time kill methods in vitro [24]. While many of the in vitro studies were performed in methicillin-resistant S. aureus (MRSA), multiple hypotheses have emerged about the mechanism behind the observed synergy. First, the ability of beta-lactam drugs to reduce the net positive charge on the organism’s cell membrane may increase daptomycin binding and efficacy [25]. Second, daptomycin may be able to inhibit bacterial peptidoglycan synthesis, thereby preventing penicillin-binding protein cross-linking and enhancing beta-lactam activity. Third, given that some strains of MSSA may express relatively low amounts of mecA [26], the addition of daptomycin may be of benefit. However, there is a paucity of clinical data that address the effect of daptomycin and beta-lactam combination therapy in the management of MSSA BSI. While certain institutions have reported using daptomycin along with an ASBL [27] for MSSA, the combination has not been formally evaluated in a clinical trial. Our objective in this study was to determine the efficacy of daptomycin when given as an adjunct with an ASBL for the treatment of patients with MSSA BSI.
METHODS
Trial Design
We conducted the Daptomycin as Adjunctive Therapy for Staphylococcus aureus Bacteremia in Hospital (DASH) study, a randomized, double-blind, placebo-controlled trial. The protocol was designed by the study investigators and has been published previously [28]. It reflects the standard of care at the study sites in the management of hospitalized patients with MSSA BSI in addition to evaluation of the usage of adjunctive daptomycin (Supplementary Appendix). Two academic hospitals in Montreal, Canada, participated in the study. The McGill University Health Center (MUHC) Research Ethics Board approved the protocol. The authors vouch for the data and analysis, as well as for the fidelity of this report to the study protocol. This article is reported according to the Consolidated Standards for Reporting of Trials [29].
Participants
We recruited adult patients (aged ≥18 years) with a documented monomicrobial BSI caused by MSSA. Patients were enrolled within a maximum of 72 hours from the first positive blood culture collected. Patients were excluded if they were moribund due to other illnesses and were expected to die within 5 days according to the treating team, they were clinically appropriate for admission to a critical care service but were not going to receive such care due to advanced directives, they were unable to receive an ASBL or daptomycin, they required additional antibiotic therapy other than an ASBL according to the treating team, or they were receiving or were going to receive open-label daptomycin. These exclusion criteria precluded individuals with known type I hypersensitivity to ASBL or daptomycin, as well as those with polymicrobial infections that required additional antibiotic therapy.
Interventions
All participants recruited in the study received standard-of-care treatment for MSSA BSI that, at our institution, consists of the administration of a beta-lactam antibiotic, removal of intravascular devices, obtaining a cardiac echocardiogram, consultation with an infectious diseases specialist, and additional ancillary investigations to identify and treat metastatic infections as needed. The choice of ASBL, either cloxacillin or cefazolin, was made at the treating team’s discretion in consultation with the infectious diseases service.
Study participants were randomized to receive adjunctive daptomycin or matching placebo intravenously once daily for 5 days. Daptomycin was prescribed at a dose of 6 mg/kg. This dose was chosen based on previous studies of daptomycin for MSSA BSI [21] and is the Health Canada monograph approved dose for BSI. In obese patients, the actual body weight is used, which is in keeping with the monograph. Participants with abnormal renal function received daptomycin or placebo on an alternate schedule per guideline dosing recommendations (see Supplementary Appendix). During the study period, the MUHC research pharmacy and the study investigators monitored the patient’s renal function and provided blinded dosing modifications to the study drugs as needed.
The study protocol required routine blood samples to be obtained to document clearance of the BSI and to monitor for adverse drug effects. Two sets of blood cultures were obtained daily for 5 days or until microbiological clearance was confirmed. As per institutional policy, the first blood culture set contained both an aerobic and an anaerobic bottle, whereas the second set contained only an aerobic bottle. A total of 30 mL of blood was obtained as per current recommendations [30]. Complete blood counts, serum electrolytes, creatinine, creatine kinase, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, gamma-glutamyl transferase, and bilirubin were monitored. Written informed consent was obtained from all participants or their surrogate decision-makers before enrollment in the trial. All patients had baseline demographic and clinical data recorded.
Outcomes
The primary outcome of the study was the total duration of MSSA BSI, compared with placebo, decreased the duration of MSSA BSI in hospitalized patients. The total duration of bacteremia was determined based on the time difference between the first positive culture and the first negative culture measured in fractional days using the date and time of blood culture collection from our electronic medical records system. Because we collect daily (not hourly) blood cultures, they become negative at some time point between the last positive culture that was collected and the time the first negative culture is subsequently collected. As culture times may be dependent on phlebotomy and nursing workloads and to be uniformly consistent, we assign the time the blood culture became negative as the midpoint between the last positive culture and the first negative one. Secondary outcomes included all-cause mortality at 30 days; relapsed bacteremia, defined as a repeat MSSA BSI ≥48 hours and ≤30 and after first obtaining a negative blood culture; clinically relevant embolic or metastatic MSSA disease within 30 days of the first positive blood culture; and nephrotoxicity, hepatotoxicity, and rhabdomyolysis within 5 days of enrollment. A post hoc decision was made after the last patient was recruited, but prior to any analysis, in order to assess all-cause mortality at 90 days. Additionally, we report 90 days for relapse or clinically relevant embolic disease to be consistent with the published protocol. Uncomplicated BSI was defined per previous definitions and consisted of patients in whom endocarditis has been excluded, no implanted prostheses are present, follow-up blood cultures drawn 2–4 days after the initial set are sterile, the patient was afebrile within 72 hours of initiation of effective antibiotic therapy, and no evidence of metastatic infection is present on exam [31, 32]. The Supplementary Appendix includes the definitions of secondary outcomes.
Sample Size
A sensitivity analysis was performed to determine the sample size required to achieve 80% power for the primary outcome. Assuming that the duration of BSI time was normally, log-normally, or Poisson distributed, with a mean duration of bacteremia of 3 days and a standard deviation of 2 days (3 for Poisson), a sample size of approximately 102 participants would be required to detect a 1-day difference in duration of bacteremia at a significance level of 5%. Assumptions were adapted from a previously published study [11] and derived from our center’s local epidemiology. A 1-day difference in duration of bacteremia was chosen as a clinically significant difference between treatment strategies through a review of the literature and consultation with infectious diseases specialists involved in the study protocol development.
Randomization
Upon enrollment in the trial, each participant was assigned a unique study identifier that was used for randomization and statistical purposes. Randomization was performed by permuted block with variable block sizes (4–8). The randomization table was held by the central research pharmacy and was not disclosed to the study investigators.
Blinding
Treatment allocation was blinded to the patients, their clinicians, the study investigators who assessed clinical outcomes, as well as those who performed the statistical analysis. Unblinding could be requested by the treating physician on an individual basis in the event of a serious adverse event suspected to be due to the study drug. This was done by the central research pharmacy, and the study investigators remained unaware of the patient’s treatment allocation.
Statistical Methods
The final dataset was inspected for errors, outliers, and missing values. All participants had complete data for the exposure and outcomes of interest. No participant was omitted due to missing data. Participants were analyzed according to their treatment allocation in an intention-to-treat (ITT) analysis. A modified intention-to-treat (mITT) analysis was performed in participants whose blood cultures were not already negative at the time of first study drug administration. Participants who received the full study drug course according to their treatment allocation were analyzed in a per-protocol (PP) analysis. The most common reason for exclusion from the PP analysis was a change in antibiotics from cloxacillin or cefazolin to a broader-spectrum agent (11/21 patients). A full list of reasons for exclusion from the PP analysis can be found in Supplementary Materials 1.
Baseline patient characteristics are presented as proportions for categorical variables and as median and interquartile ranges for continuous variables. The primary outcome of duration of MSSA BSI was evaluated in a time-to-event analysis using Kaplan-Meier curves. Comparisons were made using the log-rank test. Secondary outcomes were compared using the Fisher exact test. All statistical analyses were performed using Stata 15.0 (StataCorp, College Station, TX), SAS 9.4 (SAS Institute Inc., Cary, NC), or R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patients
Between November 2016 and June 2019, 115 patients were enrolled in the study (Figure 1). A total of 11 patients were subsequently excluded from the study; 9 did not receive the study intervention and 2 were treated with piperacillin-tazobactam. In total, 104 patients were included in the final analysis as the ITT population. Seventy-one patients continued to have positive blood cultures at the time of study enrollment and were included in the mITT analysis. Eighty-three patients were included in the PP analysis.
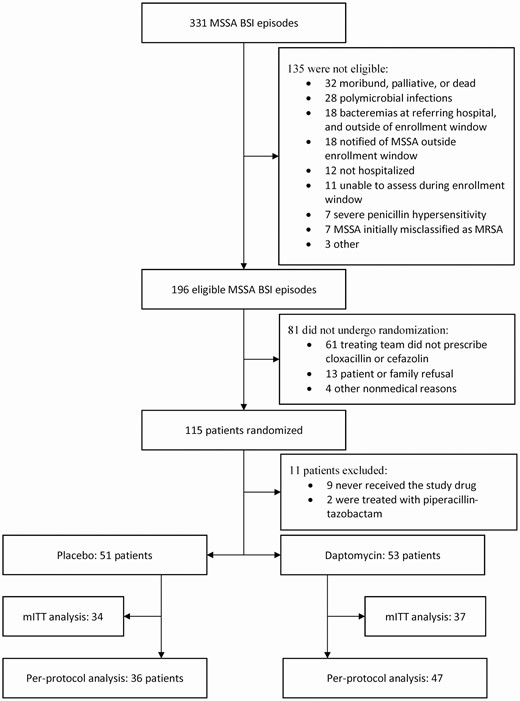
Patient flow chart. Abbreviations: BSI, bloodstream infection; mITT, modified intention-to-treat; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus.
Patient characteristics were balanced between groups and are presented in Table 1. The median age at study enrollment was 67 years (interquartile range [IQR], 56–73), and 36 participants (34.6%) were female. The median Charlson comorbidity index was 5 (IQR, 3–9). The most common sources of bacteremia were endovascular (39.4%), followed by skin and soft tissue infections (24.0%), osteomyelitis (7.7%), and pneumonia (6.7%). Most participants were treated with cefazolin (73.1%) and had a complicated BSI (78.8%). Only 3 patients reported actively using intravenous drugs: 2 in the placebo group and 1 in the daptomycin group. Median time between the first positive culture and enrollment was 47 hours. We had a 100% follow-up rate for all outcomes, including 90-day mortality.
| . | No. (%) . | ||
|---|---|---|---|
| Variable . | Placebo . | Daptomycin . | Total . |
| No. of participants | 51 | 53 | 104 |
| Patient details | |||
| Age, median (IQR), y | 65 (57–72) | 68 (55–75) | 67 (56–73) |
| Female sex | 16 (31.3) | 20 (37.7) | 36 (34.6) |
| Charlson comorbidity index, median (IQR) | 4 (3–8.5) | 5 (3–9) | 5 (3–9) |
| Active solid organ malignancy | 7 (13.7) | 11 (20.8) | 18 (17.3) |
| Active hematologic malignancy | 5 (9.8) | 4 (7.8) | 9 (8.7) |
| Prosthetic valve | 3 (5.9) | 6 (11.3) | 9 (8.7) |
| Severe valvular diseasea | 6 (11.8) | 5 (9.4) | 11 (10.6) |
| Left-sided | 3 (5.9) | 3 (5.7) | 6 (5.8) |
| Right-sided | 3 (5.9) | 2 (3.8) | 5 (4.8) |
| Other foreign body | 11 (21.6) | 12 (22.6) | 23 (22.1) |
| Intravascular | 7 (13.7) | 7 (13.2) | 14 (13.5) |
| Extravascular | 4 (7.8) | 5 (9.4) | 9 (8.7) |
| Central venous catheter when positive | 13 (25.5) | 20 (37.8) | 33 (31.7) |
| Procedure in preceding 30 days | 9 (17.6) | 9 (17.0) | 18 (17.3) |
| Intravascular device placement | 2 (3.9) | 5 (9.4) | 7 (6.7) |
| Cardiothoracic surgery | 1 (2.0) | 1 (2.0) | 2 (2.0) |
| Orthopedic/spinal surgery | 4 (7.8) | 2 (3.8) | 6 (5.8) |
| Other | 2 (3.9) | 1 (2.0) | 3 (2.9) |
| Methicillin-resistant Staphylococcus aureus colonization in preceding 30 days | 0 (0) | 1 (2.0) | 1 (1.0) |
| Infection details | |||
| Sequential organ failure assessment score on enrollment, median (IQR) | 1 (0–3.5) | 2 (0–4) | 1 (0–4) |
| qPitt bacteremia score, median (IQR) | 0 (0–0) | 0 (0–1) | 0 (0–0.25) |
| Source of bacteremia a | |||
| Definite endocarditis by Duke criteria | 7 (13.7) | 5 (9.4) | 12 (11.5) |
| Endovascular without prosthesis | 7 (13.7) | 4 (7.8) | 11 (10.6) |
| Endovascular with prosthesis | 11 (21.6) | 19 (35.8) | 30 (28.8) |
| Pneumonia | 4 (7.8) | 3 (5.7) | 7 (6.7) |
| Septic arthritis | 3 (5.9) | 1 (2.0) | 4 (3.8) |
| Osteomyelitis | 5 (9.8) | 3 (5.7) | 8 (7.7) |
| Skin and soft tissue | 11 (21.6) | 14 (26.4) | 25 (24.0) |
| Other | 3 (5.9) | 3 (5.7) | 6 (5.8) |
| Unknown | 12 (23.5) | 8 (15.1) | 20 (19.2) |
| Complicated bacteremia a | 39 (76.5) | 43 (81.1) | 82 (78.8) |
| Endocarditis not excluded | 10 (19.6) | 12 (22.6) | 22 (21.2) |
| Implanted prostheses | 16 (31.4) | 19 (35.8) | 35 (33.7) |
| Positive culture at 2–4 days | 34 (66.7) | 37 (69.8) | 71 (68.3) |
| Fever at 72 hours | 4 (7.8) | 8 (15.1) | 12 (11.5) |
| Evidence of metastatic infection | 9 (17.6) | 13 (24.5) | 22 (21.2) |
| Treatment details | |||
| Cefazolin | 35 (68.6) | 41 (77.4) | 76 (73.1) |
| Cloxacillin | 16 (31.4) | 12 (22.6) | 28 (26.9) |
| Hours from positive culture to enrollment, median (IQR) | 46 (35–50) | 49 (42–57) | 47 (39–53) |
| . | No. (%) . | ||
|---|---|---|---|
| Variable . | Placebo . | Daptomycin . | Total . |
| No. of participants | 51 | 53 | 104 |
| Patient details | |||
| Age, median (IQR), y | 65 (57–72) | 68 (55–75) | 67 (56–73) |
| Female sex | 16 (31.3) | 20 (37.7) | 36 (34.6) |
| Charlson comorbidity index, median (IQR) | 4 (3–8.5) | 5 (3–9) | 5 (3–9) |
| Active solid organ malignancy | 7 (13.7) | 11 (20.8) | 18 (17.3) |
| Active hematologic malignancy | 5 (9.8) | 4 (7.8) | 9 (8.7) |
| Prosthetic valve | 3 (5.9) | 6 (11.3) | 9 (8.7) |
| Severe valvular diseasea | 6 (11.8) | 5 (9.4) | 11 (10.6) |
| Left-sided | 3 (5.9) | 3 (5.7) | 6 (5.8) |
| Right-sided | 3 (5.9) | 2 (3.8) | 5 (4.8) |
| Other foreign body | 11 (21.6) | 12 (22.6) | 23 (22.1) |
| Intravascular | 7 (13.7) | 7 (13.2) | 14 (13.5) |
| Extravascular | 4 (7.8) | 5 (9.4) | 9 (8.7) |
| Central venous catheter when positive | 13 (25.5) | 20 (37.8) | 33 (31.7) |
| Procedure in preceding 30 days | 9 (17.6) | 9 (17.0) | 18 (17.3) |
| Intravascular device placement | 2 (3.9) | 5 (9.4) | 7 (6.7) |
| Cardiothoracic surgery | 1 (2.0) | 1 (2.0) | 2 (2.0) |
| Orthopedic/spinal surgery | 4 (7.8) | 2 (3.8) | 6 (5.8) |
| Other | 2 (3.9) | 1 (2.0) | 3 (2.9) |
| Methicillin-resistant Staphylococcus aureus colonization in preceding 30 days | 0 (0) | 1 (2.0) | 1 (1.0) |
| Infection details | |||
| Sequential organ failure assessment score on enrollment, median (IQR) | 1 (0–3.5) | 2 (0–4) | 1 (0–4) |
| qPitt bacteremia score, median (IQR) | 0 (0–0) | 0 (0–1) | 0 (0–0.25) |
| Source of bacteremia a | |||
| Definite endocarditis by Duke criteria | 7 (13.7) | 5 (9.4) | 12 (11.5) |
| Endovascular without prosthesis | 7 (13.7) | 4 (7.8) | 11 (10.6) |
| Endovascular with prosthesis | 11 (21.6) | 19 (35.8) | 30 (28.8) |
| Pneumonia | 4 (7.8) | 3 (5.7) | 7 (6.7) |
| Septic arthritis | 3 (5.9) | 1 (2.0) | 4 (3.8) |
| Osteomyelitis | 5 (9.8) | 3 (5.7) | 8 (7.7) |
| Skin and soft tissue | 11 (21.6) | 14 (26.4) | 25 (24.0) |
| Other | 3 (5.9) | 3 (5.7) | 6 (5.8) |
| Unknown | 12 (23.5) | 8 (15.1) | 20 (19.2) |
| Complicated bacteremia a | 39 (76.5) | 43 (81.1) | 82 (78.8) |
| Endocarditis not excluded | 10 (19.6) | 12 (22.6) | 22 (21.2) |
| Implanted prostheses | 16 (31.4) | 19 (35.8) | 35 (33.7) |
| Positive culture at 2–4 days | 34 (66.7) | 37 (69.8) | 71 (68.3) |
| Fever at 72 hours | 4 (7.8) | 8 (15.1) | 12 (11.5) |
| Evidence of metastatic infection | 9 (17.6) | 13 (24.5) | 22 (21.2) |
| Treatment details | |||
| Cefazolin | 35 (68.6) | 41 (77.4) | 76 (73.1) |
| Cloxacillin | 16 (31.4) | 12 (22.6) | 28 (26.9) |
| Hours from positive culture to enrollment, median (IQR) | 46 (35–50) | 49 (42–57) | 47 (39–53) |
Abbreviation: IQR, interquartile range.
aA patient may have belonged to more than 1 category.
| . | No. (%) . | ||
|---|---|---|---|
| Variable . | Placebo . | Daptomycin . | Total . |
| No. of participants | 51 | 53 | 104 |
| Patient details | |||
| Age, median (IQR), y | 65 (57–72) | 68 (55–75) | 67 (56–73) |
| Female sex | 16 (31.3) | 20 (37.7) | 36 (34.6) |
| Charlson comorbidity index, median (IQR) | 4 (3–8.5) | 5 (3–9) | 5 (3–9) |
| Active solid organ malignancy | 7 (13.7) | 11 (20.8) | 18 (17.3) |
| Active hematologic malignancy | 5 (9.8) | 4 (7.8) | 9 (8.7) |
| Prosthetic valve | 3 (5.9) | 6 (11.3) | 9 (8.7) |
| Severe valvular diseasea | 6 (11.8) | 5 (9.4) | 11 (10.6) |
| Left-sided | 3 (5.9) | 3 (5.7) | 6 (5.8) |
| Right-sided | 3 (5.9) | 2 (3.8) | 5 (4.8) |
| Other foreign body | 11 (21.6) | 12 (22.6) | 23 (22.1) |
| Intravascular | 7 (13.7) | 7 (13.2) | 14 (13.5) |
| Extravascular | 4 (7.8) | 5 (9.4) | 9 (8.7) |
| Central venous catheter when positive | 13 (25.5) | 20 (37.8) | 33 (31.7) |
| Procedure in preceding 30 days | 9 (17.6) | 9 (17.0) | 18 (17.3) |
| Intravascular device placement | 2 (3.9) | 5 (9.4) | 7 (6.7) |
| Cardiothoracic surgery | 1 (2.0) | 1 (2.0) | 2 (2.0) |
| Orthopedic/spinal surgery | 4 (7.8) | 2 (3.8) | 6 (5.8) |
| Other | 2 (3.9) | 1 (2.0) | 3 (2.9) |
| Methicillin-resistant Staphylococcus aureus colonization in preceding 30 days | 0 (0) | 1 (2.0) | 1 (1.0) |
| Infection details | |||
| Sequential organ failure assessment score on enrollment, median (IQR) | 1 (0–3.5) | 2 (0–4) | 1 (0–4) |
| qPitt bacteremia score, median (IQR) | 0 (0–0) | 0 (0–1) | 0 (0–0.25) |
| Source of bacteremia a | |||
| Definite endocarditis by Duke criteria | 7 (13.7) | 5 (9.4) | 12 (11.5) |
| Endovascular without prosthesis | 7 (13.7) | 4 (7.8) | 11 (10.6) |
| Endovascular with prosthesis | 11 (21.6) | 19 (35.8) | 30 (28.8) |
| Pneumonia | 4 (7.8) | 3 (5.7) | 7 (6.7) |
| Septic arthritis | 3 (5.9) | 1 (2.0) | 4 (3.8) |
| Osteomyelitis | 5 (9.8) | 3 (5.7) | 8 (7.7) |
| Skin and soft tissue | 11 (21.6) | 14 (26.4) | 25 (24.0) |
| Other | 3 (5.9) | 3 (5.7) | 6 (5.8) |
| Unknown | 12 (23.5) | 8 (15.1) | 20 (19.2) |
| Complicated bacteremia a | 39 (76.5) | 43 (81.1) | 82 (78.8) |
| Endocarditis not excluded | 10 (19.6) | 12 (22.6) | 22 (21.2) |
| Implanted prostheses | 16 (31.4) | 19 (35.8) | 35 (33.7) |
| Positive culture at 2–4 days | 34 (66.7) | 37 (69.8) | 71 (68.3) |
| Fever at 72 hours | 4 (7.8) | 8 (15.1) | 12 (11.5) |
| Evidence of metastatic infection | 9 (17.6) | 13 (24.5) | 22 (21.2) |
| Treatment details | |||
| Cefazolin | 35 (68.6) | 41 (77.4) | 76 (73.1) |
| Cloxacillin | 16 (31.4) | 12 (22.6) | 28 (26.9) |
| Hours from positive culture to enrollment, median (IQR) | 46 (35–50) | 49 (42–57) | 47 (39–53) |
| . | No. (%) . | ||
|---|---|---|---|
| Variable . | Placebo . | Daptomycin . | Total . |
| No. of participants | 51 | 53 | 104 |
| Patient details | |||
| Age, median (IQR), y | 65 (57–72) | 68 (55–75) | 67 (56–73) |
| Female sex | 16 (31.3) | 20 (37.7) | 36 (34.6) |
| Charlson comorbidity index, median (IQR) | 4 (3–8.5) | 5 (3–9) | 5 (3–9) |
| Active solid organ malignancy | 7 (13.7) | 11 (20.8) | 18 (17.3) |
| Active hematologic malignancy | 5 (9.8) | 4 (7.8) | 9 (8.7) |
| Prosthetic valve | 3 (5.9) | 6 (11.3) | 9 (8.7) |
| Severe valvular diseasea | 6 (11.8) | 5 (9.4) | 11 (10.6) |
| Left-sided | 3 (5.9) | 3 (5.7) | 6 (5.8) |
| Right-sided | 3 (5.9) | 2 (3.8) | 5 (4.8) |
| Other foreign body | 11 (21.6) | 12 (22.6) | 23 (22.1) |
| Intravascular | 7 (13.7) | 7 (13.2) | 14 (13.5) |
| Extravascular | 4 (7.8) | 5 (9.4) | 9 (8.7) |
| Central venous catheter when positive | 13 (25.5) | 20 (37.8) | 33 (31.7) |
| Procedure in preceding 30 days | 9 (17.6) | 9 (17.0) | 18 (17.3) |
| Intravascular device placement | 2 (3.9) | 5 (9.4) | 7 (6.7) |
| Cardiothoracic surgery | 1 (2.0) | 1 (2.0) | 2 (2.0) |
| Orthopedic/spinal surgery | 4 (7.8) | 2 (3.8) | 6 (5.8) |
| Other | 2 (3.9) | 1 (2.0) | 3 (2.9) |
| Methicillin-resistant Staphylococcus aureus colonization in preceding 30 days | 0 (0) | 1 (2.0) | 1 (1.0) |
| Infection details | |||
| Sequential organ failure assessment score on enrollment, median (IQR) | 1 (0–3.5) | 2 (0–4) | 1 (0–4) |
| qPitt bacteremia score, median (IQR) | 0 (0–0) | 0 (0–1) | 0 (0–0.25) |
| Source of bacteremia a | |||
| Definite endocarditis by Duke criteria | 7 (13.7) | 5 (9.4) | 12 (11.5) |
| Endovascular without prosthesis | 7 (13.7) | 4 (7.8) | 11 (10.6) |
| Endovascular with prosthesis | 11 (21.6) | 19 (35.8) | 30 (28.8) |
| Pneumonia | 4 (7.8) | 3 (5.7) | 7 (6.7) |
| Septic arthritis | 3 (5.9) | 1 (2.0) | 4 (3.8) |
| Osteomyelitis | 5 (9.8) | 3 (5.7) | 8 (7.7) |
| Skin and soft tissue | 11 (21.6) | 14 (26.4) | 25 (24.0) |
| Other | 3 (5.9) | 3 (5.7) | 6 (5.8) |
| Unknown | 12 (23.5) | 8 (15.1) | 20 (19.2) |
| Complicated bacteremia a | 39 (76.5) | 43 (81.1) | 82 (78.8) |
| Endocarditis not excluded | 10 (19.6) | 12 (22.6) | 22 (21.2) |
| Implanted prostheses | 16 (31.4) | 19 (35.8) | 35 (33.7) |
| Positive culture at 2–4 days | 34 (66.7) | 37 (69.8) | 71 (68.3) |
| Fever at 72 hours | 4 (7.8) | 8 (15.1) | 12 (11.5) |
| Evidence of metastatic infection | 9 (17.6) | 13 (24.5) | 22 (21.2) |
| Treatment details | |||
| Cefazolin | 35 (68.6) | 41 (77.4) | 76 (73.1) |
| Cloxacillin | 16 (31.4) | 12 (22.6) | 28 (26.9) |
| Hours from positive culture to enrollment, median (IQR) | 46 (35–50) | 49 (42–57) | 47 (39–53) |
Abbreviation: IQR, interquartile range.
aA patient may have belonged to more than 1 category.
Primary Outcome
Among the entire study population, the median duration of bacteremia was 2.04 days among patients who received daptomycin vs 1.65 days for those who received placebo. The absolute difference in the duration of bacteremia was 0.39 days (95% confidence interval [CI], −.58 to 1.4; P = .40). In a mITT analysis, the median duration of bacteremia was 3.06 days among patients who received daptomycin vs 3.0 days for those who received placebo. The absolute difference in the duration of bacteremia was 0.06 days (95% CI, −1.1 to 1.3; P = .77). Results were consistent in an analysis of the PP population (absolute difference, 0.44 days; 95% CI, −.65 to 1.53; P = .37). The time to BSI clearance was comparable between treatment groups in the ITT, mITT, and PP populations (Figure 2).
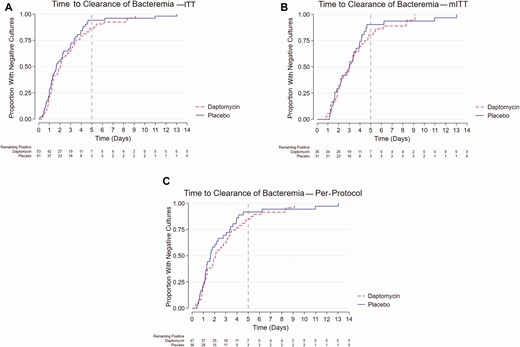
Time to clearance of bacteremia by treatment assignment in (A) ITT, (B) mITT, and (C) per-protocol analyses. Abbreviations: ITT, intention-to-treat; mITT, modified intention-to-treat.
Secondary Outcomes
Secondary outcomes are presented in Table 2 and were comparable between groups. The 90-day mortality in the placebo arm was 17.7% compared with 18.9% in the daptomycin arm (odds ratio [OR], 1.08; 95% CI, .36 to 3.35; P = 1.0). There was only 1 relapse within 30 days (in the daptomycin group), but there were 2 relapses in each arm at 90 days (P = 1). All embolic phenomenon occurred on diagnosis or within 30 days, with 9 in the placebo group and 13 in the daptomycin group (P = .47). Causes of death are reported in Table 3. While more patients died of S. aureus–related complications in the placebo group compared with the daptomycin group, this difference was not statistically significant (55.6% in the placebo group compared with 20% in the daptomycin group; OR, 0.22; 95% CI,.01 to 2.11; P = .15).
| . | No. (%) . | |||
|---|---|---|---|---|
| Variable . | Placebo (n = 51) . | Daptomycin (n = 53) . | Total (n = 104) . | P Value . |
| All-cause mortality (30 days) | 6 (11.8) | 8 (15.1) | 14 (13.5) | .62 |
| All-cause mortality (90 days) | 9 (17.7) | 10 (18.9) | 19 (18.3) | 1 |
| Relapsed bacteremia within 30 days (among those who survived bacteremia episode) | 0 (0) | 1 (1.9) | 1 (1.0) | 1 |
| Relapsed bacteremia within 90 days (among those who survived bacteremia episode) | 2 (3.9) | 2 (3.8) | 4 (3.8) | 1 |
| Embolic or metastatic MSSA disease within 30 days | 9 (17.7) | 13 (24.5) | 22 (21.2) | .47 |
| Embolic or metastatic MSSA disease within 90 days | 9 (17.7) | 13 (24.5) | 22 (21.2) | .47 |
| Nephrotoxicity on presentation | 6 (11.8) | 9 (17.0) | 15 (14.4) | .57 |
| Nephrotoxicity on treatmenta,b | 13 (28.9) | 21 (47.7) | 34 (32.7) | .15 |
| Hepatotoxicity b | 3 (5.88) | 2 (3.77) | 5 (4.8) | .675 |
| Rhabdomyolysis b | 3 (5.88) | 0 (0) | 3 (2.9) | 0.11 |
| . | No. (%) . | |||
|---|---|---|---|---|
| Variable . | Placebo (n = 51) . | Daptomycin (n = 53) . | Total (n = 104) . | P Value . |
| All-cause mortality (30 days) | 6 (11.8) | 8 (15.1) | 14 (13.5) | .62 |
| All-cause mortality (90 days) | 9 (17.7) | 10 (18.9) | 19 (18.3) | 1 |
| Relapsed bacteremia within 30 days (among those who survived bacteremia episode) | 0 (0) | 1 (1.9) | 1 (1.0) | 1 |
| Relapsed bacteremia within 90 days (among those who survived bacteremia episode) | 2 (3.9) | 2 (3.8) | 4 (3.8) | 1 |
| Embolic or metastatic MSSA disease within 30 days | 9 (17.7) | 13 (24.5) | 22 (21.2) | .47 |
| Embolic or metastatic MSSA disease within 90 days | 9 (17.7) | 13 (24.5) | 22 (21.2) | .47 |
| Nephrotoxicity on presentation | 6 (11.8) | 9 (17.0) | 15 (14.4) | .57 |
| Nephrotoxicity on treatmenta,b | 13 (28.9) | 21 (47.7) | 34 (32.7) | .15 |
| Hepatotoxicity b | 3 (5.88) | 2 (3.77) | 5 (4.8) | .675 |
| Rhabdomyolysis b | 3 (5.88) | 0 (0) | 3 (2.9) | 0.11 |
Abbreviations: MSSA, methicillin-susceptible Staphylococcus aureus.
aExcluding patients who had evidence of nephrotoxicity on presentation.
bWithin 5 days of patient enrollment into the study.
| . | No. (%) . | |||
|---|---|---|---|---|
| Variable . | Placebo (n = 51) . | Daptomycin (n = 53) . | Total (n = 104) . | P Value . |
| All-cause mortality (30 days) | 6 (11.8) | 8 (15.1) | 14 (13.5) | .62 |
| All-cause mortality (90 days) | 9 (17.7) | 10 (18.9) | 19 (18.3) | 1 |
| Relapsed bacteremia within 30 days (among those who survived bacteremia episode) | 0 (0) | 1 (1.9) | 1 (1.0) | 1 |
| Relapsed bacteremia within 90 days (among those who survived bacteremia episode) | 2 (3.9) | 2 (3.8) | 4 (3.8) | 1 |
| Embolic or metastatic MSSA disease within 30 days | 9 (17.7) | 13 (24.5) | 22 (21.2) | .47 |
| Embolic or metastatic MSSA disease within 90 days | 9 (17.7) | 13 (24.5) | 22 (21.2) | .47 |
| Nephrotoxicity on presentation | 6 (11.8) | 9 (17.0) | 15 (14.4) | .57 |
| Nephrotoxicity on treatmenta,b | 13 (28.9) | 21 (47.7) | 34 (32.7) | .15 |
| Hepatotoxicity b | 3 (5.88) | 2 (3.77) | 5 (4.8) | .675 |
| Rhabdomyolysis b | 3 (5.88) | 0 (0) | 3 (2.9) | 0.11 |
| . | No. (%) . | |||
|---|---|---|---|---|
| Variable . | Placebo (n = 51) . | Daptomycin (n = 53) . | Total (n = 104) . | P Value . |
| All-cause mortality (30 days) | 6 (11.8) | 8 (15.1) | 14 (13.5) | .62 |
| All-cause mortality (90 days) | 9 (17.7) | 10 (18.9) | 19 (18.3) | 1 |
| Relapsed bacteremia within 30 days (among those who survived bacteremia episode) | 0 (0) | 1 (1.9) | 1 (1.0) | 1 |
| Relapsed bacteremia within 90 days (among those who survived bacteremia episode) | 2 (3.9) | 2 (3.8) | 4 (3.8) | 1 |
| Embolic or metastatic MSSA disease within 30 days | 9 (17.7) | 13 (24.5) | 22 (21.2) | .47 |
| Embolic or metastatic MSSA disease within 90 days | 9 (17.7) | 13 (24.5) | 22 (21.2) | .47 |
| Nephrotoxicity on presentation | 6 (11.8) | 9 (17.0) | 15 (14.4) | .57 |
| Nephrotoxicity on treatmenta,b | 13 (28.9) | 21 (47.7) | 34 (32.7) | .15 |
| Hepatotoxicity b | 3 (5.88) | 2 (3.77) | 5 (4.8) | .675 |
| Rhabdomyolysis b | 3 (5.88) | 0 (0) | 3 (2.9) | 0.11 |
Abbreviations: MSSA, methicillin-susceptible Staphylococcus aureus.
aExcluding patients who had evidence of nephrotoxicity on presentation.
bWithin 5 days of patient enrollment into the study.
| Placebo . | Daptomycin . |
|---|---|
| Cirrhosis | Ovarian cancer |
| Infective endocarditis due to Staphylococcus aureus | Metastatic lung cancer |
| Infective endocarditis due to S. aureus | Bladder cancer |
| S. aureus bacteremia | Metastatic carcinoma |
| S. aureus bacteremia | Renal failure (chose to stop dialysis) |
| Acute myeloid leukemia | Stroke |
| Died between dialysis sessions (unknown cause) on day 77 | S. aureus bacteremia |
| Renal failure (chose not to start dialysis) | Bacteremic S. aureus pneumonia |
| S. aureus pneumonia/advanced dementia | Renal failure (chose not to start dialysis) |
| Congestive heart failure |
| Placebo . | Daptomycin . |
|---|---|
| Cirrhosis | Ovarian cancer |
| Infective endocarditis due to Staphylococcus aureus | Metastatic lung cancer |
| Infective endocarditis due to S. aureus | Bladder cancer |
| S. aureus bacteremia | Metastatic carcinoma |
| S. aureus bacteremia | Renal failure (chose to stop dialysis) |
| Acute myeloid leukemia | Stroke |
| Died between dialysis sessions (unknown cause) on day 77 | S. aureus bacteremia |
| Renal failure (chose not to start dialysis) | Bacteremic S. aureus pneumonia |
| S. aureus pneumonia/advanced dementia | Renal failure (chose not to start dialysis) |
| Congestive heart failure |
Mortality directly attributable to S. aureus are in bold.
| Placebo . | Daptomycin . |
|---|---|
| Cirrhosis | Ovarian cancer |
| Infective endocarditis due to Staphylococcus aureus | Metastatic lung cancer |
| Infective endocarditis due to S. aureus | Bladder cancer |
| S. aureus bacteremia | Metastatic carcinoma |
| S. aureus bacteremia | Renal failure (chose to stop dialysis) |
| Acute myeloid leukemia | Stroke |
| Died between dialysis sessions (unknown cause) on day 77 | S. aureus bacteremia |
| Renal failure (chose not to start dialysis) | Bacteremic S. aureus pneumonia |
| S. aureus pneumonia/advanced dementia | Renal failure (chose not to start dialysis) |
| Congestive heart failure |
| Placebo . | Daptomycin . |
|---|---|
| Cirrhosis | Ovarian cancer |
| Infective endocarditis due to Staphylococcus aureus | Metastatic lung cancer |
| Infective endocarditis due to S. aureus | Bladder cancer |
| S. aureus bacteremia | Metastatic carcinoma |
| S. aureus bacteremia | Renal failure (chose to stop dialysis) |
| Acute myeloid leukemia | Stroke |
| Died between dialysis sessions (unknown cause) on day 77 | S. aureus bacteremia |
| Renal failure (chose not to start dialysis) | Bacteremic S. aureus pneumonia |
| S. aureus pneumonia/advanced dementia | Renal failure (chose not to start dialysis) |
| Congestive heart failure |
Mortality directly attributable to S. aureus are in bold.
Adverse Events
There were no significant differences in the proportion of patients who developed renal failure, hepatotoxicity, or rhabdomyolysis between groups. No serious adverse events related to the study drug were reported during the trial. The study drug was stopped in 1 patient due to treating physician concerns over adverse events. In that instance, the drug was the placebo.
DISCUSSION
Patients with MSSA BSI remain at significant risk of morbidity and mortality. Previous in vitro studies have suggested that adding daptomycin to an ASBL results in synergistic activity against S. aureus [24]. However, our results demonstrate that this combination has no advantage over ASBL monotherapy for the management of patients with MSSA BSIs. While the addition of daptomycin to ASBL was safe and well tolerated, it did not reduce the duration of bacteremia. It also did not reduce 90-day mortality or prevent recurrent episodes of MSSA BSI. Our results thus support current treatment guidelines [9, 31] and demonstrate that daptomycin should not be added to ASBL in the management of patients with MSSA BSI.
The all-cause mortality rate in our study is comparable to other contemporaneous cohorts and recent randomized, controlled trials and highlights the urgent need to improve clinical outcomes in patients with S. aureus bacteremia. While there are generally accepted best practices with regard to managing these patients, there remain important knowledge gaps regarding antimicrobial selection. For example, there is an ongoing debate as to whether a semisynthetic penicillin or a first-generation cephalosporin should be used preferentially in patients with MSSA BSI. While we await the results of a large randomized, controlled trial to address this question, our study adds to the growing body of literature that shows that combination antibiotic therapy should not be considered routinely for S. aureus bacteremia. Rather, novel treatment strategies should be sought to improve patient outcomes.
Our study has several strengths. First, we performed a randomized, double-blind, placebo-controlled study that was designed, coordinated, and conducted by trainees in infectious diseases and medical microbiology. This trial did not receive industry sponsorship and was devoid of financial bias. To broaden the generalizability of our results, we used standardized definitions for our study end points [33]. To minimize confounding, the standard of care for the treatment of MSSA BSI at our institutions was maintained throughout the study period. Our results are not at risk of attrition bias, as follow-up was complete for the entire study population.
Nonetheless, this study has several limitations. First, we used a surrogate end point as the primary outcome. A recent randomized, controlled trial on the adjunctive use of cefazolin in addition to vancomycin failed in treatment of MRSA [34]. While adjunctive cefazolin resulted in faster bloodstream clearance at day 5, this did not translate to improved outcome, including survival. The adjunctive use of cefazolin with vancomycin was associated with a rise in acute kidney injury, which may explain this discrepancy. Nevertheless, the causal effect of a longer bacteremia on mortality is not as clear as previously thought. However, it is still a commonly used clinical criteria to guide therapy [35], and our trial shows that daptomycin adjunctive treatment will not alter the infection course in prolonged MSSA BSIs. Furthermore, the secondary outcomes of our trial, including 90-day mortality and relapsed bacteremia, were comparable between groups. Second, we were only able to recruit 34.7% of patients with MSSA BSIs during the study period. Certain critically ill patients died prior to enrollment while others were on additional antimicrobial therapy in the setting of polymicrobial infections. However, our recruitment proportion is comparable or higher than that of contemporaneous treatment trials of S. aureus bacteremia [9, 12, 18] and reflect the challenges of performing a randomized, controlled trial in this patient population. Third, a significant proportion of patients were no longer bacteremic at the time of randomization and treatment initiation. However, the results of the primary outcome were comparable among the ITT, mITT, and PP populations. While the median duration of bacteremia was slightly lower than expected in the overall study population, it was as predicted in the mITT population. Fourth, randomization occurred within 72 hours of index blood culture (median, 47 hours; IQR, 39–53), and most patients received antibiotics prior to enrollment. The study was not designed to test differences in empirical therapy; rather, we looked at definitive therapy once MSSA was confirmed. Finally, we did not control the poststudy antibiotic therapy and cannot make any inferences on poststudy care and the resultant impact on the secondary outcomes.
It is paramount to perform randomized, controlled trials to confirm or refute findings observed in case-series and cohort studies. Our results do not support the routine addition of daptomycin to an ASBL in the treatment of patients with MSSA BSI. Given that combination therapies have not shown therapeutic benefits, new management approaches are required for the treatment of patients with MSSA BSI. To avoid repeating the mistakes of the past, the testing of these strategies in properly designed studies is paramount prior to their introduction into clinical practice.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Gilbert Matte, Jasmine Mian, and all of their colleagues at the McGill University Health Center (MUHC) research pharmacy for their crucial support during this trial.
Financial support. This work was supported by the Association of Physicians of MUHC. The funding agency had no role in designing the study, analyzing the data, writing the manuscript, or submitting the manuscript for publication.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
Author notes
M. P. C., A. L., and G. B.-L. contributed equally to this work.




