-
PDF
- Split View
-
Views
-
Cite
Cite
Klara M Posfay-Barbe, Diego O Andrey, Julien Virzi, Patrick Cohen, Fiona Pigny, Ana R Goncalves, Selina Pinosch, Laurence Lacroix, Silvia Stringhini, Laurent Kaiser, Nicolas Vuilleumier, Arnaud G L’Huillier, Prevalence of Immunoglobulin G (IgG) Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Evaluation of a Rapid MEDsan IgG Test in Children Seeking Medical Care, Clinical Infectious Diseases, Volume 72, Issue 7, 1 April 2021, Pages e192–e195, https://doi.org/10.1093/cid/ciaa1702
Close - Share Icon Share
Abstract
In 208 children seeking medical care, the seropositivity rate of anti–SARS-CoV-2 IgG antibodies was 8.7%, suggesting an infection rate similar to that observed in adults but >100-fold the incidence of RT-PCR–confirmed pediatric cases. Compared with the gold-standard combined ELISA + immunofluorescence, the MEDsan IgG rapid diagnostic test performed accurately.
Children are underrepresented in terms of recognized acute coronavirus disease 2019 (COVID-19) cases, either because of a lower infection rate [1, 2] and/or because of more frequent asymptomatic or atypical presentations leading to underdiagnosis of the acute phase [3]. In this context, seroprevalence studies are helpful for identifying the proportion of children who have been infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), irrespective of clinical testing practices. The region of Geneva was severely affected, with an estimated reverse transcription–polymerase chain reaction (RT-PCR)–confirmed 1000 cases per 100 000 inhabitants and reaching a seroprevalence of approximately 10% in the general population [4]. The first aim of this work was to evaluate the prevalence of SARS-CoV-2 immunoglobulin G (IgG) in children who presented to our institution for non-COVID-19–related blood testing in the month following the local epidemic peak of infections. The other aim of the study was to evaluate the SARS-CoV-2 IgG detection performance of the MEDsan COVID-19 IgG rapid diagnostic test (RDT) in children (MEDsan GmbH, Hamburg, Germany), which was recently shown to display a satisfying diagnostic accuracy when compared with centralized tests in adults [5]. Multiple COVID-19 serology rapid tests are currently being developed and widely distributed, often without extensive or published performance assessments, especially in children.
METHODS
Study Design
All patients younger than 16 years with blood collected at Geneva University Hospitals between 1 and 30 April, and for whom leftover ethylenediaminetetraacetic acid (EDTA) plasma was available, were eligible, with the exception of neonates. Patients who presented to the pediatric emergency room (ER; n = 86), who were admitted to the hospital (n = 64) or visiting outpatient clinics (n=58) were enrolled. The study was approved by the local ethics committee (protocol #2020-0835). Assessment of seropositivity rate was performed using a commercial enzyme-linked immunosorbent assay (ELISA) with confirmation by recombinant immunofluorescence assay (rIFA) and used as a gold standard against which the MEDsan COVID-19 IgG RDT was challenged. Further details are available in the Supplementary Methods.
ELISA
ELISA was performed using the Euroimmun IgG assay (Euroimmun AG, Lubeck, Germany) targeting the S1 domain of the spike protein [6]. Quantitative results were interpreted following previously published cutoffs, with values less than 0.8 considered as negative, between 0.8 and 1.5 as indeterminate, and 1.5 or higher as positive. Indeterminate and positive ELISAs were confirmed by rIFA, as previously described [4, 7].
Spike-Expressing Recombinant Immunofluorescence Assay
Recombinant immunofluorescence assay was performed by determination of the IgG antibody response against the complete spike (S) protein (both S1 and S2 domains) of SARS-CoV-2, as previously validated for SARS-CoV-2 [7]. Results were assessed by 2 independent readers with satisfactory interobserver κ-coefficients [7].
Rapid Diagnostic Test
For the RDT IgG, 5 μL of plasma was applied to the cassette, and results read after 10 minutes but no later than 15 minutes, as per the manufacturer’s instruction.
RESULTS
SARS-CoV-2 IgG Prevalence in Children
Among the 316 children with blood collected in our institution during the study period, leftover plasma was available and used for this study in 208 patients (65.8%), with a median age of 9.0 years (Supplementary Table 1). Among those, 19 (9.1%) were tested as seropositive, 8 (3.8%) as indeterminate, and 181 (87.0%), as seronegative by ELISA. A total of 16 and 2 of the respectively 19 positive and 8 indeterminate tests were confirmed positive by rIFA, leading to a seropositivity rate of 8.7% (18/208) in the dataset. The positivity rate increased from 1.6% (1/61) between 1 and 10 April, to 10.8% (7/65) between 11 and 20 April, and to 12.2% (10/82) between 21 and 30 April (Figure 1). In comparison, the cumulative incidence of RT-PCR–confirmed cases younger than 16 years was less than 0.08% at the end of the study period (Figure 1), suggesting that only 1 out of 100 children infected by SARS-CoV-2 is clinically diagnosed. Median age did not significantly differ between IgG-positive (10.6 years; interquartile range, 8.0–13.7 years) and IgG-negative children (8.8 [3.5–13.0] years; P = .149). However, there was a trend towards a lower seropositivity rate in children younger than 10 years (5.4%; 6/111) when compared with older children (12.4%; 12/97; P = .075). The seropositivity rate did not differ according to where the patient was sampled (Supplementary Table 1).
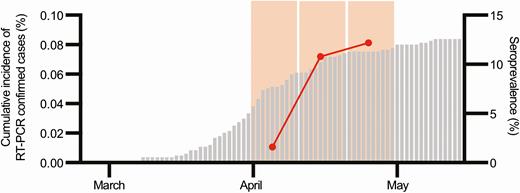
Evaluation of the seroprevalence of IgG against SARS-CoV-2 with time in patients younger than 16 years in comparison to the cumulative incidence of RT-PCR–confirmed cases younger than 16 years. The gray bars represent the cumulative incidence of RT-PCR–confirmed SARS-CoV-2 infections in patients younger than 16 years. The red dots represent the prevalence of IgG against SARS-CoV-2 in our dataset of children younger than 16 years. The wide light orange bars represent the 3 study periods (1–10, 11–20, and 21–30 April). Of note, serological tests were performed in children who had blood collected in our institution regardless of the indication for blood collection, whereas RT-PCR tests were performed in children who met the definition for suspected COVID-19, which was the presence of any respiratory symptom or fever. Abbreviations: COVID-19, coronavirus disease 2019; IgG, immunoglobulin G; RT-PCR, reverse transcription–polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Rapid Diagnostic Test Performance
Using the MEDsan RDT, IgG results were positive in 26 (12.5%) and negative in 182 (87.5%) children. The performance of SARS-CoV-2 RDT IgG detection using RDT against ELISA + rIFA showed a sensitivity and specificity of 88.9% (16/18; 95% confidence interval [CI], 64–98%]) and 94.7% (180/190; 95% CI, 90–97%), respectively. The positive-predictive value (PPV) and negative-predictive value (NPV) were 61.5% (16/26; 95% CI, 41–79%) and 98.9% (180/182; 95% CI, 96–100%), respectively. The Kendall correlation coefficient between the RDT and ELISA + rIFA was 0.71 (P < .001).
DISCUSSION
The key finding of the present study is that the seropositivity rate in children presenting to the hospital during this period was of the same magnitude than what was observed in adults over the same period [4], suggesting that the SARS-CoV-2 infection rate is similar in children and in adults. In our dataset, the overall prevalence of IgG antibodies against SARS-CoV-2 during the month following the epidemic peak was 8.7% and increased from 1.6% to 12.2% over a short period. In comparison, seroprevalence increased from 4.8% to 10.8% during the 5 weeks following the peak in the large seroprevalence study conducted in our region [4]. Similarly, another large seroprevalence study in a region less heavily affected has shown a seroprevalence of 2.8% among children, in line with the adult seroprevalence [8]. In our study, despite a similar overall infection rate when compared with adults [4], children younger than 10 years were 2.3-fold less likely to be seropositive than those aged 10–16 years. Even though these results were not significant, they suggest that either younger children were less exposed to SARS-CoV-2 during the first wave or they are less susceptible to SARS-CoV-2 infection because of reduced angiotensin-converting enzyme 2 (ACE-2) receptor density in the upper airways, a stronger innate immune system, and/or cross-reactive immunity provided by recent exposure to human coronaviruses. Along the same lines as our data, seropositivity rates in our region’s population study were, respectively, 0.8% and 9.6% in children aged 5–9 years and 10–19 years over the 5 weeks following the epidemic peak [4] and 4.3% and 7.9%, respectively, over the 12 weeks following the peak (S. Stringhini, personal communication, 28 October 2020). Altogether, available data suggest that, even though children have lower RT-PCR–confirmed secondary attack rates inside households [1, 2, 9], they might be infected as frequently as adults [4, 8]. Interestingly, the seropositivity rate in our dataset at the end of the study was more than 100-fold the cumulative incidence of RT-PCR–confirmed cases younger than 16 years old at the same period, suggesting that, despite the absence of RT-PCR reagents shortage during the epidemic and wide RT-PCR pediatric testing criteria [10], only approximately 1% of the pediatric COVID-19 cases were diagnosed. This ratio of 1% is in line with other pediatric populational data [8].
There are limitations to this pediatric seroprevalence study. First, our sample is possibly not representative of the general pediatric population because patients were enrolled in a clinical setting. Some of the enrolled patients might have sought medical attention for an illness of possible infectious origin with or without a SARS-CoV-2 RT-PCR test, thus conceivably increasing the pre-test probability of SARS-CoV-2 infection compared with the overall child population. Nevertheless, we believe this is a minor bias, because the prevalence of IgG antibodies was not lower in elective outpatients than in patients from the ER or those admitted in our hospital. Second, the relatively small sample size and the lower number of preschool-aged children compared with older children do not allow to definitively conclude whether the first group is less susceptible to the virus. This pilot study provides the basis for larger population-based studies enrolling preschool children to better evaluate the impact of public health policies on seroprevalence in younger age groups.
The study also shows that the performance of the MEDsan IgG RDT in children is acceptable, with an NPV of 99%, in line with previously published NPVs of 97–100% [5, 11]. The sensitivity and specificity were 89% and 95%, respectively, in our dataset, which is in the range of previously reported sensitivities and specificities [5, 11, 12]. As expected, the PPV of 62% was similar than published PPVs, with a 10% seroprevalence [11], but lower than the PPV of 95% reported in the published method validation study [5], emphasizing the importance of the prevalence on PPV. In addition, in the previous validation study by Andrey et al [5], plasma samples were obtained from hospitalized patients, who are likely to have higher antibody levels than those with milder disease. Finally, this RDT performance assessment was obtained against a validated ELISA method combined with immunofluorescence, unlike most SARS-CoV-2 seropositivity detection studies which lack reliable, validated IgG detection reference methods as a comparator. Altogether, these data show that, in the absence of centralized test availability, the MEDsan IgG RDT could be an acceptable option for rapid assessment of SARS-CoV-2 IgG seroprevalence in children. The rapid turnaround time without the need of a laboratory and the possibility to use capillary blood make it particularly convenient for children.
This RDT performance evaluation has 1 limitation, since the use of plasma might overestimate sensitivity and NPV when compared with whole blood, which would be used in capillary tests. Nevertheless, at least in adults, plasma and whole-blood samples showed similar results when tested with the MEDsan RDT [5].
In conclusion, our data show an 8.7% seropositivity rate of SARS-CoV-2 IgG in children, which is in line with adult data derived from the same region [4]. There was, however, a trend towards a lower rate of seropositivity among children younger than 10 years, suggesting a lower infection rate in younger children. Our serological data also suggest that only a small percentage of pediatric COVID-19 cases were diagnosed by RT-PCR. We also showed that the MEDsan IgG RDT performs accurately in the pediatric setting and could be—despite its limitations—considered for individual testing or even sero-surveys in difficult-to-reach populations.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Olivier Golaz for his help in selecting the biological specimens. They thank the Office of the Surgeon General for the use of the data in the Actionable Registry of the Geneva Outpatients with SARS-CoV-2.
Financial support. This work was supported by the Department of Woman, Child and Adolescent Medicine and the Department of Diagnostics, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland.
Potential conflicts of interest. K. M. P.-B. reports personal fees to her institution by the MSD Advisory Board and Pfizer Advisory Board, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- enzyme-linked immunosorbent assay
- coronavirus
- adult
- child
- fluorescent antibody technique
- pediatrics
- reverse transcriptase polymerase chain reaction
- immunoglobulin g
- infections
- antibodies
- severe acute respiratory syndrome
- igg antibody
- gold standard
- rapid screening test
- seroprevalence
- sars-cov-2
- covid-19




