-
PDF
- Split View
-
Views
-
Cite
Cite
Vanessa W Stevens, Karim Khader, Kelly Echevarria, Richard E Nelson, Yue Zhang, Makoto Jones, Tristan T Timbrook, Matthew H Samore, Michael A Rubin, Use of Oral Vancomycin for Clostridioides difficile Infection and the Risk of Vancomycin-Resistant Enterococci, Clinical Infectious Diseases, Volume 71, Issue 3, 1 August 2020, Pages 645–651, https://doi.org/10.1093/cid/ciz871
Close - Share Icon Share
Abstract
Vancomycin is now a preferred treatment for all cases of Clostridioides difficile infection (CDI), regardless of disease severity. Concerns remain that a large-scale shift to oral vancomycin may increase selection pressure for vancomycin-resistant Enterococci (VRE). We evaluated the risk of VRE following oral vancomycin or metronidazole treatment among patients with CDI.
We conducted a retrospective cohort study of patients with CDI in the US Department of Veterans Affairs health system between 1 January 2006 and 31 December 2016. Patients were included if they were treated with metronidazole or oral vancomycin and had no history of VRE in the previous year. Missing data were handled by multiple imputation of 50 datasets. Patients treated with oral vancomycin were compared to those treated with metronidazole after balancing on patient characteristics using propensity score matching in each imputed dataset. Patients were followed for VRE isolated from a clinical culture within 3 months.
Patients treated with oral vancomycin were no more likely to develop VRE within 3 months than metronidazole-treated patients (adjusted relative risk, 0.96; 95% confidence interval [CI], .77 to 1.20), equating to an absolute risk difference of −0.11% (95% CI, −.68% to .47%). Similar results were observed at 6 months.
Our results suggest that oral vancomycin and metronidazole are equally likely to impact patients’ risk of VRE. In the setting of stable CDI incidence, replacement of metronidazole with oral vancomycin is unlikely to be a significant driver of increased risk of VRE at the patient level.
In this multicenter, retrospective cohort study of patients with Clostridioides difficile infection, the use of oral vancomycin did not increase the risk of vancomycin-resistant Enterococci infection at 3 or 6 months compared to metronidazole.
Clostridioides difficile infection (CDI) remains one of the most common and challenging healthcare-associated infections worldwide. An estimated half-million new cases and 30 000 attributable deaths occur in the United States annually [1]. In its 2013 Antimicrobial Resistance Threat Report, the Centers for Disease Control and Prevention (CDC) designated CDI as 1 of 3 urgent threats to public health [2].
Metronidazole has long been considered the first-line agent of choice for CDI treatment due to its low cost and presumed equivalence to vancomycin. However, a recent clinical trial indicates higher initial clinical cure rates with vancomycin among CDI episodes, mostly driven by stronger effects in patients with severe disease [3]. The newest clinical practice guidelines from the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America recommend treating all initial episodes of CDI with vancomycin [4] rather than using metronidazole for mild to moderate infections and vancomycin for initially severe cases as was recommended in the 2010 clinical practice guidelines [5]. This represents a significant shift in clinical practice given that 70%–90% of initial CDI episodes in the United States are treated with metronidazole at present [6–8].
The release of the new guidelines has sparked an interesting discussion on the role of outcomes other than efficacy and on how large differences in efficacy should be to justify transitioning an entire population to more intensive treatment. In particular, the cost of treatment, insurance coverage and access, and selection for vancomycin-resistant Enterococci (VRE) remain valid concerns. The role of vancomycin in the etiology of VRE infection is somewhat unclear. Results of a metaanalysis suggest an increased risk of VRE with antecedent vancomycin exposure, but there was substantial heterogeneity across studies [9]. The literature comprises studies with small sample sizes, biases due to time-varying confounding, and other methodological limitations. Oral vancomycin makes up only a very small proportion of all vancomycin use, resulting in few estimates of its independent effect on the risk of VRE. Most studies of vancomycin and the risk of VRE were conducted in hospital settings, whereas the majority of CDIs are now diagnosed in the community. Both oral vancomycin and metronidazole have been shown to promote overgrowth of VRE, but large-scale studies are lacking [10].
Treatment choices should be based on a careful weighing of all of the risks and benefits of those treatments, including the risk of death, healthcare utilization, and costs. For antibiotics, the risks also include the possibility of selecting for resistant infections that have impacts beyond the individual patient and may increase transmission within healthcare facilities. We sought to fill gaps in the evidence on how oral vancomycin and metronidazole impact the risk of developing a clinically relevant VRE infection in order to help anticipate the population-level impact of a large-scale shift in prescribing to oral vancomycin for all cases of CDI and to allow for a detailed assessment of the risk–benefit profile of existing CDI treatments.
METHODS
Study Design and Participants
We conducted a retrospective, propensity-matched cohort study of inpatients, long-term care residents, and outpatients diagnosed with CDI in the US Department of Veterans Affairs Health System between 1 January 2006 and 31 December 2016. Patients were included if they had a positive laboratory test indicating the presence of toxin or toxin genes by enzyme immunoassay (EIA) or polymerase chain reaction (PCR) in a stool sample, had been treated with oral vancomycin or intravenous or oral metronidazole within 2 days prior to 14 days after a CDI diagnosis, and had no prior history of VRE identified from any source in the prior year. Untreated patients and those who received both vancomycin and metronidazole were excluded. Only the first eligible episode of CDI for each patient was considered. The University of Utah School of Medicine Institutional Review Board and the VA (Veterans Affairs) Salt Lake City Health Care System Research and Development Committee reviewed and approved the study.
Study Data
All study data were obtained from the VA Corporate Data Warehouse. Information was collected on patient demographics including age at the time of CDI diagnosis, gender, and race/ethnicity. Baseline comorbidity was measured by applying the Charlson comorbidity index [11] to inpatient and outpatient International Classification of Diseases, 9th and 10th revisions, codes assigned in the previous year. CDI episodes were categorized according to likely location of symptom onset (community or first 3 days in acute or long-term care, acute care beyond 3 days, or long-term care beyond 3 days), episode type (primary incident, recurrent, or secondary incident [12]), and year of diagnosis. Maximum white blood cell (WBC) counts and maximum and average baseline serum creatinine (SCr) values were assessed as markers of CDI severity [4, 5]. Diagnosis in the intensive care unit (ICU) was also considered. Proton pump inhibitor, fluoroquinolone, and cephalosporin exposures in the prior 3 months and immune suppressive agents and chemotherapy in the prior month were evaluated through inpatient bar code medication administration data and outpatient pharmacy prescriptions filled. History of vancomycin and metronidazole use were measured as the number of vancomycin and metronidazole days in the last year.
The primary exposure was the receipt of oral vancomycin or oral or intravenous metronidazole within 2 days before to 14 days after a CDI diagnosis to capture changes in treatment during the course of therapy. The primary outcomes of interest were positive VRE clinical (ie, nonsurveillance) cultures in the 3 and 6 months following initiation of treatment. We also evaluated VRE isolated from blood samples and surveillance cultures at 3 months.
Sensitivity Analyses
VRE surveillance practices varied considerably across VA facilities. More than half and 80% of all positive screens came from 4 and 10 (of 129) facilities, respectively. We performed sensitivity analyses excluding the 10 facilities that perform the majority of screening tests. Because duration of treatment can vary, particularly for vancomycin taper doses among multiply-recurrent cases, we also performed a subgroup analysis limiting the study cohort to incident cases only. In our final analysis, we evaluated only CDI cases diagnosed after implementation of the Methicillin-Resistant Staphylococcus aureus Prevention Initiative in 2007 [13].
Statistical Analyses
Because of the importance of CDI severity (as measured by WBC and SCr) in determining treatment and because a nontrivial proportion of patients had missing values for these laboratory tests, we performed multivariate imputation using chained equations [14] to estimate missing values for WBC and SCr under the missing-at-random (MAR) assumption. The MAR assumption holds when the reason for missing data and the values themselves are related to measured information [15]. We hypothesized that the lack of laboratory values was a function of milder illness and being diagnosed with CDI in nonacute settings where the intensity of diagnostic testing is lower than in the hospital [16]. We generated 50 imputed datasets. Predictors of the missing variables included patient demographics (age, sex, race), VA station (facility), clinical and CDI episode characteristics, as well as the main exposure (oral vancomycin or metronidazole) and outcomes (VRE) in accordance with recommended methods [17].
Propensity scores were generated using logistic regression to estimate the log(odds) of receiving vancomycin as a function of year of diagnosis; episode type; onset location; age at diagnosis; maximum WBC; average baseline SCr (defined as the mean SCr value in the 90 days prior to diagnosis); maximum SCr; Charlson comorbidity score; immune suppressive drugs or chemotherapy in the prior 30 days; fluoroquinolone, cephalosporin, or proton pump inhibitor use in the prior 3 months; days of vancomycin and metronidazole exposure and history of hospitalization in the past year; and CDI treatment with rifaximin. Matching was performed within each of the 50 imputed datasets using greedy nearest neighbor matching and a caliper width of 0.2 standard deviations [18]. Each vancomycin-treated patient was matched to up to 2 metronidazole-treated patients. The average treatment effect of oral vancomycin compared to metronidazole on the risk of VRE was estimated in each dataset separately and combined across the imputations using Rubin’s rules [19]. In each imputed set, adjusted relative risks (RRs) and risk differences (RDs) were estimated from Poisson regression [20, 21] with log link within a generalized estimating equations framework with an independence working correlation structure clustered at the VA facility (station) level.
All statistical analyses were performed in R (v3.5.1). Multiple imputation was carried out using the mice package (v3.3.0) [22]. Covariate balance across imputed datasets was assessed using the cobalt package (v3.5.0) [23]. Propensity score matching within each dataset was performed using MatchIt (v3.0.2) [24]. Adjusted RRs and RDs and 95% confidence intervals (CIs) were estimated using geepack (v1.2-1) [25]. RR estimates and 95% CIs were combined across imputed datasets using mitml (v0.3.6) [26]. Combined RD estimates and 95% CIs were calculated manually [21].
RESULTS
From 1 January 2006 through 31 December 2016, 82 405 patients met the case definition of CDI and had no history of VRE (Figure 1). In this prematching cohort, the use of oral vancomycin alone or in combination increased from approximately 10% of patients with CDI in 2006 to approximately 30% in 2016, while the use of metronidazole alone or in combination decreased from approximately 90% to approximately 75%. Over the same period, the 3-month risk of VRE from a clinical culture among these patients decreased from 4.8% to 1.2%. Overall, approximately 6% (5269) of patients received oral vancomycin alone and 68.3% (56 268) received metronidazole alone within 14 days of CDI. VRE screening occurred in 1.9% of vancomycin- and 1.4% of metronidazole-treated patients within 6 months after CDI diagnosis. Among patients who developed VRE within 1 year after CDI, the median (interquartile range) time to VRE was 34 (85) days and similar between treatment groups (Supplementary Figure 1). Figure 2 demonstrates the trends in vancomycin prescribing and 3-month risk of VRE in clinical cultures over time by the location of CDI onset. The use of oral vancomycin increased the most among patients with onset in the acute care setting, but similar increases were seen in all 3 groups. The risk of VRE at 3 months decreased in all 3 groups but was most pronounced among patients with hospital onset.
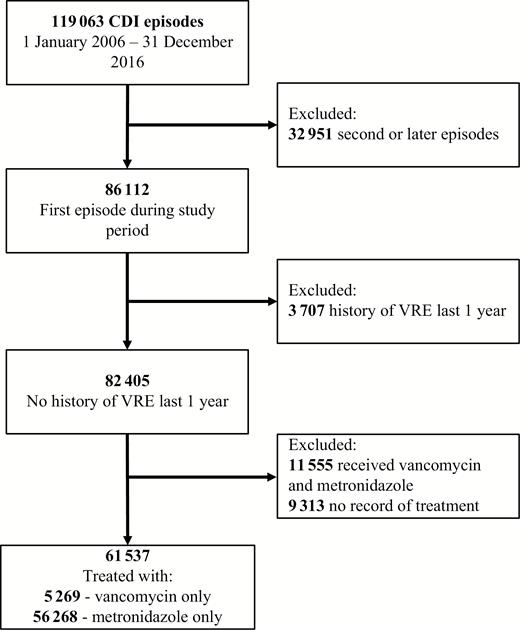
Eligibility and selection of patients with Clostridioides difficile infection in the US Department of Veterans Affairs Health System between 1 January 2006 and 31 December 2016. Abbreviations: CDI, Clostridioides difficile infection; VRE, vancomycin-resistant Enterococci.
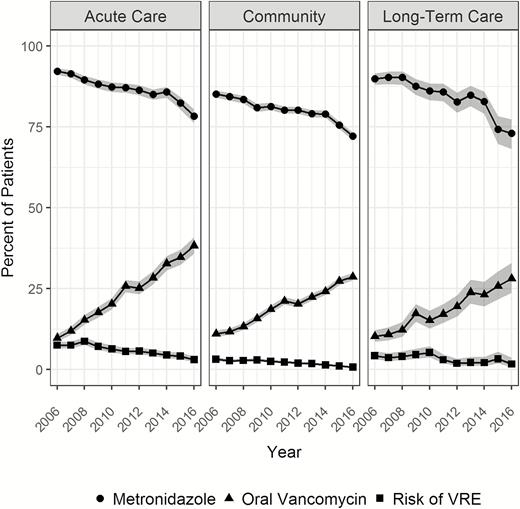
Trends in metronidazole and oral vancomycin use and the risk of vancomycin-resistant Enterococci among patients with Clostridioides difficile infection across healthcare settings in the US Department of Veterans Affairs, 2006–2016. Abbreviation: VRE, vancomycin-resistant Enterococci.
Data were missing in 20 407 (24.7%) of maximum WBC, 13 780 (16.7%) of maximum SCr, and 11 721 (14.2%) of average baseline SCr values. Multiple imputation resulted in 50 datasets with 100% complete data.
On average across the 50 sets, 5267 of 5269 (99.9%) patients treated with vancomycin alone were matched to 1 or more metronidazole-treated patients for an average total of 15 780 patients included in the analysis. After matching, patients were well balanced with respect to all observed covariates (Supplementary Figure 2). Table 1 describes the characteristics of the propensity-matched sample from a randomly chosen imputation. The average age of patients who received metronidazole and vancomycin was 69.0 and 68.0 years, respectively. Nearly all (99% of) cases were initial incident CDI, and 70% of cases onset in the community.
Demographic and Clinical Characteristics of Propensity-Matched Clostridioides difficile Infection Patients Treated With Metronidazole or Oral Vancomycin
| Characteristic . | Metronidazole, n (%) . | Vancomycin, n (%) . |
|---|---|---|
| No. of patients | 10 510 | 5266 |
| Year | ||
| 2006 | 439 (4.2) | 181 (3.4) |
| 2007 | 419 (4.0) | 176 (3.3) |
| 2008 | 482 (4.6) | 218 (4.1) |
| 2009 | 512 (4.9) | 261 (5.0) |
| 2010 | 606 (5.8) | 310 (5.9) |
| 2011 | 826 (7.9) | 487 (9.2) |
| 2012 | 985 (9.4) | 470 (8.9) |
| 2013 | 1129 (10.7) | 600 (11.4) |
| 2014 | 1417 (13.5) | 667 (12.7) |
| 2015 | 1680 (16.0) | 864 (16.4) |
| 2016 | 2015 (19.2) | 1032 (19.6) |
| Episode type | ||
| Primary incident | 1040 0 (99.0) | 5207 (98.9) |
| Recurrent | 38 (0.4) | 21 (0.4) |
| Secondary incident | 72 (0.7) | 38 (0.7) |
| Onset | ||
| Acute care facility | 2235 (21.3) | 1102 (20.9) |
| Community | 7328 (69.7) | 3694 (70.1) |
| Long-term care facility | 947 (9.0) | 470 (8.9) |
| Diagnosis in the intensive care unit | 1066 (10.1) | 522 (9.9) |
| Age at diagnosis, median (IQR), y | 69.00 (62.00, 79.00) | 68.00 (62.00, 79.00) |
| Maximum white blood cell count, median (IQR), thousands, per cubic millimeter | 10.60 (7.50, 15.00) | 11.20 (7.60, 16.60) |
| Average baseline SCr, median (IQR), milligrams per deciliter | 1.10 (0.87, 1.50) | 1.10 (0.85, 1.50) |
| Maximum SCr, median (IQR), milligrams per deciliter | 1.20 (0.90, 1.86) | 1.21 (0.90, 1.90) |
| Charlson score, median (IQR) | 2.00 (0.00, 4.00) | 2.00 (0.00, 4.00) |
| Days of vancomycin last 1 year, median (IQR) | 0.00 (0.00, 3.00) | 0.00 (0.00, 3.00) |
| Days of metronidazole last 1 year, median (IQR) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| Immune suppressive medications last 1 month | 543 (5.2) | 261 (5.0) |
| Chemotherapy last 1 month | 306 (2.9) | 145 (2.8) |
| Fluoroquinolones last 3 months | 3349 (31.9) | 1696 (32.2) |
| Cephalosporins last 3 months | 3496 (33.3) | 1736 (33.0) |
| Proton pump inhibitors last 3 months | 3450 (32.8) | 1755 (33.3) |
| CDI treatment with rifaximin | 160 (1.5) | 89 (1.7) |
| History of CDI | 48 (0.5) | 33 (0.6) |
| History of hospitalization last 1 year | 6148 (58.5) | 3101 (58.9) |
| Male sex | 9944 (94.6) | 4991 (94.8) |
| Characteristic . | Metronidazole, n (%) . | Vancomycin, n (%) . |
|---|---|---|
| No. of patients | 10 510 | 5266 |
| Year | ||
| 2006 | 439 (4.2) | 181 (3.4) |
| 2007 | 419 (4.0) | 176 (3.3) |
| 2008 | 482 (4.6) | 218 (4.1) |
| 2009 | 512 (4.9) | 261 (5.0) |
| 2010 | 606 (5.8) | 310 (5.9) |
| 2011 | 826 (7.9) | 487 (9.2) |
| 2012 | 985 (9.4) | 470 (8.9) |
| 2013 | 1129 (10.7) | 600 (11.4) |
| 2014 | 1417 (13.5) | 667 (12.7) |
| 2015 | 1680 (16.0) | 864 (16.4) |
| 2016 | 2015 (19.2) | 1032 (19.6) |
| Episode type | ||
| Primary incident | 1040 0 (99.0) | 5207 (98.9) |
| Recurrent | 38 (0.4) | 21 (0.4) |
| Secondary incident | 72 (0.7) | 38 (0.7) |
| Onset | ||
| Acute care facility | 2235 (21.3) | 1102 (20.9) |
| Community | 7328 (69.7) | 3694 (70.1) |
| Long-term care facility | 947 (9.0) | 470 (8.9) |
| Diagnosis in the intensive care unit | 1066 (10.1) | 522 (9.9) |
| Age at diagnosis, median (IQR), y | 69.00 (62.00, 79.00) | 68.00 (62.00, 79.00) |
| Maximum white blood cell count, median (IQR), thousands, per cubic millimeter | 10.60 (7.50, 15.00) | 11.20 (7.60, 16.60) |
| Average baseline SCr, median (IQR), milligrams per deciliter | 1.10 (0.87, 1.50) | 1.10 (0.85, 1.50) |
| Maximum SCr, median (IQR), milligrams per deciliter | 1.20 (0.90, 1.86) | 1.21 (0.90, 1.90) |
| Charlson score, median (IQR) | 2.00 (0.00, 4.00) | 2.00 (0.00, 4.00) |
| Days of vancomycin last 1 year, median (IQR) | 0.00 (0.00, 3.00) | 0.00 (0.00, 3.00) |
| Days of metronidazole last 1 year, median (IQR) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| Immune suppressive medications last 1 month | 543 (5.2) | 261 (5.0) |
| Chemotherapy last 1 month | 306 (2.9) | 145 (2.8) |
| Fluoroquinolones last 3 months | 3349 (31.9) | 1696 (32.2) |
| Cephalosporins last 3 months | 3496 (33.3) | 1736 (33.0) |
| Proton pump inhibitors last 3 months | 3450 (32.8) | 1755 (33.3) |
| CDI treatment with rifaximin | 160 (1.5) | 89 (1.7) |
| History of CDI | 48 (0.5) | 33 (0.6) |
| History of hospitalization last 1 year | 6148 (58.5) | 3101 (58.9) |
| Male sex | 9944 (94.6) | 4991 (94.8) |
Abbreviations: CDI, Clostridioides difficile infection; IQR, interquartile range; SCr, serum creatinine.
Demographic and Clinical Characteristics of Propensity-Matched Clostridioides difficile Infection Patients Treated With Metronidazole or Oral Vancomycin
| Characteristic . | Metronidazole, n (%) . | Vancomycin, n (%) . |
|---|---|---|
| No. of patients | 10 510 | 5266 |
| Year | ||
| 2006 | 439 (4.2) | 181 (3.4) |
| 2007 | 419 (4.0) | 176 (3.3) |
| 2008 | 482 (4.6) | 218 (4.1) |
| 2009 | 512 (4.9) | 261 (5.0) |
| 2010 | 606 (5.8) | 310 (5.9) |
| 2011 | 826 (7.9) | 487 (9.2) |
| 2012 | 985 (9.4) | 470 (8.9) |
| 2013 | 1129 (10.7) | 600 (11.4) |
| 2014 | 1417 (13.5) | 667 (12.7) |
| 2015 | 1680 (16.0) | 864 (16.4) |
| 2016 | 2015 (19.2) | 1032 (19.6) |
| Episode type | ||
| Primary incident | 1040 0 (99.0) | 5207 (98.9) |
| Recurrent | 38 (0.4) | 21 (0.4) |
| Secondary incident | 72 (0.7) | 38 (0.7) |
| Onset | ||
| Acute care facility | 2235 (21.3) | 1102 (20.9) |
| Community | 7328 (69.7) | 3694 (70.1) |
| Long-term care facility | 947 (9.0) | 470 (8.9) |
| Diagnosis in the intensive care unit | 1066 (10.1) | 522 (9.9) |
| Age at diagnosis, median (IQR), y | 69.00 (62.00, 79.00) | 68.00 (62.00, 79.00) |
| Maximum white blood cell count, median (IQR), thousands, per cubic millimeter | 10.60 (7.50, 15.00) | 11.20 (7.60, 16.60) |
| Average baseline SCr, median (IQR), milligrams per deciliter | 1.10 (0.87, 1.50) | 1.10 (0.85, 1.50) |
| Maximum SCr, median (IQR), milligrams per deciliter | 1.20 (0.90, 1.86) | 1.21 (0.90, 1.90) |
| Charlson score, median (IQR) | 2.00 (0.00, 4.00) | 2.00 (0.00, 4.00) |
| Days of vancomycin last 1 year, median (IQR) | 0.00 (0.00, 3.00) | 0.00 (0.00, 3.00) |
| Days of metronidazole last 1 year, median (IQR) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| Immune suppressive medications last 1 month | 543 (5.2) | 261 (5.0) |
| Chemotherapy last 1 month | 306 (2.9) | 145 (2.8) |
| Fluoroquinolones last 3 months | 3349 (31.9) | 1696 (32.2) |
| Cephalosporins last 3 months | 3496 (33.3) | 1736 (33.0) |
| Proton pump inhibitors last 3 months | 3450 (32.8) | 1755 (33.3) |
| CDI treatment with rifaximin | 160 (1.5) | 89 (1.7) |
| History of CDI | 48 (0.5) | 33 (0.6) |
| History of hospitalization last 1 year | 6148 (58.5) | 3101 (58.9) |
| Male sex | 9944 (94.6) | 4991 (94.8) |
| Characteristic . | Metronidazole, n (%) . | Vancomycin, n (%) . |
|---|---|---|
| No. of patients | 10 510 | 5266 |
| Year | ||
| 2006 | 439 (4.2) | 181 (3.4) |
| 2007 | 419 (4.0) | 176 (3.3) |
| 2008 | 482 (4.6) | 218 (4.1) |
| 2009 | 512 (4.9) | 261 (5.0) |
| 2010 | 606 (5.8) | 310 (5.9) |
| 2011 | 826 (7.9) | 487 (9.2) |
| 2012 | 985 (9.4) | 470 (8.9) |
| 2013 | 1129 (10.7) | 600 (11.4) |
| 2014 | 1417 (13.5) | 667 (12.7) |
| 2015 | 1680 (16.0) | 864 (16.4) |
| 2016 | 2015 (19.2) | 1032 (19.6) |
| Episode type | ||
| Primary incident | 1040 0 (99.0) | 5207 (98.9) |
| Recurrent | 38 (0.4) | 21 (0.4) |
| Secondary incident | 72 (0.7) | 38 (0.7) |
| Onset | ||
| Acute care facility | 2235 (21.3) | 1102 (20.9) |
| Community | 7328 (69.7) | 3694 (70.1) |
| Long-term care facility | 947 (9.0) | 470 (8.9) |
| Diagnosis in the intensive care unit | 1066 (10.1) | 522 (9.9) |
| Age at diagnosis, median (IQR), y | 69.00 (62.00, 79.00) | 68.00 (62.00, 79.00) |
| Maximum white blood cell count, median (IQR), thousands, per cubic millimeter | 10.60 (7.50, 15.00) | 11.20 (7.60, 16.60) |
| Average baseline SCr, median (IQR), milligrams per deciliter | 1.10 (0.87, 1.50) | 1.10 (0.85, 1.50) |
| Maximum SCr, median (IQR), milligrams per deciliter | 1.20 (0.90, 1.86) | 1.21 (0.90, 1.90) |
| Charlson score, median (IQR) | 2.00 (0.00, 4.00) | 2.00 (0.00, 4.00) |
| Days of vancomycin last 1 year, median (IQR) | 0.00 (0.00, 3.00) | 0.00 (0.00, 3.00) |
| Days of metronidazole last 1 year, median (IQR) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| Immune suppressive medications last 1 month | 543 (5.2) | 261 (5.0) |
| Chemotherapy last 1 month | 306 (2.9) | 145 (2.8) |
| Fluoroquinolones last 3 months | 3349 (31.9) | 1696 (32.2) |
| Cephalosporins last 3 months | 3496 (33.3) | 1736 (33.0) |
| Proton pump inhibitors last 3 months | 3450 (32.8) | 1755 (33.3) |
| CDI treatment with rifaximin | 160 (1.5) | 89 (1.7) |
| History of CDI | 48 (0.5) | 33 (0.6) |
| History of hospitalization last 1 year | 6148 (58.5) | 3101 (58.9) |
| Male sex | 9944 (94.6) | 4991 (94.8) |
Abbreviations: CDI, Clostridioides difficile infection; IQR, interquartile range; SCr, serum creatinine.
Table 2 shows the results from multivariable models within the propensity-matched cohorts. Patients treated with oral vancomycin were no more likely to develop VRE within 3 months than metronidazole-treated patients (adjusted RR [aRR], 0.96; 95% CI, .77 to 1.20), equating to an absolute RD of −0.11% (95% CI, −.68% to .47%). RR estimates were similar when surveillance tests were included at 3 months (aRR, 0.98; 95% CI, .79 to 1.21) and when clinical cultures were included at 6 months (aRR, 1.05; 95% CI, .85 to 1.28). Patients treated with vancomycin appeared to be at lower risk of a VRE bloodstream infection at 3 months, but a small increase in risk is also compatible with our data (aRR, 0.56; 95% CI, .3 to 1.05). Adjusted RDs varied from −0.22% (95% CI, −.45% to .00%) for the risk of VRE bloodstream infections to 0.14% (95% CI, −.50% to .78%) for the risk of VRE in clinical cultures at 6 months. Results of other sensitivity analyses are consistent with the primary analyses (Supplementary Table 1).
Adjusted Relative Risks and Risk Differences Comparing the Impact of Vancomycin to Metronidazole on the Risk of Vancomycin-Resistant Enterococci
| Outcome . | Relative Riska . | 95% CIa . | Risk Differencea (%) . | 95% CIa . |
|---|---|---|---|---|
| VRE clinical culture at 3 months | 0.96 | (.77 to 1.20) | −0.11 | (−.68 to 0.47) |
| VRE bloodstream infection at 3 months | 0.56 | (.3 to 1.05) | −0.22 | (−.45 to 0.00) |
| VRE from any source at 3 months | 0.98 | (.79 to 1.21) | −0.07 | (−.69 to 0.55) |
| VRE in clinical culture at 6 months | 1.05 | (.85 to 1.28) | 0.14 | (−.5 to 0.78) |
| Outcome . | Relative Riska . | 95% CIa . | Risk Differencea (%) . | 95% CIa . |
|---|---|---|---|---|
| VRE clinical culture at 3 months | 0.96 | (.77 to 1.20) | −0.11 | (−.68 to 0.47) |
| VRE bloodstream infection at 3 months | 0.56 | (.3 to 1.05) | −0.22 | (−.45 to 0.00) |
| VRE from any source at 3 months | 0.98 | (.79 to 1.21) | −0.07 | (−.69 to 0.55) |
| VRE in clinical culture at 6 months | 1.05 | (.85 to 1.28) | 0.14 | (−.5 to 0.78) |
Abbreviations: CI, confidence interval; VRE, vancomycin-resistant Enterococci.
aAverage across 50 imputed and propensity score matched datasets.
Adjusted Relative Risks and Risk Differences Comparing the Impact of Vancomycin to Metronidazole on the Risk of Vancomycin-Resistant Enterococci
| Outcome . | Relative Riska . | 95% CIa . | Risk Differencea (%) . | 95% CIa . |
|---|---|---|---|---|
| VRE clinical culture at 3 months | 0.96 | (.77 to 1.20) | −0.11 | (−.68 to 0.47) |
| VRE bloodstream infection at 3 months | 0.56 | (.3 to 1.05) | −0.22 | (−.45 to 0.00) |
| VRE from any source at 3 months | 0.98 | (.79 to 1.21) | −0.07 | (−.69 to 0.55) |
| VRE in clinical culture at 6 months | 1.05 | (.85 to 1.28) | 0.14 | (−.5 to 0.78) |
| Outcome . | Relative Riska . | 95% CIa . | Risk Differencea (%) . | 95% CIa . |
|---|---|---|---|---|
| VRE clinical culture at 3 months | 0.96 | (.77 to 1.20) | −0.11 | (−.68 to 0.47) |
| VRE bloodstream infection at 3 months | 0.56 | (.3 to 1.05) | −0.22 | (−.45 to 0.00) |
| VRE from any source at 3 months | 0.98 | (.79 to 1.21) | −0.07 | (−.69 to 0.55) |
| VRE in clinical culture at 6 months | 1.05 | (.85 to 1.28) | 0.14 | (−.5 to 0.78) |
Abbreviations: CI, confidence interval; VRE, vancomycin-resistant Enterococci.
aAverage across 50 imputed and propensity score matched datasets.
DISCUSSION
With release of the new clinical practice guidelines, the treatment of CDI is likely to shift substantially in the coming years. Vancomycin was the first US Food and Drug Administration–approved drug for CDI but quickly became second choice to the less expensive metronidazole. This initial shift away from vancomycin is thought to be at least partly in response to CDC guidelines on preventing the spread of vancomycin resistance by limiting its use, including for antibiotic-associated diarrhea [27, 28]. The new treatment recommendations were made largely on the basis of clinical efficacy from randomized, controlled trials [29]. However, there is still some debate on the classification of metronidazole as a nonpreferred agent among patients with mild disease. In a Letter to the Editor, Fabre and colleagues highlighted the relative lack of data demonstrating vancomycin’s superiority in mild CDI, higher cost, impact on the intestinal flora, and potential selective pressure for vancomycin resistance among Enterococci as reasons to continue treating low-risk patients with metronidazole [30]. In their reply, the guideline authors underscored the lack of validated criteria to distinguish mild, moderate, and severe infections and incomplete understanding of the relative impact of vancomycin and metronidazole on VRE [29]. To address the latter gap, we present the results of a large, multicenter observational study comparing CDI patients treated with vancomycin or metronidazole and followed longitudinally for VRE infections from clinical and surveillance sources in the next 3–6 months.
Our results suggest that oral vancomycin does not increase the risk of VRE when compared to metronidazole for the treatment of CDI. These findings do not preclude the possibility that both metronidazole and vancomycin play a role in the epidemiology of VRE, only that there is no evidence to support preference of metronidazole over vancomycin in CDI management with respect to the risk of VRE. A number of antibiotics have been implicated in the promotion of VRE infection or colonization, including extended-spectrum cephalosporins, antianaerobes, and intravenous vancomycin. Although de novo glycopeptide resistance in an individual treated patient is rare, vancomycin may influence the likelihood of colonization and transmission between patients, particularly in healthcare facilities [31]. While intravenous vancomycin has been studied extensively and with conflicting results [9, 31], less is known about how oral vancomycin, with its much higher intestinal concentrations, influences the risk of VRE. Metronidazole has also previously been noted to promote VRE colonization and overgrowth [10, 32], but data directly comparing oral vancomycin to metronidazole, which are most useful for guiding CDI treatment decisions, are sparse.
Nationally representative studies on the epidemiology of VRE are lacking, and available data suggest variability by study setting [33]. The incidence of VRE decreased in VA acute care facilities between 2007 and 2010 on ICU and non-ICU wards [13] during which time metronidazole and intravenous vancomycin accounted for 7%–8% and 16%–19% of inpatient antibiotic use, respectively, and remained fairly stable [34]. We noted similar declines in the risk of VRE infections among CDI patients, a population typically at elevated risk of other healthcare-associated infections [35, 36], between 2008 and 2010 with continued declines through 2016. Over the same period, oral vancomycin monotherapy increased while metronidazole use decreased appreciably.
Taken together, these results suggest that oral vancomycin and metronidazole are equally likely to impact patients’ risk of VRE. In the setting of stable CDI incidence, replacement of metronidazole with oral vancomycin is unlikely to be a significant driver of increased risk of VRE at the individual patient level. From the population perspective, continued antimicrobial stewardship and infection control efforts will likely have greater impacts on VRE acquisition and transmission.
To our knowledge, this is the largest study to date to directly compare the risk of VRE following oral vancomycin or metronidazole exposure in the context of CDI treatment. The VA is the largest integrated health system in the United States, caring for millions of veterans each year in the inpatient, long-term care, and outpatient settings. Our analysis includes patients diagnosed with CDI at more than 1200 hospitals and clinics.
As a retrospective observational study, our results must be interpreted in light of some important limitations. We used propensity score matching to address imbalances in measured confounders. However, since patients were not randomized to treatment, the possibility of unmeasured confounding remains. Although the VA records information on healthcare encounters in the inpatient, outpatient, and long-term care settings, capture is not perfect, particularly for patients using non-VA services. This potential misclassification could have impacted our study results in unpredictable ways. VRE colonization before and after CDI is common and may influence the risk of developing a clinically meaningful VRE infection. We were unable to systematically evaluate VRE colonization status due to the limited use of screening in VA facilities. Our study was not designed to evaluate impacts of treatment on the transmission of VRE, and additional work is needed to fully appreciate the impacts of oral vancomycin and vancomycin on the dynamics of vancomycin-resistance among Enterococci. Because veterans are a unique patient population, largely male and with a greater comorbidity burden, our results must be generalized to nonveteran populations with caution.
Finally, we defined CDI using laboratory results alone in accordance with commonly used electronic surveillance methods [37]. Laboratory testing for C. difficile changed from predominantly EIA to predominantly PCR over the course of the study [38, 39]. By including only patients who received metronidazole or oral vancomycin, we captured patients whose providers considered their test results as suggestive enough of clinically relevant infection to warrant treatment.
Conclusions
Treatment decisions are multifactorial and should consider outcomes beyond efficacy. For CDI, relevant issues include patient preferences, costs, insurance coverage, recurrence, mortality, and antimicrobial resistance at the individual and population levels. Existing evidence suggests that both vancomycin and metronidazole potentially play a role in the acquisition and transmission of VRE. Our results indicate that the magnitude of these effects is similar between drugs among patients with CDI. We observed no increase in the risk of VRE from clinical or surveillance sources at 3 or 6 months post-CDI treatment for patients treated with vancomycin compared to metronidazole. From the perspective of preventing the spread of antimicrobial resistance, the best antibiotic is no antibiotic. Future research efforts should focus on the identification of patients with self-limiting CDI who may not need antibiotic therapy and on balancing the trade-offs of vancomycin and metronidazole with a broader range of important risks and benefits.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: 28th European Congress on Clinical Microbiology and Infectious Diseases. Madrid, Spain, 21–25 April 2018. Presentation number O0263.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Department of Veterans Affairs or the US government.
Financial support. This work was supported in part by a Center of Innovation grant (CIN 13–414, principal investigator [PI], M. H. S.) and a Career Development award (CDA 11–210, PI, R. E. N.) from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development.
Potential conflicts of interest. All authors have no conflicts of interest to report. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




