-
PDF
- Split View
-
Views
-
Cite
Cite
Kuei-Lien Tien, Wang-Huei Sheng, Shiouh-Chu Shieh, Yen-Ping Hung, Hwei-Fang Tien, Yi-Hsuan Chen, Li-Jung Chien, Jann-Tay Wang, Chi-Tai Fang, Yee-Chun Chen, Chlorhexidine Bathing to Prevent Central Line–Associated Bloodstream Infections in Hematology Units: A Prospective, Controlled Cohort Study, Clinical Infectious Diseases, Volume 71, Issue 3, 1 August 2020, Pages 556–563, https://doi.org/10.1093/cid/ciz874
Close - Share Icon Share
Abstract
Chlorhexidine (CHG) bathing decreases the incidence of bloodstream infections in intensive care units, but its effect has been understudied in patients with hematological malignancies in noncritical care units.
Adults with hematological malignancies hospitalized for cytotoxic chemotherapy in noncritical care units were offered daily 2% CHG bathing. We compared outcomes of patients who chose CHG bathing (CHG group) with outcomes of those who did not choose CHG bathing (usual-care group). The primary outcome was gram-positive cocci–related, skin flora–related, or central line–associated bloodstream infection. The negative control outcome was gut-origin bacteremia.
The CHG group (n = 485) had a crude incidence rate of the primary outcome that was 60% lower than the rate for the usual-care group (n = 408; 3.4 vs 8.4 per 1000 patient-days, P = .02) but had a similar crude incidence rate of the negative control outcome (4.5 vs 3.2 per 1000 patient-days; P = .10). In multivariable analyses, CHG bathing was associated with a 60% decrease in the primary outcome (adjusted hazard ratio [HR], 0.4; P < .001). In contrast, CHG bathing had no effect on the negative control outcome (adjusted HR, 1.1; P = .781). CHG bathing was well tolerated by participants in the CHG group.
CHG bathing could be a highly effective approach for preventing gram-positive cocci–related, skin flora–related, or central line–associated bacteremia in patients with hematological malignancies who are hospitalized for cytotoxic chemotherapy in noncritical care units.
Patients with hematological malignancies hospitalized for myelosuppressive chemotherapy are at high risk of life-threatening healthcare-associated infections, despite the use of oral prophylactic antibiotics [1, 2]. The indwelling central venous catheter is a major source of bloodstream infections (BSIs) in such patients [3, 4]. Even with routine cleansing and disinfection of skin before placement and use of indwelling catheters [3, 5], central line-associated BSI (CLABSI) remains a leading cause of excess mortality, morbidity, cost, and prolonged hospitalization in patients with hematological malignancies [6–9]. There is an unmet need for a more effective strategy for preventing healthcare-associated infection in such patients.
Daily bathing with 2% chlorhexidine (CHG) is a simple and effective way to decrease microbial load on the skin. Systematic reviews and metaanalyses of randomized trials concluded that CHG bathing prevents 50%–56% of CLABSIs in patients in intensive care units (ICUs) [10–20]. However, for patients in noncritical care, general medical, or surgical units (including cancer patients in oncology units), a cluster-randomized trial involving half a million patients found that CHG bathing did not decrease the incidence of BSI [21]. The effect of CHG bathing has not been assessed in patients with hematological malignancies in noncritical care units.
In this prospective, concurrent, controlled cohort study, we aimed to assess the effect of daily CHG bathing on healthcare-associated bloodstream infections in patients with hematological malignancies hospitalized for cytotoxic chemotherapy.
METHODS
Study Setting
National Taiwan University Hospital (NTUH) is an academic center with 2300 beds . NTUH provides both primary care and tertiary referral care, with 3 million outpatient visits and 91 645 admissions in 2014. NTUH is the leading hematological treatment center in Taiwan. A prospective, hospital-wide surveillance of healthcare-associated infections (HAIs) was initiated in 1981 at this hospital. A web-based HAI management system was established and integrated into the medical information system in 2007, and a rule-based healthcare-associated BSI surveillance and classification system was implemented on 1 October 2010 [22]. To develop the detection rules in the candidate-detection subsystem, we adapted the objective components of the National Health Care Safety Network definitions established by the US Centers for Disease Control and Prevention and modified them according to the national consensus and local practice. The performance of this web-based automatic system has been validated [22]. Blood culture practices at this hospital have been described previously [22].
Despite implementation of a central line insertion and maintenance care bundle (the main components were hand hygiene, aseptic technique, local disinfectants at insertion, maximum sterile surface, avoidance of the femoral area for central venous catheter placement, and early removal of catheters) [3] since 2009, patients with hematological malignancies hospitalized in noncritical care hematology wards still had a baseline incidence of healthcare-associated BSIs (approximately 10/1000 patient-days during 2013–2014) that was 2 times higher than that of general patients in ICUs during the same period. One-third of these healthcare-associated BSI episodes were CLABSIs or skin flora–associated BSIs (criterion 1 for laboratory-confirmed BSI [LCBI 1] or criterion 2 for laboratory-confirmed BSI [LCBI 2]); nearly all of the remaining events were gut-origin Escherichia coli or Klebsiella pneumoniae BSIs (criterion 1 for mucosal barrier injury LCBI [MBI-LCBI 1]) [23].
Study Design
This was a prospective, concurrent, controlled cohort study within the context of a healthcare quality improvement campaign. Adults (aged ≥20 years in Taiwan) with hematological malignancies hospitalized in noncritical care units for receipt of intravenous cytotoxic chemotherapy at NTUH from December 2015 through June 2016 were eligible. All eligible patients were offered information on daily CHG bathing. All patients who chose CHG bathing were provided with 2% CHG-containing skin cleanser solution (independent patients) or a 2% CHG-impregnated paper towel (bed-ridden patients). We compared the incidence of the primary outcome and the negative control outcome of patients who chose CHG bathing (CHG group) and of patients who did not choose CHG bathing (usual-care group) during the same period.
Ethical Statement
The NTUH Research Ethics Committee approved the study protocol a priori. All participants in the CHG group gave written informed consent. Patients in the usual-care group received standard medical care and standard HAI monitoring. The Research Ethics Committee waived informed consent for patients in the usual-care group.
Baseline Care
All hospitalized patients received the same care bundle for prevention of CLABSI. Uncoated central venous catheters were used in noncritical care units. Before placement, 2% CHG (in 70% ethanol) was used for skin antisepsis. Senior physicians placed the catheters via the jugular or subclavian route without ultrasound guidance. After placement, the insertion site was covered with 2% CHG-impregnated dressings. Physicians prescribed prophylactic antibiotics according to guidelines (levofloxacin or ciprofloxacin are preferred in patients at high risk of prolonged neutropenia) [24] and instructed patients to bathe daily using over-the-counter non–CHG-based antibacterial soap or cleansing lotion.
CHG Bathing
Participants in the CHG group were provided with 2% CHG-containing skin cleanser solution (PBF Biotech, Taipei, Taiwan) for daily bathing (for those who were independent in their activities of daily living) or a 2% CHG-impregnated paper towel (PBF Biotech, Taipei, Taiwan) for a daily wiping bath (for those who were bed-ridden). One of the authors instructed those who were independent in their activities of daily living to apply the 2% CHG cleanser solution to their bodies after an initial water rinse and then allow the 2% CHG to remain on the body surface for 30 seconds before washing with water and drying with a clean towel. The 2% CHG was applied from the chin downward until all body surfaces below the neck were covered, with extra focus on skin folds and sweat areas. For bed-ridden participants, research nurses instructed caregivers to perform a wiping bath with a 2% CHG-impregnated paper towel over all body surfaces below the jawline and then allow the 2% CHG to dry naturally without washing with water. The researchers visited patients daily to check adherence (reporting from patients and caregivers) and monitor adverse events (inspection for rash or allergic reactions).
Primary and Negative Control Outcomes
The primary outcome was the first occurrence of a healthcare-associated BSI (after 48 hours hospitalization) caused by common gram-positive cocci–related BSI (Staphylococcus aureus or group A Streptococcus, cultured from at least 1 set of blood cultures; LCBI 1), skin flora–related BSI (LCBI 2), or CLABSI [22, 23]. CLABSI was defined as a BSI episode that occurred when a central venous catheter was in place or had been removed during the previous 48 hours [3, 22, 23]. The negative control outcome was the first occurrence of gut-origin bacteremia, which was operatively defined as healthcare-associated E. coli or K. pneumoniae bacteremia (the 2 most common enteric bacteria, which accounted for nearly all gut-origin bacteremia at NTUH) that occurred after the start of cytotoxic chemotherapy (MBI-LCBI-1) [23].
Adverse Event
At enrollment, each participant was instructed to report any adverse events to the attending nurses. The researchers visited participants every day to record any adverse event.
Data Collection
We systematically collected baseline demographic and clinical data, including hematological diagnosis, types of cytotoxic chemotherapy, indwelling central venous catheters or devices such as a port-A-cath, types of oral prophylactic antibiotics, carriage of multidrug-resistant organisms, and the presence of diabetes mellitus. We also obtained neutrophil count at nadir after cytotoxic chemotherapy as an indicator of the severity of treatment-related myelosuppression.
Statistical Analyses
With a baseline healthcare-associated BSI incidence of 10/1000 patient-days (one-third of events were CLABSI or skin flora–associated BSIs) and an estimated average hospital stay of 30 days, 872 participants, at a minimum, would be required to detect a 50% decrease in the cumulative incidence of the primary outcome by the χ2 test with a power of 0.8. Statistical analyses were performed using SAS ver9.4 (SAS Institute Inc, Cary, NC). Continuous and categorical variables were compared using the Student t test (or Mann-Whitney test) and χ2 test (or Fisher exact test), respectively. Incidence rates were compared using Poisson regression. For the time-to-event analyses, patient-day at risk was counted from the day of enrollment to the day when outcomes occurred (event) or when the patient was discharged from the hospital (censored). The primary outcome and the negative control outcome were analyzed separately. For multivariable Cox regression, all variables were initially included in the maximum model. We applied the stepwise selection procedure to determine the optimum model, with CHG bathing and fluoroquinolone prophylaxis forced into the model if these 2 clinically relevant variables were not retained by the stepwise algorithm. We did post hoc analyses for the subgroup of participants who used central lines or port devices. We also analyzed the determinants for the length of hospital stay. All tests were 2-sided, and a P value < .05 was considered statistically significant.
RESULTS
We screened 1078 patients with hematological malignancies (Figure 1). Of the 893 eligible patients, 485 chose CHG bathing (CHG group) and 408 patients did not choose CHG bathing (usual-care group). Table 1 compares the demographic and clinical characteristics of the 2 groups. Participants in the CHG group were more likely to have a hematological diagnosis of leukemia, to receive induction chemotherapy, to experience neutropenia during hospitalization, and to use central lines or port devices for venous access than those in the usual-care group (all P < .001). All participants completed the study and were evaluable at the end of the study (Figure 1).
Patients Characteristics for the Chlorhexidine Group and the Usual-care Group
| Characteristic . | Chlorhexidine Group (n = 485) . | Usual-care Group (n = 408) . | P Value . |
|---|---|---|---|
| Sex | .196 | ||
| Male | 256 (52.8) | 233 (57.1) | |
| Female | 229 (47.2) | 175 (42.9) | |
| Age, y | |||
| Median (interquartile range) | 51 (38–62) | 57 (44–66) | <.001a |
| Hematological diagnosis | <.001a | ||
| Lymphoma | 177 (36.5) | 268 (65.7) | |
| Hodgkin disease | 17 (9.6) | 23 (8.6) | |
| Non-Hodgkin lymphoma | 160 (90.4) | 245 (91.4) | |
| Leukemia | 253 (52.2) | 121 (29.7) | |
| Acute myelogenous leukemia | 156 (61.7) | 77 (63.6) | |
| Acute lymphocytic leukemia | 73 (28.9) | 29 (24.0) | |
| Other typesb | 24 (9.4) | 15 (12.4) | |
| Othersc | 55 (11.3) | 19 (4.6) | |
| Relapsed/refractory diseases | 73 (15.0) | 53 (13.0) | .378 |
| Types of chemotherapy | <.001a | ||
| Induction | 146 (30.1) | 43 (10.5) | |
| Consolidation | 339 (69.9) | 365(89.5) | |
| Post-chemotherapy neutropenia (<500/μL) | 260 (53.6) | 91 (22.3) | <.001a |
| Autologous peripheral blood stem cell transplantation | 25 (5.2) | 4 (1.0) | <.001a |
| Use of central line | |||
| Only CVCd | 120 (24.7) | 70 (17.2) | .006a |
| Only port-A-cath | 227 (46.8) | 136 (33.3) | <.001a |
| CVCd and port-A-cath | 60 (12.4) | 18 (4.4) | <.001a |
| Nonee | 78 (16.1) | 184 (45.1) | <.001a |
| Oral prophylactic antibiotics | |||
| Ciprofloxacin or levofloxacin | 130 (26.8) | 33 (8.1) | <.001 a |
| Sulfamethoxazole/trimethoprim | 208 (42.9) | 100 (24.5) | <.001 a |
| Multidrug-resistant organism carriage at enrollmentf | 84 (17.3) | 43 (10.5) | .004 a |
| Vancomycin-resistant enterococci | 18 (3.7) | 13 (3.2) | .669 |
| Clostridium difficile | 14 (3.0) | 3 (0.8) | .019 a |
| Diabetes mellitus | 36 (7.4) | 46 (11.3) | .047 a |
| Activities of daily livingg | .010 a | ||
| Completely independent | 422 (87.0) | 318 (77.9) | |
| Mild dependence | 5 (1.0) | 8 (2.0) | |
| Moderate dependence | 36 (7.4) | 48 (11.8) | |
| Heavy reliance | 18 (3.7) | 26 (6.4) | |
| Completely dependent | 4 (0.8) | 8 (2.0) | |
| Hospital stay (days), mean ± standard deviation | 26.2 ± 26.6 | 15.8 ± 27.8 | <.001 a |
| In-hospital mortality | 23 (4.7) | 22 (5.4) | .658 |
| Characteristic . | Chlorhexidine Group (n = 485) . | Usual-care Group (n = 408) . | P Value . |
|---|---|---|---|
| Sex | .196 | ||
| Male | 256 (52.8) | 233 (57.1) | |
| Female | 229 (47.2) | 175 (42.9) | |
| Age, y | |||
| Median (interquartile range) | 51 (38–62) | 57 (44–66) | <.001a |
| Hematological diagnosis | <.001a | ||
| Lymphoma | 177 (36.5) | 268 (65.7) | |
| Hodgkin disease | 17 (9.6) | 23 (8.6) | |
| Non-Hodgkin lymphoma | 160 (90.4) | 245 (91.4) | |
| Leukemia | 253 (52.2) | 121 (29.7) | |
| Acute myelogenous leukemia | 156 (61.7) | 77 (63.6) | |
| Acute lymphocytic leukemia | 73 (28.9) | 29 (24.0) | |
| Other typesb | 24 (9.4) | 15 (12.4) | |
| Othersc | 55 (11.3) | 19 (4.6) | |
| Relapsed/refractory diseases | 73 (15.0) | 53 (13.0) | .378 |
| Types of chemotherapy | <.001a | ||
| Induction | 146 (30.1) | 43 (10.5) | |
| Consolidation | 339 (69.9) | 365(89.5) | |
| Post-chemotherapy neutropenia (<500/μL) | 260 (53.6) | 91 (22.3) | <.001a |
| Autologous peripheral blood stem cell transplantation | 25 (5.2) | 4 (1.0) | <.001a |
| Use of central line | |||
| Only CVCd | 120 (24.7) | 70 (17.2) | .006a |
| Only port-A-cath | 227 (46.8) | 136 (33.3) | <.001a |
| CVCd and port-A-cath | 60 (12.4) | 18 (4.4) | <.001a |
| Nonee | 78 (16.1) | 184 (45.1) | <.001a |
| Oral prophylactic antibiotics | |||
| Ciprofloxacin or levofloxacin | 130 (26.8) | 33 (8.1) | <.001 a |
| Sulfamethoxazole/trimethoprim | 208 (42.9) | 100 (24.5) | <.001 a |
| Multidrug-resistant organism carriage at enrollmentf | 84 (17.3) | 43 (10.5) | .004 a |
| Vancomycin-resistant enterococci | 18 (3.7) | 13 (3.2) | .669 |
| Clostridium difficile | 14 (3.0) | 3 (0.8) | .019 a |
| Diabetes mellitus | 36 (7.4) | 46 (11.3) | .047 a |
| Activities of daily livingg | .010 a | ||
| Completely independent | 422 (87.0) | 318 (77.9) | |
| Mild dependence | 5 (1.0) | 8 (2.0) | |
| Moderate dependence | 36 (7.4) | 48 (11.8) | |
| Heavy reliance | 18 (3.7) | 26 (6.4) | |
| Completely dependent | 4 (0.8) | 8 (2.0) | |
| Hospital stay (days), mean ± standard deviation | 26.2 ± 26.6 | 15.8 ± 27.8 | <.001 a |
| In-hospital mortality | 23 (4.7) | 22 (5.4) | .658 |
Data are mean (standard deviation) or n (%). Continuous and categorical variables were compared using the Student t test (or Mann-Whitney test) and χ2 test (or Fisher exact test), respectively.
Abbreviation: CVC, central venous catheter.
aStatistically significant.
bChronic myelogenous leukemia (n = 10), chronic lymphocytic leukemia (n = 5), hairy cell leukemia (n = 1), myeloid leukemia (n = 4), lymphoid leukemia (n = 2), acute leukemia of unspecified cell (n = 9), leukemia unspecified (n = 4), other (n = 4).
cMultiple myeloma (n = 63), myelodysplastic syndrome (n = 4), histiocytic sarcoma (n = 5), Langerhans cell histiocytosis (n = 1), idiopathic thrombocytopenic purpura (n = 1).
dBefore the onset of infection.
eThese patients used peripheral intravenous lines to receive cytotoxic chemotherapy.
fMultidrug-resistant organism carriage included methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococcus, carbapenem-resistant Acinetobacter baumannii complex, and carbapenem-resistant Enterobacteriaceae.
gActivities of daily living assessed on the basis of the Pap scale [25].
Patients Characteristics for the Chlorhexidine Group and the Usual-care Group
| Characteristic . | Chlorhexidine Group (n = 485) . | Usual-care Group (n = 408) . | P Value . |
|---|---|---|---|
| Sex | .196 | ||
| Male | 256 (52.8) | 233 (57.1) | |
| Female | 229 (47.2) | 175 (42.9) | |
| Age, y | |||
| Median (interquartile range) | 51 (38–62) | 57 (44–66) | <.001a |
| Hematological diagnosis | <.001a | ||
| Lymphoma | 177 (36.5) | 268 (65.7) | |
| Hodgkin disease | 17 (9.6) | 23 (8.6) | |
| Non-Hodgkin lymphoma | 160 (90.4) | 245 (91.4) | |
| Leukemia | 253 (52.2) | 121 (29.7) | |
| Acute myelogenous leukemia | 156 (61.7) | 77 (63.6) | |
| Acute lymphocytic leukemia | 73 (28.9) | 29 (24.0) | |
| Other typesb | 24 (9.4) | 15 (12.4) | |
| Othersc | 55 (11.3) | 19 (4.6) | |
| Relapsed/refractory diseases | 73 (15.0) | 53 (13.0) | .378 |
| Types of chemotherapy | <.001a | ||
| Induction | 146 (30.1) | 43 (10.5) | |
| Consolidation | 339 (69.9) | 365(89.5) | |
| Post-chemotherapy neutropenia (<500/μL) | 260 (53.6) | 91 (22.3) | <.001a |
| Autologous peripheral blood stem cell transplantation | 25 (5.2) | 4 (1.0) | <.001a |
| Use of central line | |||
| Only CVCd | 120 (24.7) | 70 (17.2) | .006a |
| Only port-A-cath | 227 (46.8) | 136 (33.3) | <.001a |
| CVCd and port-A-cath | 60 (12.4) | 18 (4.4) | <.001a |
| Nonee | 78 (16.1) | 184 (45.1) | <.001a |
| Oral prophylactic antibiotics | |||
| Ciprofloxacin or levofloxacin | 130 (26.8) | 33 (8.1) | <.001 a |
| Sulfamethoxazole/trimethoprim | 208 (42.9) | 100 (24.5) | <.001 a |
| Multidrug-resistant organism carriage at enrollmentf | 84 (17.3) | 43 (10.5) | .004 a |
| Vancomycin-resistant enterococci | 18 (3.7) | 13 (3.2) | .669 |
| Clostridium difficile | 14 (3.0) | 3 (0.8) | .019 a |
| Diabetes mellitus | 36 (7.4) | 46 (11.3) | .047 a |
| Activities of daily livingg | .010 a | ||
| Completely independent | 422 (87.0) | 318 (77.9) | |
| Mild dependence | 5 (1.0) | 8 (2.0) | |
| Moderate dependence | 36 (7.4) | 48 (11.8) | |
| Heavy reliance | 18 (3.7) | 26 (6.4) | |
| Completely dependent | 4 (0.8) | 8 (2.0) | |
| Hospital stay (days), mean ± standard deviation | 26.2 ± 26.6 | 15.8 ± 27.8 | <.001 a |
| In-hospital mortality | 23 (4.7) | 22 (5.4) | .658 |
| Characteristic . | Chlorhexidine Group (n = 485) . | Usual-care Group (n = 408) . | P Value . |
|---|---|---|---|
| Sex | .196 | ||
| Male | 256 (52.8) | 233 (57.1) | |
| Female | 229 (47.2) | 175 (42.9) | |
| Age, y | |||
| Median (interquartile range) | 51 (38–62) | 57 (44–66) | <.001a |
| Hematological diagnosis | <.001a | ||
| Lymphoma | 177 (36.5) | 268 (65.7) | |
| Hodgkin disease | 17 (9.6) | 23 (8.6) | |
| Non-Hodgkin lymphoma | 160 (90.4) | 245 (91.4) | |
| Leukemia | 253 (52.2) | 121 (29.7) | |
| Acute myelogenous leukemia | 156 (61.7) | 77 (63.6) | |
| Acute lymphocytic leukemia | 73 (28.9) | 29 (24.0) | |
| Other typesb | 24 (9.4) | 15 (12.4) | |
| Othersc | 55 (11.3) | 19 (4.6) | |
| Relapsed/refractory diseases | 73 (15.0) | 53 (13.0) | .378 |
| Types of chemotherapy | <.001a | ||
| Induction | 146 (30.1) | 43 (10.5) | |
| Consolidation | 339 (69.9) | 365(89.5) | |
| Post-chemotherapy neutropenia (<500/μL) | 260 (53.6) | 91 (22.3) | <.001a |
| Autologous peripheral blood stem cell transplantation | 25 (5.2) | 4 (1.0) | <.001a |
| Use of central line | |||
| Only CVCd | 120 (24.7) | 70 (17.2) | .006a |
| Only port-A-cath | 227 (46.8) | 136 (33.3) | <.001a |
| CVCd and port-A-cath | 60 (12.4) | 18 (4.4) | <.001a |
| Nonee | 78 (16.1) | 184 (45.1) | <.001a |
| Oral prophylactic antibiotics | |||
| Ciprofloxacin or levofloxacin | 130 (26.8) | 33 (8.1) | <.001 a |
| Sulfamethoxazole/trimethoprim | 208 (42.9) | 100 (24.5) | <.001 a |
| Multidrug-resistant organism carriage at enrollmentf | 84 (17.3) | 43 (10.5) | .004 a |
| Vancomycin-resistant enterococci | 18 (3.7) | 13 (3.2) | .669 |
| Clostridium difficile | 14 (3.0) | 3 (0.8) | .019 a |
| Diabetes mellitus | 36 (7.4) | 46 (11.3) | .047 a |
| Activities of daily livingg | .010 a | ||
| Completely independent | 422 (87.0) | 318 (77.9) | |
| Mild dependence | 5 (1.0) | 8 (2.0) | |
| Moderate dependence | 36 (7.4) | 48 (11.8) | |
| Heavy reliance | 18 (3.7) | 26 (6.4) | |
| Completely dependent | 4 (0.8) | 8 (2.0) | |
| Hospital stay (days), mean ± standard deviation | 26.2 ± 26.6 | 15.8 ± 27.8 | <.001 a |
| In-hospital mortality | 23 (4.7) | 22 (5.4) | .658 |
Data are mean (standard deviation) or n (%). Continuous and categorical variables were compared using the Student t test (or Mann-Whitney test) and χ2 test (or Fisher exact test), respectively.
Abbreviation: CVC, central venous catheter.
aStatistically significant.
bChronic myelogenous leukemia (n = 10), chronic lymphocytic leukemia (n = 5), hairy cell leukemia (n = 1), myeloid leukemia (n = 4), lymphoid leukemia (n = 2), acute leukemia of unspecified cell (n = 9), leukemia unspecified (n = 4), other (n = 4).
cMultiple myeloma (n = 63), myelodysplastic syndrome (n = 4), histiocytic sarcoma (n = 5), Langerhans cell histiocytosis (n = 1), idiopathic thrombocytopenic purpura (n = 1).
dBefore the onset of infection.
eThese patients used peripheral intravenous lines to receive cytotoxic chemotherapy.
fMultidrug-resistant organism carriage included methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococcus, carbapenem-resistant Acinetobacter baumannii complex, and carbapenem-resistant Enterobacteriaceae.
gActivities of daily living assessed on the basis of the Pap scale [25].
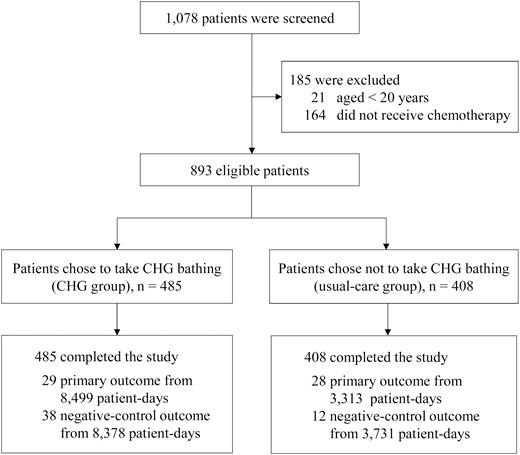
Flow chart of patient enrollment. Abbreviation: CHG, chlorhexidine.
Patients in the CHG group had a 60% lower crude incidence of the primary outcome compared with patients in the usual-care group (3.4 vs 8.5 per 1000 patient-days; P = .02). Figure 2 shows the Kaplan-Meier estimates for probability of the primary outcome in the 2 groups (P < .001, log-rank test). In contrast, neither incidence rate of the negative control outcome (4.5 vs 3.2 per 1000 patient-days; P = .10) nor Kaplan-Meier estimates for the probability of the negative control outcome (P = .688, log-rank test; Figure 3) differed between the 2 groups. The Supplementary Material provides details on the microbiological data for the primary outcome (Supplementary Table 1) and the negative control outcome (Supplementary Table 2).
| Variable . | Univariable Analysis . | Multivariable Analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P Value . | Adjusted HR a (95% CI) . | P Value . | |
| 2% chlorhexidine daily bathing | 0.4 (.2–.6) | <.001 | 0.4 (.2–.6) | <.001b |
| Sex, male | 1.0 (.6–1.8) | .889 | - | - |
| Age >65 years | 0.7 (.3–1.6) | .393 | - | - |
| Hematological diagnosis, lymphoma vs other diagnosis | 0.4 (.2–.9) | .027 | - | - |
| Relapsed/refractory diseases | 1.5 (.8–2.9) | .202 | … | … |
| Post-chemotherapy neutropenia (<500/μL) | 13.1 (1.8–95.3) | .011 | 13.9 (1.9–101.9) | .010b |
| Autologous peripheral blood stem cell transplantation | 0.9 (.3–2.9) | .869 | … | … |
| Chemotherapy, induction vs other types | 1.6 (1.0–2.8) | .068 | - | - |
| Use of central venous catheter | 3.5 (2.1–5.9) | <.001 | 2.7 (1.6–4.6) | <.00b |
| Use of port-A-cath | 0.3 (.2–.7) | .299 | - | - |
| Oral ciprofloxacin/levofloxacin for prophylaxis | 0.6 (.3–1.1) | .094 | 0.6 (.3–1.0) | .057 |
| Oral sulfamethoxazole/trimethoprim for prophylaxis | 1.7 (1.0–3.0) | .068 | - | - |
| Multidrug-resistant organism carriage | 0.8 (.4–1.5) | .444 | - | - |
| Diabetes mellitus | 1.6 (.7–3.8) | .251 | - | - |
| In-hospital mortality | 1.9 (1.0–3.8) | .054 | - | - |
| Variable . | Univariable Analysis . | Multivariable Analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P Value . | Adjusted HR a (95% CI) . | P Value . | |
| 2% chlorhexidine daily bathing | 0.4 (.2–.6) | <.001 | 0.4 (.2–.6) | <.001b |
| Sex, male | 1.0 (.6–1.8) | .889 | - | - |
| Age >65 years | 0.7 (.3–1.6) | .393 | - | - |
| Hematological diagnosis, lymphoma vs other diagnosis | 0.4 (.2–.9) | .027 | - | - |
| Relapsed/refractory diseases | 1.5 (.8–2.9) | .202 | … | … |
| Post-chemotherapy neutropenia (<500/μL) | 13.1 (1.8–95.3) | .011 | 13.9 (1.9–101.9) | .010b |
| Autologous peripheral blood stem cell transplantation | 0.9 (.3–2.9) | .869 | … | … |
| Chemotherapy, induction vs other types | 1.6 (1.0–2.8) | .068 | - | - |
| Use of central venous catheter | 3.5 (2.1–5.9) | <.001 | 2.7 (1.6–4.6) | <.00b |
| Use of port-A-cath | 0.3 (.2–.7) | .299 | - | - |
| Oral ciprofloxacin/levofloxacin for prophylaxis | 0.6 (.3–1.1) | .094 | 0.6 (.3–1.0) | .057 |
| Oral sulfamethoxazole/trimethoprim for prophylaxis | 1.7 (1.0–3.0) | .068 | - | - |
| Multidrug-resistant organism carriage | 0.8 (.4–1.5) | .444 | - | - |
| Diabetes mellitus | 1.6 (.7–3.8) | .251 | - | - |
| In-hospital mortality | 1.9 (1.0–3.8) | .054 | - | - |
..., this variable did not enter the final multivariable analysis.
Abbreviations: CI, confidence interval; HR, hazard ratio.
aAdjusted using the Cox proportional hazards model (proportional hazard assumption test, P = .455).
bStatistically significant.
| Variable . | Univariable Analysis . | Multivariable Analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P Value . | Adjusted HR a (95% CI) . | P Value . | |
| 2% chlorhexidine daily bathing | 0.4 (.2–.6) | <.001 | 0.4 (.2–.6) | <.001b |
| Sex, male | 1.0 (.6–1.8) | .889 | - | - |
| Age >65 years | 0.7 (.3–1.6) | .393 | - | - |
| Hematological diagnosis, lymphoma vs other diagnosis | 0.4 (.2–.9) | .027 | - | - |
| Relapsed/refractory diseases | 1.5 (.8–2.9) | .202 | … | … |
| Post-chemotherapy neutropenia (<500/μL) | 13.1 (1.8–95.3) | .011 | 13.9 (1.9–101.9) | .010b |
| Autologous peripheral blood stem cell transplantation | 0.9 (.3–2.9) | .869 | … | … |
| Chemotherapy, induction vs other types | 1.6 (1.0–2.8) | .068 | - | - |
| Use of central venous catheter | 3.5 (2.1–5.9) | <.001 | 2.7 (1.6–4.6) | <.00b |
| Use of port-A-cath | 0.3 (.2–.7) | .299 | - | - |
| Oral ciprofloxacin/levofloxacin for prophylaxis | 0.6 (.3–1.1) | .094 | 0.6 (.3–1.0) | .057 |
| Oral sulfamethoxazole/trimethoprim for prophylaxis | 1.7 (1.0–3.0) | .068 | - | - |
| Multidrug-resistant organism carriage | 0.8 (.4–1.5) | .444 | - | - |
| Diabetes mellitus | 1.6 (.7–3.8) | .251 | - | - |
| In-hospital mortality | 1.9 (1.0–3.8) | .054 | - | - |
| Variable . | Univariable Analysis . | Multivariable Analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P Value . | Adjusted HR a (95% CI) . | P Value . | |
| 2% chlorhexidine daily bathing | 0.4 (.2–.6) | <.001 | 0.4 (.2–.6) | <.001b |
| Sex, male | 1.0 (.6–1.8) | .889 | - | - |
| Age >65 years | 0.7 (.3–1.6) | .393 | - | - |
| Hematological diagnosis, lymphoma vs other diagnosis | 0.4 (.2–.9) | .027 | - | - |
| Relapsed/refractory diseases | 1.5 (.8–2.9) | .202 | … | … |
| Post-chemotherapy neutropenia (<500/μL) | 13.1 (1.8–95.3) | .011 | 13.9 (1.9–101.9) | .010b |
| Autologous peripheral blood stem cell transplantation | 0.9 (.3–2.9) | .869 | … | … |
| Chemotherapy, induction vs other types | 1.6 (1.0–2.8) | .068 | - | - |
| Use of central venous catheter | 3.5 (2.1–5.9) | <.001 | 2.7 (1.6–4.6) | <.00b |
| Use of port-A-cath | 0.3 (.2–.7) | .299 | - | - |
| Oral ciprofloxacin/levofloxacin for prophylaxis | 0.6 (.3–1.1) | .094 | 0.6 (.3–1.0) | .057 |
| Oral sulfamethoxazole/trimethoprim for prophylaxis | 1.7 (1.0–3.0) | .068 | - | - |
| Multidrug-resistant organism carriage | 0.8 (.4–1.5) | .444 | - | - |
| Diabetes mellitus | 1.6 (.7–3.8) | .251 | - | - |
| In-hospital mortality | 1.9 (1.0–3.8) | .054 | - | - |
..., this variable did not enter the final multivariable analysis.
Abbreviations: CI, confidence interval; HR, hazard ratio.
aAdjusted using the Cox proportional hazards model (proportional hazard assumption test, P = .455).
bStatistically significant.
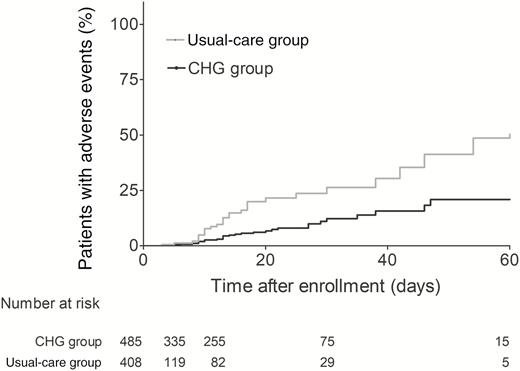
Kaplan-Meier estimates for probability of the primary outcome. Abbreviation: CHG, chlorhexidine.

Kaplan-Meier estimates for probability of the negative control outcome. Abbreviation: CHG, chlorhexidine.
Multivariable analysis shows that CHG bathing was independently associated with a 60% lower hazard of the primary outcome (adjusted hazards ratio [HR], 0.4; 95% confident interval [CI], 0.2–0.6; P < .001) after adjusting for neutropenia, use of a central venous catheter, and oral prophylactic fluoroquinolones (Table 2). In contrast, CHG bathing had no effect on the incidence of the negative control outcome (adjusted HR, 1.1; 95% CI, 0.6–2.1; P = .781; Table 3). Exclusion of participants who used peripheral lines to receive chemotherapy (and thus did not have central lines or port devices) from the analysis did not alter the estimated effects of CHG bathing (Supplementary Tables 3 and 4).
| Variable . | Univariable Analysis . | Multivariable Analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P Value . | Adjusted HRa (95% CI) . | P Value . | |
| 2% chlorhexidine daily bathing | 1.1 (.6–2.2) | .690 | 1.1 (.6–2.1) | .781 |
| Sex, male | 1.5 (.9–2.6) | .163 | - | - |
| Age >65 years | 1.5 (.8–3.1) | .236 | - | - |
| Hematological diagnosis, lymphoma vs other diagnosis | 0.5 (.2–1.0) | .055 | - | - |
| Relapsed/refractory diseases | 1.0 (.5–2.1) | .942 | … | … |
| Post-chemotherapy neutropenia (<500/μL) | 13.0 (1.8–94.2) | .011 | 10.7 (1.4–79.8) | .021b |
| Autologous peripheral blood stem cell transplantation | 1.0 (.3–3.1) | .956 | … | … |
| Chemotherapy, induction vs other types | 3.1 (1.7–5.5) | <.001 | 2.3 (1.3–4.3) | .006b |
| Use of central venous catheter | 0.9 (.5–1.6) | .627 | - | - |
| Use of port-A-cath | 1.2 (.7–2.2) | .420 | - | - |
| Oral ciprofloxacin/levofloxacin for prophylaxis | 0.6 (.3–1.1) | .080 | 0.4 (.2–.8) | .008b |
| Oral sulfamethoxazole/trimethoprim for prophylaxis | 0.9 (.5–1.5) | .646 | - | - |
| Diabetes mellitus | 2.3 (1.0–5.0) | .045 | - | - |
| Multidrug-resistant organism carriage | 0.9 (.5–1.7) | .646 | - | - |
| In-hospital mortality | 0.8 (.3–2.3) | .698 | - | - |
| Variable . | Univariable Analysis . | Multivariable Analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P Value . | Adjusted HRa (95% CI) . | P Value . | |
| 2% chlorhexidine daily bathing | 1.1 (.6–2.2) | .690 | 1.1 (.6–2.1) | .781 |
| Sex, male | 1.5 (.9–2.6) | .163 | - | - |
| Age >65 years | 1.5 (.8–3.1) | .236 | - | - |
| Hematological diagnosis, lymphoma vs other diagnosis | 0.5 (.2–1.0) | .055 | - | - |
| Relapsed/refractory diseases | 1.0 (.5–2.1) | .942 | … | … |
| Post-chemotherapy neutropenia (<500/μL) | 13.0 (1.8–94.2) | .011 | 10.7 (1.4–79.8) | .021b |
| Autologous peripheral blood stem cell transplantation | 1.0 (.3–3.1) | .956 | … | … |
| Chemotherapy, induction vs other types | 3.1 (1.7–5.5) | <.001 | 2.3 (1.3–4.3) | .006b |
| Use of central venous catheter | 0.9 (.5–1.6) | .627 | - | - |
| Use of port-A-cath | 1.2 (.7–2.2) | .420 | - | - |
| Oral ciprofloxacin/levofloxacin for prophylaxis | 0.6 (.3–1.1) | .080 | 0.4 (.2–.8) | .008b |
| Oral sulfamethoxazole/trimethoprim for prophylaxis | 0.9 (.5–1.5) | .646 | - | - |
| Diabetes mellitus | 2.3 (1.0–5.0) | .045 | - | - |
| Multidrug-resistant organism carriage | 0.9 (.5–1.7) | .646 | - | - |
| In-hospital mortality | 0.8 (.3–2.3) | .698 | - | - |
..., This variable did not enter the final multivariable analysis.
Abbreviations: CI, confidence interval; HR, hazard ratio.
aAdjusted using the Cox proportional hazards model analysis (proportional hazard assumption test, P = .960).
bStatistically significant.
| Variable . | Univariable Analysis . | Multivariable Analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P Value . | Adjusted HRa (95% CI) . | P Value . | |
| 2% chlorhexidine daily bathing | 1.1 (.6–2.2) | .690 | 1.1 (.6–2.1) | .781 |
| Sex, male | 1.5 (.9–2.6) | .163 | - | - |
| Age >65 years | 1.5 (.8–3.1) | .236 | - | - |
| Hematological diagnosis, lymphoma vs other diagnosis | 0.5 (.2–1.0) | .055 | - | - |
| Relapsed/refractory diseases | 1.0 (.5–2.1) | .942 | … | … |
| Post-chemotherapy neutropenia (<500/μL) | 13.0 (1.8–94.2) | .011 | 10.7 (1.4–79.8) | .021b |
| Autologous peripheral blood stem cell transplantation | 1.0 (.3–3.1) | .956 | … | … |
| Chemotherapy, induction vs other types | 3.1 (1.7–5.5) | <.001 | 2.3 (1.3–4.3) | .006b |
| Use of central venous catheter | 0.9 (.5–1.6) | .627 | - | - |
| Use of port-A-cath | 1.2 (.7–2.2) | .420 | - | - |
| Oral ciprofloxacin/levofloxacin for prophylaxis | 0.6 (.3–1.1) | .080 | 0.4 (.2–.8) | .008b |
| Oral sulfamethoxazole/trimethoprim for prophylaxis | 0.9 (.5–1.5) | .646 | - | - |
| Diabetes mellitus | 2.3 (1.0–5.0) | .045 | - | - |
| Multidrug-resistant organism carriage | 0.9 (.5–1.7) | .646 | - | - |
| In-hospital mortality | 0.8 (.3–2.3) | .698 | - | - |
| Variable . | Univariable Analysis . | Multivariable Analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P Value . | Adjusted HRa (95% CI) . | P Value . | |
| 2% chlorhexidine daily bathing | 1.1 (.6–2.2) | .690 | 1.1 (.6–2.1) | .781 |
| Sex, male | 1.5 (.9–2.6) | .163 | - | - |
| Age >65 years | 1.5 (.8–3.1) | .236 | - | - |
| Hematological diagnosis, lymphoma vs other diagnosis | 0.5 (.2–1.0) | .055 | - | - |
| Relapsed/refractory diseases | 1.0 (.5–2.1) | .942 | … | … |
| Post-chemotherapy neutropenia (<500/μL) | 13.0 (1.8–94.2) | .011 | 10.7 (1.4–79.8) | .021b |
| Autologous peripheral blood stem cell transplantation | 1.0 (.3–3.1) | .956 | … | … |
| Chemotherapy, induction vs other types | 3.1 (1.7–5.5) | <.001 | 2.3 (1.3–4.3) | .006b |
| Use of central venous catheter | 0.9 (.5–1.6) | .627 | - | - |
| Use of port-A-cath | 1.2 (.7–2.2) | .420 | - | - |
| Oral ciprofloxacin/levofloxacin for prophylaxis | 0.6 (.3–1.1) | .080 | 0.4 (.2–.8) | .008b |
| Oral sulfamethoxazole/trimethoprim for prophylaxis | 0.9 (.5–1.5) | .646 | - | - |
| Diabetes mellitus | 2.3 (1.0–5.0) | .045 | - | - |
| Multidrug-resistant organism carriage | 0.9 (.5–1.7) | .646 | - | - |
| In-hospital mortality | 0.8 (.3–2.3) | .698 | - | - |
..., This variable did not enter the final multivariable analysis.
Abbreviations: CI, confidence interval; HR, hazard ratio.
aAdjusted using the Cox proportional hazards model analysis (proportional hazard assumption test, P = .960).
bStatistically significant.
Oral prophylaxis with levofloxacin or ciprofloxacin reduced the incidence of the primary outcome by 40% (adjusted HR, 0.6; 95% CI, 0.3–1.0; P = .057) and that of the negative control outcome (gut-origin bacteremia) by 60% (adjusted HR, 0.4; 95% CI, 0.2–0.8; P = .008; Tables 2 and 3).
Participants who experienced BSIs (primary or negative control outcome) had a much longer hospital stay than those who did not (mean, 46.6 days vs 18.7 days; P < .001). For those who did not experience a BSI, the intensity of chemotherapy (induction chemotherapy, neutropenia after chemotherapy), receipt of autologous peripheral blood stem cell transplantation, and carriage of multidrug-resistant organisms were major determinants for a longer hospital stay (Supplementary Table 5). These 4 unfavorable host conditions were more common in the CHG group (Table 1).
Daily CHG bathing was well tolerated, with >90% adherence by patients in the CHG group. No rash or allergy occurred. No adverse events other than skin dryness was reported.
DISCUSSION
In this study, we are the first to show that daily CHG bathing is associated with a 60% lower hazard for gram-positive cocci–related BSI, skin flora–related BSI, or, CLABSIs in patients with hematological malignancies hospitalized for cytotoxic chemotherapy in noncritical care units. In contrast, daily CHG bathing had no effect on the negative control outcome, that is, gut-origin bacteremia. This strongly supports the conclusion that the effect is genuine, rather than a result of confounding by patient characteristics.
Length of hospital stay is an important determinant for the risk of nosocomial infection [1]. In a randomized trial, Noto et al showed that the benefit of daily 2% CHG bathing was negligible for low-risk patients who stayed for an average of 2.5 days in an ICU [26]. However, for higher-risk patients who stayed for an average of 5 days or more in an ICU, metaanalysis of randomized controlled trials showed that CHG bathing prevented 50%–56% of CLABSIs [10–18]. Patients with hematological malignancies who received myelosuppressive chemotherapy in noncritical care units stayed in the hospital for an average of 26.2 days in the present study. We observed that for such high-risk patients, CHG bathing has a highly significant protective effect similar to that seen in ICUs.
Use of indwelling catheters or other medical devices is another important determinant for the risk of nosocomial infection [1]. The ABATE Infection trial demonstrated that CHG bathing is not beneficial for low-risk patients in noncritical care units [21]. A subgroup analysis of the ABATE trial did show a decrease in BSIs among higher-risk patients with central lines or other medical devices, but the subgroup analysis was post hoc [21]. Unlike low-risk patients in noncritical care units, most patients with hematological malignancies depend on indwelling central catheters to ensure venous access for cytotoxic chemotherapy [27, 28]. For such high-risk patients, our prospective study provides evidence that CHG bathing does decrease the incidence of gram-positive cocci–related, skin flora–related, and catheter–associated BSIs and, therefore, could be considered as part of routine care in the future.
CHG has good bactericidal activity against gram-positive bacteria but is less effective against gram-negative bacteria [29]. To be effective, use of an adequate CHG concentration in a prepackaged cleanser solution is important. In the present study, participants in the usual-care group were allowed to use over-the-counter antibacterial cleansing lotion. Some of these products were marketed as containing CHG; however, the actual CHG concentration was not listed on the label and was probably minimal. A recent randomized trial showed that bathing with a 4% CHG soap-like solution, compared with standard soap, significantly reduced hospital-acquired infections in intensive care settings [30]. There is still no direct comparison between 2% and 4% CHG for daily bathing, which may require further studies.
A recent study showed that compared with no antibacterial prophylaxis, fluoroquinolone prophylaxis is highly effective for the prevention of CLABSI in autologous stem cell transplant patients [31]. In the present study, compared with other prophylactic antibiotics, use of a board-spectrum fluoroquinolone prophylaxis regimen was associated with a clinically significant 40% and 60% reduction in the primary outcome and gut-origin bacteremia, respectively (Tables 2 and 3). Our data confirmed the effectiveness of fluoroquinolones as oral antibacterial prophylaxis. However, fluoroquinolone prophylaxis has been associated with an increased risk of Clostridium difficile superinfection and the emergence of fluoroquinolone-resistant bacteria [30]. Daily 2% CHG bathing will be a welcome addition to current infection prevention strategies in order to ensure the safety of patients with hematological malignancies after receipt of cytotoxic chemotherapy.
When antimicrobial prophylaxis fails to prevent the occurrence of catheter-associated bacteremia in patients with hematological malignancies, physicians rely on empirical antimicrobial therapy and use regimens that typically include glycopeptide antibiotics such as vancomycin or teicoplanin. However, widespread vancomycin or teicoplanin use would cause a selective pressure that would allow the emergence of vancomycin-resistant Enterococci (VRE), a common problem in many hematological centers. Our study shows that CHG bathing cut the incidence of catheter-associated bacteremia by 60% and had the potential to reduce glycopeptide use, which in turn would decrease selective pressure and thus help to mitigate the problem of VRE in hospitals.
There were important limitations in our study. First, this was a prospective, controlled cohort study, not a randomized, controlled trial, although the use of a negative control outcome in data analyses provided some reassurance that the observed effect was not due to participation bias. Second, 2 CHG bathing protocols, this is, self-bathing with CHG for ambulatory patients and a CHG-impregnated paper towel wiping bath for bedridden patients, were used. However, both protocols used the same concentration of CHG, 2%. Third, we were not able to forbid patients in the usual-care group from using 2% CHG-containing cleansing solution (which was openly accessible in the units). In randomized trials, this contamination would cause a bias toward the null when assessing the effect of CHG bathing. Thus, the observed 60% efficacy of CHG bathing in this study could be a conservative estimate. Finally, adherence data were based on self-reporting from patients because most participants in the CHG group were independent in their activities of daily living and therefore performed CHG bathing on their own rather than with the assistance of a nurse. Nevertheless, actual adherence was likely very high due to the daily visits and adherence reminders by the researchers. In addition, an adherence rate of less than 100% would lead to a bias toward the null, which made our result more robust.
Our study was not designed as a randomized trial because of the perceived lack of clinical equipoise (based on the well-demonstrated efficacy of CHG bathing in ICUs) [10–20] when we planned the study. Participants decided on their own whether to perform CHG bathing. As a result, significant difference existed between the 2 groups. Some were disadvantageous to the CHG group (more likely to have a diagnosis of leukemia, to receive induction chemotherapy, to experience neutropenia during hospitalization, to use central lines for venous access, and to stay in the hospital for a longer time), while others were disadvantageous to the usual-care group (older, fewer fluoroquinolone prophylaxis, more diabetes mellitus, and less independence in activities of daily living). The use of a negative-control endpoint helps to support the finding that the observed effect was CHG-related but did not reach a certainty of evidence as high as that from randomized trials. Thus, further randomized trials are needed to confirm our results. The lack of effect of CHG bathing in noncritical care units, in general, that was demonstrated in the ABATE Infection trial (data released in 2019) [21] justifies randomized trials on CHG bathing at noncritical care hematology units.
In conclusion, we showed that daily 2% CHG bathing could be a simple, safe, and highly effective intervention to prevent gram-positive cocci–related, skin flora–related, and central catheter–associated BSIs in patients with hematological malignancies who are hospitalized for cytotoxic chemotherapy in noncritical care units. Further randomized, controlled trials to confirm the protective effect of daily CHG bathing in noncritical care hematology units are warranted.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. C. T. F. and J. T. W. supervised this study. K. L. T., W. H. S., J. T. W., and C. T. F. designed the study. K. L. T., S. C. S., and Y. P. H. enrolled patients and supervised implementation of chlorhexidine bathing. Y. C. C. established the rule-based healthcare-associated infection surveillance system. H. F. T. supervised the medical care of hematology patients and provided administrative support. W. H. S. and L. J. C. supervised the care bundle for prevention of catheter-associated bloodstream infection. Y. H. C. calculated the sample size. K. L. T., J. T. W., and C. T. F. performed statistical analysis. C. T. F. and K. L. T. wrote the manuscript. All authors critically reviewed and approved the submitted version of manuscript.
Acknowledgments. The authors are grateful for the assistance provided by the hematology staff at National Taiwan University Hospital, Taipei, Taiwan. A preliminary version (in traditional Chinese) was prepared for the Master of Public Health thesis of the first author, K. L. T., at National Taiwan University (2017).
Disclaimer. The funding sources had no role in study design, data collection, data analyses, interpretation, or manuscript writing. The corresponding author had full access to all of the study data and took final responsibility for the decision to submit for publication.
Financial support. This work was supported by the Ministry of Health and Welfare, Taiwan (MOHW-107-TDU-B-211-123002). PBF Biotech (Taipei, Taiwan) provided 2% chlorhexidine.
Potential conflicts of interest. All authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
J. T. W. and C. T. F. contributed equally.




