-
PDF
- Split View
-
Views
-
Cite
Cite
Joyce Wang, Marco Cassone, Kristen Gibson, Bonnie Lansing, Lona Mody, Evan S Snitkin, Krishna Rao, Gut Microbiota Features on Nursing Home Admission Are Associated With Subsequent Acquisition of Antibiotic-resistant Organism Colonization, Clinical Infectious Diseases, Volume 71, Issue 12, 15 December 2020, Pages 3244–3247, https://doi.org/10.1093/cid/ciaa662
Close - Share Icon Share
Abstract
Nursing home (NH) patients often acquire colonization with antibiotic-resistant organisms (AROs). We show that patients exposed to broad-spectrum antibiotics during previous hospitalizations have elevated enterococcal relative abundances on NH admission and higher risk of subsequent ARO acquisition. Our findings suggest that interventions preventing ARO spread should extend beyond NH doors.
Antibiotic resistance affects vulnerable populations in healthcare settings disproportionately [1]. Within the healthcare system, post–acute care settings such as nursing homes (NHs) have been recognized as an important reservoir for antibiotic-resistant organisms (AROs) [2]. Nursing home patients are often older adults with complicated medical conditions, and the acquisition of enteric AROs such as vancomycin-resistant Enterococcus (VRE) or resistant gram-negative bacteria (R-GNB) is common in this patient population [3].
The role of intestinal microbiota in mediating the risk of ARO colonization has become increasingly appreciated in clinical settings where microbiota disruptions often precede ARO colonization [4]. While most research and intervention efforts have focused on improving appropriate antibiotic use in the NH setting, the impact of frequent antibiotic exposure during previous acute care hospitalization on ARO acquisition during NH stay has been mostly overlooked [3, 5]. We hypothesized that, due to the lasting effects of antibiotic exposure, antibiotic use at connected healthcare facilities could impact patient risk of ARO acquisition once in NHs. To test this hypothesis, we conducted a prospective, longitudinal cohort study to examine patient clinical factors and gut microbiota features at the time of NH admission and their associations with the risk of acquisition of VRE and/or R-GNB within 14 days of study enrollment.
METHODS
Study Population
From September 2016–August 2018, we enrolled patients from 6 NHs in southeast Michigan within 14 days of admission as part of a larger National Institutes of Health–funded trial. Colonization status was determined by culture swabs collected from multiple body sites including hands, nares, oropharynx, suprapubic catheter site, groin, perirectal area, and wounds, at enrollment (day 0), day 7, and day 14 using microbiological procedures described previously [3]. Our analysis focused on patients (1) colonized with neither VRE nor R-GNB at the time of study enrollment, (2) who had a perirectal sample collected using ESwab (Copan Diagnostics, Murrieta, CA) collected at enrollment, and (3) who had at least 1 follow-up visit with culture samples. Patient clinical data were collected at enrollment by trained research personnel using hospital transfer records and/or NH medical data. All subjects or their proxy provided a written informed consent. This study was approved by the University of Michigan Institutional Review Board.
16S rRNA Gene Sequencing and Analysis
The processing and sequencing of perirectal specimens are described in detail in Supplementary Methods. The resulting sequences were clustered into operational taxonomic units (OTUs) at the genus level, with a 97% similarity threshold against the SILVA 16S rRNA database using mothur [6]. OTUs typically associated with the skin microbiota, including Staphylococcus, Corynebacterium, and Propionibacterium, were removed from downstream analyses.
Statistical Analysis
All data analyses were performed in R (version 3.6.1; R Foundation for Statistical Computing). The primary outcome was the acquisition of VRE or R-GNB within 14 days of study enrollment. Exposures of interest included patient clinical factors and microbiota features at enrollment. Patient characteristics associated with outcome were determined by linear or logistic regression analyses. For microbiota features, we calculated the relative abundance of each OTU in each patient. As perirectal samples are low in biomass and rare OTUs may not be well represented across samples, we conservatively restricted our analyses to the 50 most abundant OTUs in this cohort. Linear regression analyses were used to examine associations between the relative abundance of each OTU and antibiotic exposure. Logistic regression analyses were used to identify associations between outcome and individual OTUs, and the abundance of anaerobes and butyrate-producers (see Supplementary Methods for details). Log transformation of continuous variables was performed if data distribution appeared to be skewed upon histograms. For each OTU associated with ARO acquisition with an unadjusted P value of < .05, multivariable logistic regression analyses were used to identify patient factors that modified the effect of the OTU by more than 10%. To assess variables representing more global measures of microbiota state, we adapted a recently developed Microbiome Health Index (MHI) [7]. An MHI of 0 after log transformation indicates a balanced abundance between taxa associated with protection (Bacteroidia and Clostridia) and dysbiosis (Gammaproteobacteria and Bacilli). An MHI above 0 suggests higher abundances of protective taxa, and vice versa.
RESULTS
Exposure to Antibiotics Disruptive to the Gut Microbiota Prior to Nursing Home Admission Is Associated With Antibiotic-resistant Organism Acquisition During Nursing Home Stay
Of 243 patients enrolled in the study, 61 patients met our inclusion criteria: no VRE or R-GNB colonization on enrollment, a perirectal swab collected at enrollment, and at least 1 follow-up within 14 days (Supplementary Figure 1). Most patients (73%) were enrolled within 7 days of NH admission (median, 6.0 days; range, 1–13 days). Within 14 days of enrollment, 18 (29.5%) acquired AROs (3 VRE, 13 R-GNB, 2 both) (Supplementary Figure 2). In bivariable analyses, exposure to high-risk antibiotic classes with previously demonstrated associations with gut microbiota disruption [8], including third-/fourth-generation cephalosporins, quinolones, lincosamides, penicillin combinations, carbapenems, and oral vancomycin prior to enrollment, was a significant risk factor associated with ARO acquisition (odds ratio [OR], 4.20; 95% confidence interval [CI], 1.08–17.63) (Supplementary Table 1). Notably, the majority of these high-risk antibiotics were initiated during hospitalization prior to admission to an NH (n = 11/12) for infection-related reasons, and patients who received high-risk antibiotics tended to have a longer hospital stay and have had a urinary catheter compared with those who did not (Supplementary Figure 3, Supplementary Table 2).
Microbiota Features at Enrollment Differed Between Patients With and Without Antibiotic-resistant Organism Acquisition During Nursing Home Stay
Following the observation that the receipt of high-risk antibiotics prior to NH admission was associated with risk for ARO acquisition, we next asked if this exposure had resulted in detectable disruption to the gut microbiota on admission to NHs. To assess this, we performed 16S sequencing on perirectal swabs taken on enrollment. Considering only the 50 most abundant gut-associated OTUs, we found that exposure to high-risk antibiotics prior to enrollment was associated with decreased relative abundance of several OTUs associated with the Clostridia, Bacteroidia, and Actinobacteria classes, and increased relative abundance of Enterococcus (Figure 1A).
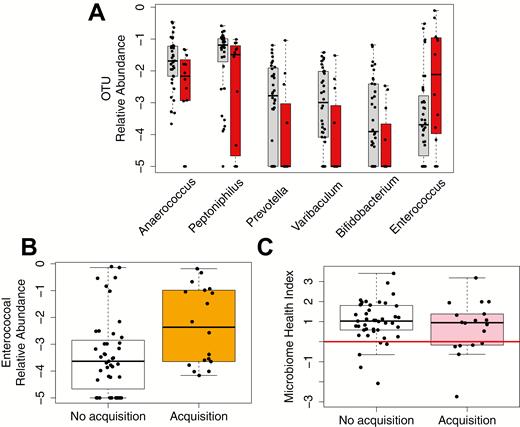
Microbiota features are associated with exposure to antibiotics during prior hospitalization and the risk of acquiring an enteric ARO within 14 days. A, Relative abundances of OTUs significantly affected by prior exposure to high-risk antibiotics (P < .05). Without exposure: gray; with exposure: red. Anaerococus and Peptoniphilus belong to class Clostridia, Prevotella belongs to class Bacteroidia, Varibaculum and Bifidobacterium belong to class Actinobacteria, and Enterococcus belongs to class Bacilli. B, Relative abundances of Enterococcus in patients who did not acquire an ARO in 14 days (white) and those who did (orange). C, Microbiome Health Index in patients who did not acquire an ARO in 14 days (white) and those who did (pink). The red line indicates a MHI of 0. Medians and interquartile ranges are shown in boxplots. The y axes are presented on a base-10 logarithmic scale. Abbreviations: ARO, antibiotic-resistant organism; OTU, operational taxonomic unit.
Next, we looked directly at whether there were OTUs associated with risk for ARO acquisition and found that patients who acquired an ARO during NH stay had a significantly increased relative abundance of Enterococcus at the time of enrollment compared with those who did not acquire an ARO (log-transformed mean ± SD, −2.31 ± 1.46 vs − 3.41 ± 1.44; P = .012) (Figure 1B). Incorporating clinical variables, including age, sex, race, body mass index, functional status, log-transformed comorbidity scores, log-transformed length of previous hospital stay, or urinary catheter use within the past 30 days, in multivariable models did not significantly improve model fit, and the statistical significance of Enterococcus was retained, suggesting that altered Enterococcus abundance was an independent risk factor for ARO acquisition. Patients who acquired an ARO had lower relative abundances of anaerobes and butyrate producers at enrollment than those who did not, but the difference was not statistically significant. Last, examination of the MHI revealed that 6 out 18 (33.33%) of the patients who acquired an ARO had an MHI of less than 0, significantly higher than the 5 out of 43 (11.63%) patients with no acquisition (chi-square test, P = .04) (Figure 1C). The association between an MHI of less than 0 and risk of acquisition remained significant after adjusting for hospital length of stay and exposure to a urinary catheter within the past 30 days, both potential confounders that modified the effect of MHI by more than 10% (adjusted OR, 6.85; 95% CI, 1.46–39.24).
DISCUSSION
With an aging population in the United States, patients are more medically complex and likely to require post–acute care after hospitalization. By reviewing patient medical records, we found that recent exposure to high-risk antibiotics, mostly initiated during prior hospitalization and more frequent in patients with longer hospital stay and urinary catheter use, was associated with the risk of ARO acquisition. As broad-spectrum antibiotics can have a profound impact on microbiota health, we further examined microbiota features and identified differences between patient groups at study enrollment.
Our finding that patients with elevated Enterococcus abundances were at a higher risk of enteric ARO acquisition was consistent with previous studies demonstrating that antibiotic therapy can promote high colonization density of VRE and R-GNB [4, 9]. Of note, while Enterococcus was detected in all 18 patients who acquired an enteric ARO, only 3 acquired VRE and the rest acquired either R-GNB (n = 13) alone or R-GNB with VRE (n = 2), suggesting that enterococcal expansion is a biomarker for the gut microbiota’s susceptibility to colonization by enteric ARO, likely from within-facility ARO transmission and/or proliferation of previous ARO colonization below the level of detection. Several patients with elevated Enterococcus abundances did not acquire an ARO within 14 days, potentially representing an at-risk population who had a lower intensity of ARO exposure from other patients, healthcare workers, or the environment.
Our characterization of patients traversing the healthcare network demonstrates that exposure to high-risk antibiotics on NH admission influenced patient gut microbiota features and the risk of ARO acquisition during NH stay. Our study highlights the connectedness of the healthcare network and suggests that the future study of coordinated infection-prevention approaches between healthcare partners would be a fruitful endeavor [10].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the patients and their families and nursing homes who participated in this study; we thank members of the Mody laboratory for data collection and analysis, Drs Christine Bassis and Jonathan Golob for technical and scientific discussions, and the Microbial Systems Molecular Biology Laboratory at the University of Michigan for performing 16S rRNA sequencing.
Financial support. This work was supported by the Centers for Disease Control and Prevention (contract number BAA 2016-N-17812) to E. S. S.; the National Institutes of Health (grant numbers R01AG041780, K24AG050685) to L. M.; the National Institute on Aging (grant number P30 AG024824) to L. M.; the University of Michigan Host Microbiome Initiative Microbiome Explorers Program (to E. S. S.); a Canadian Institutes of Health Research fellowship (grant number 201711MFE-396343-165736) to J. W.; and the Michigan Institute for Clinical and Health Research Postdoctoral Translational Scholars Program (to J. W.).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
Author notes
E. S. S. and K. R. contributed equally.




