-
PDF
- Split View
-
Views
-
Cite
Cite
Leila S Hojat, Mary T Bessesen, Misha Huang, Margaret Reid, Bryan C Knepper, Matthew A Miller, Katherine C Shihadeh, Randolph V Fugit, Timothy C Jenkins, Effectiveness of Shorter Versus Longer Durations of Therapy for Common Inpatient Infections Associated With Bacteremia: A Multicenter, Propensity-Weighted Cohort Study, Clinical Infectious Diseases, Volume 71, Issue 12, 15 December 2020, Pages 3071–3078, https://doi.org/10.1093/cid/ciz1197
Close - Share Icon Share
Abstract
National guidelines for pneumonia (PNA), urinary tract infection (UTI), and acute bacterial skin and skin structure infection (ABSSSI) do not address treatment duration for infections associated with bacteremia. We evaluated clinical outcomes of patients receiving shorter (5–9 days) versus longer (10–15 days) duration of antibiotics.
This was a multicenter retrospective cohort study of inpatients with uncomplicated PNA, UTI, or ABSSSI and associated bacteremia. The primary outcome was clinical failure, a composite of rehospitalization, reinitiation of antibiotics, or all-cause mortality within 30 days of antibiotic completion. Secondary outcomes included individual components of the primary outcome, Clostridioides difficile infection, and antibiotic-related adverse effects necessitating change in therapy. A propensity score-weighted logistic regression model was used to mitigate potential bias associated with nonrandom assignment of treatment duration.
Of 408 patients included, 123 received a shorter treatment duration (median 8 days) and 285 received a longer duration (median 13 days). In the propensity-weighted analysis, the probability of the primary outcome was 13.5% in the shorter group and 11.1% in the longer group (average treatment effect, 2.4%; odds ratio [OR], 1.25; 95% confidence interval [CI], .65–2.40; P = .505). However, shorter courses were associated with higher probability of restarting antibiotics (OR, 1.62; 95% CI, 1.01–2.61; P = .046) and C. difficile infection (OR, 4.01; 95% CI, 2.21–7.59; P < .0001).
Shorter courses of antibiotic treatment for PNA, UTI, and ABSSSI with bacteremia were not associated with increased overall risk of clinical failure; however, prospective studies are needed to further evaluate the effectiveness of shorter treatment durations.
Antibiotics are prescribed to approximately half of all hospitalized patients, most of which are intended for treatment of a known or suspected infection [1]. The most common infections treated in hospitalized patients include pneumonia (PNA), urinary tract infections (UTI), and acute bacterial skin and skin structure infections (ABSSSI). These are associated with bacteremia in an estimated 5–9% [2, 3], 23–33% [4, 5], and 1–5% [6, 7] of cases, respectively. Infectious Diseases Society of America guidelines advocate for short courses of antibiotics for these syndromes in uncomplicated cases [8–10]. However, the guidelines do not address duration of treatment for these infections when associated with bacteremia. As a result, there is significant heterogeneity in the approach to treating common infections involving bacteremia [11–13].
Longer durations of therapy may increase the risk of adverse events, Clostridioides difficile infection [14, 15], and antimicrobial resistance [16–19]. Recent studies have suggested that shorter durations of therapy for uncomplicated infections with bacteremia are as effective as longer durations; however, these have largely focused on Enterobacteriaceae bacteremia associated with urinary tract infection [20–22]. Additional data are needed to further delineate the appropriate duration of therapy for a wider breadth of infections associated with bacteremia.
The objectives of this study were to evaluate the effectiveness of shorter versus longer durations of therapy for common infections associated with bacteremia in the hospital setting. Additionally, we aimed to assess whether infection type, infecting pathogen, antibiotic class, and route of antibiotic administration modified the effectiveness of shorter versus longer treatment durations. We hypothesized that there would be no difference in clinical effectiveness between the shorter and longer duration groups and that there would be fewer adverse events in the shorter duration group.
METHODS
Study Setting and Population
The study took place at 3 hospitals in the Denver metropolitan area. These included a 555-bed urban public safety-net hospital (Hospital A), a 698-bed university hospital, which serves as the major tertiary referral center for the Rocky Mountain region (Hospital B), and a 182-bed Veterans Affairs hospital, which serves as a referral center for a 5-state area (Hospital C).
Study Design
This was a retrospective cohort study including adults age 18 years and older hospitalized due to PNA, UTI, or ABSSSI with associated bacteremia between April 1, 2016, and November 30, 2017. Due to a smaller number of patients at Hospital C, cases were included beginning from April 1, 2013. Patients were eligible for inclusion if they had a positive blood culture attributed by the treating clinician to PNA, UTI, or ABSSSI. Patients were excluded from analysis if they were treated with antibiotics for <5 days or >15 days or if they died prior to completion of the intended treatment course. Additional exclusion criteria included: positive blood culture after the index positive blood culture day, Staphylococcus aureus or Staphylococcus lugdunensis bacteremia, fungemia, growth limited to contaminant organisms (coagulase-negative Staphylococcus, diphtheroids, Micrococcus, Bacillus, or Cutibacterium species), concomitant infection requiring antibiotic treatment, complicated infection, or hospitalization exceeding 60 days. Complicated infection was defined as pleural space involvement, renal or perinephric abscess, undrained cutaneous abscess >5 cm, endovascular or metastatic focus of infection, intravascular prosthetic device presence, deep tissue involvement, necrosis, extensive burns, anatomical obstruction, transplanted organ, or otherwise structurally abnormal infected organ.
At each hospital, cases were identified through an electronic search of all positive blood cultures. To achieve a target sample size of 500 cases, at Hospitals A and C, all identified cases were reviewed to determine eligibility. Due to a greater number of potentially eligible cases from Hospital B, a randomly selected subset of cases was reviewed. For patients who had multiple episodes of infection over the study period, only the initial episode was reviewed. Using a structured data collection instrument, a single reviewer (L.H.) extracted the following data from the electronic medical record: demographic characteristics, comorbidities, antibiotic allergies, markers of infection severity, microbiologic and radiographic data, antibiotic therapy, and clinical outcomes. Antibiotic therapy was classified as empiric, definitive inpatient, or definitive outpatient therapy, which referred to initial treatment choice, inpatient treatment tailored to susceptibility data, and antibiotics prescribed at discharge, respectively. Cases in which therapy was completed during the inpatient stay did not have definitive outpatient therapy. To determine clinical outcomes, all medical records within 30 days of completion of the antibiotic course were reviewed for prespecified clinical outcomes of interest. The study was approved by the Colorado Multiple Institutional Review Board.
Classification and Outcomes
Patients were classified as having received a shorter or longer duration of therapy if they received 5–9 days or 10–15 days of initial antibiotic treatment, respectively. The administration of ≥1 doses of at least 1 antibiotic on a calendar day was considered 1 treatment day. Antibiotics given prior to the positive blood culture, if any, were included in the treatment duration. The duration of therapy after discharge was recorded based on the number antibiotic treatment days prescribed by the clinician.
The primary outcome measure was clinical failure, defined as a composite of rehospitalization or resumption of antibiotics for the original infection or all-cause mortality within 30 days of antibiotic completion. The primary outcome was analyzed among the following prespecified stratifications: infection type (PNA, UTI, or ABSSSI); gram-positive or gram-negative bacteremia; predominantly fluoroquinolone or beta-lactam therapy (≥50% of treatment days), and predominantly intravenous versus oral therapy (≥50% of treatment days). Secondary outcome measures included the individual components of the composite primary outcome, C. difficile infection, and adverse drug events resulting in a change or early cessation of antibiotics.
Data Analysis
Inverse probability of treatment weighting was used to mitigate selection bias resulting from patients being nonrandomly assigned to shorter or longer duration of therapy. This approach utilized 2 logistic regression models to assess the association of duration of therapy with patient outcomes. The first modeled a patient’s probability of being in the shorter group based on observable confounding characteristics, regardless of the actual group to which the patient was assigned. This probability was then used to statistically balance both groups in the second model, which evaluated the effect of duration of therapy on patient outcomes such that duration of therapy represented their only difference. Weights were constructed as the inverse of the probability of being in the shorter duration group (1/p) among those actually in the shorter group, and as the inverse of the probability of being in the longer group (1/1-p) among those in the longer group.
The propensity score model included basic demographic and hospitalization characteristics along with quadratic terms for age, Charlson comorbidity index, and Pitt bacteremia score. We assessed the balance of the covariates between shorter and longer duration groups using standardized differences and variance ratios. Standardized differences of <10% and variance ratios <10% from 1.0 were considered negligible [23].
To evaluate if the effect of duration of therapy on clinical failure varied by our prespecified stratifications, we included an interaction between the variable of interest and the indicator for being in the shorter duration group. Standard errors were clustered at the hospital level in the propensity-weighted model to account for the lack of independence among patients receiving care at the same hospital. We aimed to include at least 50 cases meeting the primary endpoint of clinical failure. By estimating a clinical failure rate of 10% based on prior literature, we planned to include a total of 500 cases.
RESULTS
We identified 3434 hospitalized patients with a positive blood culture during the study period. Of these, 2850 met exclusion criteria as detailed in Figure 1. After removal of 18 cases used for data collection pilot testing, 155 cases that were not randomly selected from Hospital B, and 3 cases with hospitalizations exceeding 60 days, a total of 408 eligible patients were included for analysis; 123 received a shorter duration of therapy and 285 received a longer duration. The median treatment duration was 8 days (interquartile range [IQR], 7–9) in the shorter group and 13 days (IQR, 11–14) in the longer group. The median age at hospitalization in the entire cohort was 61 (IQR, 48–72), and median length of stay was 5 days (IQR, 4–8). Documentation from clinical visits beyond the 30-day follow-up period was available for 106 (86%) patients in the shorter group and 235 (83%) in the longer group. Additional characteristics are presented in Tables 1 and 2. Smoking, obesity, and diabetes were the most common comorbidities; other comorbidities were uncommon. The mean Pitt bacteremia score was low. UTI and gram-negative organisms accounted for most of the infections. There was a predominance of beta-lactam over fluoroquinolone antibiotic class and oral over intravenous administration route.
Demographic and Clinical Characteristics of Patients Hospitalized With Pneumonia, UTI, or ABSSSI and Associated Bacteremia Treated with a Shorter (5–9 Days) or Longer (10–15 Days) Course of Antibiotics
| Characteristic . | Shorter Course . | Longer Course . |
|---|---|---|
| . | N = 123 (%) . | N = 285 (%) . |
| Age, mean (SD) | 60.9 (15.9) | 59.5 (17.2) |
| Female sex | 63 (51.2) | 127 (44.6) |
| Comorbid conditions | ||
| Diabetes | 35 (28.5) | 103 (36.1) |
| Obesitya | 43 (35) | 88 (30.9) |
| Current or former smoker | 70 (56.9) | 184 (64.6) |
| COPD | 22 (17.9) | 43 (15.1) |
| End-stage renal disease | 4 (3.3) | 6 (2.1) |
| HIV infection | 1 (0.8) | 13 (4.6) |
| Peripheral artery disease | 3 (2.4) | 8 (2.8) |
| Venous stasis | 5 (4.1) | 18 (6.3) |
| Known MRSA infection | 5 (4.1) | 13 (4.6) |
| Cirrhosis | 6 (4.9) | 18 (6.3) |
| Connective tissue disease | 10 (8.1) | 14 (4.9) |
| Heme malignancy | 2 (1.6) | 5 (1.8) |
| Solid tumor malignancy | 10 (8.1) | 43 (15.1) |
| Chronic urinary catheterization | 5 (4.1) | 22 (7.7) |
| Spinal cord injury | 2 (1.6) | 3 (1.1) |
| Immunosuppressive medication | 6 (4.9) | 22 (7.7) |
| Prior infection requiring treatmentb | 68 (55.3) | 142 (49.8) |
| Pneumonia | 13 (10.6) | 36 (12.6) |
| UTI | 43 (35) | 92 (32.3) |
| ABSSSI | 26 (21.1) | 61 (21.4) |
| Penicillin allergy | 26 (21.1) | 45 (15.8) |
| Non-penicillin antibiotic allergy | 8 (6.5) | 48 (16.8) |
| Charlson comorbidity index, 0–14, mean (SD) | 3.4 (2.5) | 3.6 (2.8) |
| Pitt bacteremia score 0–8, mean (SD) | 1.1 (1.4) | 1.3 (1.5) |
| Characteristic . | Shorter Course . | Longer Course . |
|---|---|---|
| . | N = 123 (%) . | N = 285 (%) . |
| Age, mean (SD) | 60.9 (15.9) | 59.5 (17.2) |
| Female sex | 63 (51.2) | 127 (44.6) |
| Comorbid conditions | ||
| Diabetes | 35 (28.5) | 103 (36.1) |
| Obesitya | 43 (35) | 88 (30.9) |
| Current or former smoker | 70 (56.9) | 184 (64.6) |
| COPD | 22 (17.9) | 43 (15.1) |
| End-stage renal disease | 4 (3.3) | 6 (2.1) |
| HIV infection | 1 (0.8) | 13 (4.6) |
| Peripheral artery disease | 3 (2.4) | 8 (2.8) |
| Venous stasis | 5 (4.1) | 18 (6.3) |
| Known MRSA infection | 5 (4.1) | 13 (4.6) |
| Cirrhosis | 6 (4.9) | 18 (6.3) |
| Connective tissue disease | 10 (8.1) | 14 (4.9) |
| Heme malignancy | 2 (1.6) | 5 (1.8) |
| Solid tumor malignancy | 10 (8.1) | 43 (15.1) |
| Chronic urinary catheterization | 5 (4.1) | 22 (7.7) |
| Spinal cord injury | 2 (1.6) | 3 (1.1) |
| Immunosuppressive medication | 6 (4.9) | 22 (7.7) |
| Prior infection requiring treatmentb | 68 (55.3) | 142 (49.8) |
| Pneumonia | 13 (10.6) | 36 (12.6) |
| UTI | 43 (35) | 92 (32.3) |
| ABSSSI | 26 (21.1) | 61 (21.4) |
| Penicillin allergy | 26 (21.1) | 45 (15.8) |
| Non-penicillin antibiotic allergy | 8 (6.5) | 48 (16.8) |
| Charlson comorbidity index, 0–14, mean (SD) | 3.4 (2.5) | 3.6 (2.8) |
| Pitt bacteremia score 0–8, mean (SD) | 1.1 (1.4) | 1.3 (1.5) |
Abbreviations: ABSSSI, acute bacterial skin and skin structure infection; BMI, body mass index; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; MRSA, methicillin-resistant Staphylococcus aureus; SD, standard deviation; UTI, urinary tract infection.
aObesity defined as BMI ≥ 30.
bRefers to having any instances of these infectious syndromes in the past treated with antibiotics
Demographic and Clinical Characteristics of Patients Hospitalized With Pneumonia, UTI, or ABSSSI and Associated Bacteremia Treated with a Shorter (5–9 Days) or Longer (10–15 Days) Course of Antibiotics
| Characteristic . | Shorter Course . | Longer Course . |
|---|---|---|
| . | N = 123 (%) . | N = 285 (%) . |
| Age, mean (SD) | 60.9 (15.9) | 59.5 (17.2) |
| Female sex | 63 (51.2) | 127 (44.6) |
| Comorbid conditions | ||
| Diabetes | 35 (28.5) | 103 (36.1) |
| Obesitya | 43 (35) | 88 (30.9) |
| Current or former smoker | 70 (56.9) | 184 (64.6) |
| COPD | 22 (17.9) | 43 (15.1) |
| End-stage renal disease | 4 (3.3) | 6 (2.1) |
| HIV infection | 1 (0.8) | 13 (4.6) |
| Peripheral artery disease | 3 (2.4) | 8 (2.8) |
| Venous stasis | 5 (4.1) | 18 (6.3) |
| Known MRSA infection | 5 (4.1) | 13 (4.6) |
| Cirrhosis | 6 (4.9) | 18 (6.3) |
| Connective tissue disease | 10 (8.1) | 14 (4.9) |
| Heme malignancy | 2 (1.6) | 5 (1.8) |
| Solid tumor malignancy | 10 (8.1) | 43 (15.1) |
| Chronic urinary catheterization | 5 (4.1) | 22 (7.7) |
| Spinal cord injury | 2 (1.6) | 3 (1.1) |
| Immunosuppressive medication | 6 (4.9) | 22 (7.7) |
| Prior infection requiring treatmentb | 68 (55.3) | 142 (49.8) |
| Pneumonia | 13 (10.6) | 36 (12.6) |
| UTI | 43 (35) | 92 (32.3) |
| ABSSSI | 26 (21.1) | 61 (21.4) |
| Penicillin allergy | 26 (21.1) | 45 (15.8) |
| Non-penicillin antibiotic allergy | 8 (6.5) | 48 (16.8) |
| Charlson comorbidity index, 0–14, mean (SD) | 3.4 (2.5) | 3.6 (2.8) |
| Pitt bacteremia score 0–8, mean (SD) | 1.1 (1.4) | 1.3 (1.5) |
| Characteristic . | Shorter Course . | Longer Course . |
|---|---|---|
| . | N = 123 (%) . | N = 285 (%) . |
| Age, mean (SD) | 60.9 (15.9) | 59.5 (17.2) |
| Female sex | 63 (51.2) | 127 (44.6) |
| Comorbid conditions | ||
| Diabetes | 35 (28.5) | 103 (36.1) |
| Obesitya | 43 (35) | 88 (30.9) |
| Current or former smoker | 70 (56.9) | 184 (64.6) |
| COPD | 22 (17.9) | 43 (15.1) |
| End-stage renal disease | 4 (3.3) | 6 (2.1) |
| HIV infection | 1 (0.8) | 13 (4.6) |
| Peripheral artery disease | 3 (2.4) | 8 (2.8) |
| Venous stasis | 5 (4.1) | 18 (6.3) |
| Known MRSA infection | 5 (4.1) | 13 (4.6) |
| Cirrhosis | 6 (4.9) | 18 (6.3) |
| Connective tissue disease | 10 (8.1) | 14 (4.9) |
| Heme malignancy | 2 (1.6) | 5 (1.8) |
| Solid tumor malignancy | 10 (8.1) | 43 (15.1) |
| Chronic urinary catheterization | 5 (4.1) | 22 (7.7) |
| Spinal cord injury | 2 (1.6) | 3 (1.1) |
| Immunosuppressive medication | 6 (4.9) | 22 (7.7) |
| Prior infection requiring treatmentb | 68 (55.3) | 142 (49.8) |
| Pneumonia | 13 (10.6) | 36 (12.6) |
| UTI | 43 (35) | 92 (32.3) |
| ABSSSI | 26 (21.1) | 61 (21.4) |
| Penicillin allergy | 26 (21.1) | 45 (15.8) |
| Non-penicillin antibiotic allergy | 8 (6.5) | 48 (16.8) |
| Charlson comorbidity index, 0–14, mean (SD) | 3.4 (2.5) | 3.6 (2.8) |
| Pitt bacteremia score 0–8, mean (SD) | 1.1 (1.4) | 1.3 (1.5) |
Abbreviations: ABSSSI, acute bacterial skin and skin structure infection; BMI, body mass index; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; MRSA, methicillin-resistant Staphylococcus aureus; SD, standard deviation; UTI, urinary tract infection.
aObesity defined as BMI ≥ 30.
bRefers to having any instances of these infectious syndromes in the past treated with antibiotics
Hospitalization Characteristics and Antibiotic Treatment for Patients with Pneumonia, UTI, or ABSSSI and Associated With Bacteremia
| . | Shorter Course N = 123 . | Longer Course N = 285 . |
|---|---|---|
| . | N (%) . | N (%) . |
| Hospital A | 58 (47.2) | 108 (37.9) |
| Hospital B | 48 (39) | 120 (42.1) |
| Hospital C | 17 (13.8) | 57 (20) |
| Infection type | ||
| Pneumonia | 37 (30.1) | 67 (23.5) |
| UTI | 69 (56.1) | 175 (61.4) |
| ABSSSI | 17 (13.8) | 43 (15.1) |
| Admitted to ICU | 34 (27.6) | 85 (29.8) |
| Admitting team | ||
| Medicine teaching | 91 (74) | 216 (75.8) |
| Medicine nonteaching | 22 (17.9) | 45 (15.8) |
| Surgical service | 7 (5.7) | 22 (7.7) |
| Other | 3 (2.4) | 2 (0.7) |
| Consultation during admission | ||
| Medicine | 1 (0.8) | 6 (2.1) |
| Infectious diseases | 17 (13.8) | 68 (23.9) |
| Surgical service | 12 (9.8) | 43 (15.1) |
| Gram-negative bacteremia | 76 (61.8) | 192 (67.4) |
| Gram-positive bacteremia | 47 (38.2) | 93 (32.6) |
| Predominant antibiotic class | ||
| Beta-lactam | 82 (66.7) | 175 (61.4) |
| Fluoroquinolone | 40 (32.5) | 84 (29.5) |
| Predominant route of antibiotic administration | ||
| Intravenous | 72 (58.5) | 99 (34.7) |
| Oral | 85 (69.1) | 215 (75.4) |
| Length of stay, mean (SD) | 7.4 (8.4) | 7.7 (7.2) |
| . | Shorter Course N = 123 . | Longer Course N = 285 . |
|---|---|---|
| . | N (%) . | N (%) . |
| Hospital A | 58 (47.2) | 108 (37.9) |
| Hospital B | 48 (39) | 120 (42.1) |
| Hospital C | 17 (13.8) | 57 (20) |
| Infection type | ||
| Pneumonia | 37 (30.1) | 67 (23.5) |
| UTI | 69 (56.1) | 175 (61.4) |
| ABSSSI | 17 (13.8) | 43 (15.1) |
| Admitted to ICU | 34 (27.6) | 85 (29.8) |
| Admitting team | ||
| Medicine teaching | 91 (74) | 216 (75.8) |
| Medicine nonteaching | 22 (17.9) | 45 (15.8) |
| Surgical service | 7 (5.7) | 22 (7.7) |
| Other | 3 (2.4) | 2 (0.7) |
| Consultation during admission | ||
| Medicine | 1 (0.8) | 6 (2.1) |
| Infectious diseases | 17 (13.8) | 68 (23.9) |
| Surgical service | 12 (9.8) | 43 (15.1) |
| Gram-negative bacteremia | 76 (61.8) | 192 (67.4) |
| Gram-positive bacteremia | 47 (38.2) | 93 (32.6) |
| Predominant antibiotic class | ||
| Beta-lactam | 82 (66.7) | 175 (61.4) |
| Fluoroquinolone | 40 (32.5) | 84 (29.5) |
| Predominant route of antibiotic administration | ||
| Intravenous | 72 (58.5) | 99 (34.7) |
| Oral | 85 (69.1) | 215 (75.4) |
| Length of stay, mean (SD) | 7.4 (8.4) | 7.7 (7.2) |
Abbreviations: ABSSSI, acute bacterial skin and skin structure infection; ED, emergency department; ICU, intensive care unit; SD, standard deviation; UTI, urinary tract infection.
Hospitalization Characteristics and Antibiotic Treatment for Patients with Pneumonia, UTI, or ABSSSI and Associated With Bacteremia
| . | Shorter Course N = 123 . | Longer Course N = 285 . |
|---|---|---|
| . | N (%) . | N (%) . |
| Hospital A | 58 (47.2) | 108 (37.9) |
| Hospital B | 48 (39) | 120 (42.1) |
| Hospital C | 17 (13.8) | 57 (20) |
| Infection type | ||
| Pneumonia | 37 (30.1) | 67 (23.5) |
| UTI | 69 (56.1) | 175 (61.4) |
| ABSSSI | 17 (13.8) | 43 (15.1) |
| Admitted to ICU | 34 (27.6) | 85 (29.8) |
| Admitting team | ||
| Medicine teaching | 91 (74) | 216 (75.8) |
| Medicine nonteaching | 22 (17.9) | 45 (15.8) |
| Surgical service | 7 (5.7) | 22 (7.7) |
| Other | 3 (2.4) | 2 (0.7) |
| Consultation during admission | ||
| Medicine | 1 (0.8) | 6 (2.1) |
| Infectious diseases | 17 (13.8) | 68 (23.9) |
| Surgical service | 12 (9.8) | 43 (15.1) |
| Gram-negative bacteremia | 76 (61.8) | 192 (67.4) |
| Gram-positive bacteremia | 47 (38.2) | 93 (32.6) |
| Predominant antibiotic class | ||
| Beta-lactam | 82 (66.7) | 175 (61.4) |
| Fluoroquinolone | 40 (32.5) | 84 (29.5) |
| Predominant route of antibiotic administration | ||
| Intravenous | 72 (58.5) | 99 (34.7) |
| Oral | 85 (69.1) | 215 (75.4) |
| Length of stay, mean (SD) | 7.4 (8.4) | 7.7 (7.2) |
| . | Shorter Course N = 123 . | Longer Course N = 285 . |
|---|---|---|
| . | N (%) . | N (%) . |
| Hospital A | 58 (47.2) | 108 (37.9) |
| Hospital B | 48 (39) | 120 (42.1) |
| Hospital C | 17 (13.8) | 57 (20) |
| Infection type | ||
| Pneumonia | 37 (30.1) | 67 (23.5) |
| UTI | 69 (56.1) | 175 (61.4) |
| ABSSSI | 17 (13.8) | 43 (15.1) |
| Admitted to ICU | 34 (27.6) | 85 (29.8) |
| Admitting team | ||
| Medicine teaching | 91 (74) | 216 (75.8) |
| Medicine nonteaching | 22 (17.9) | 45 (15.8) |
| Surgical service | 7 (5.7) | 22 (7.7) |
| Other | 3 (2.4) | 2 (0.7) |
| Consultation during admission | ||
| Medicine | 1 (0.8) | 6 (2.1) |
| Infectious diseases | 17 (13.8) | 68 (23.9) |
| Surgical service | 12 (9.8) | 43 (15.1) |
| Gram-negative bacteremia | 76 (61.8) | 192 (67.4) |
| Gram-positive bacteremia | 47 (38.2) | 93 (32.6) |
| Predominant antibiotic class | ||
| Beta-lactam | 82 (66.7) | 175 (61.4) |
| Fluoroquinolone | 40 (32.5) | 84 (29.5) |
| Predominant route of antibiotic administration | ||
| Intravenous | 72 (58.5) | 99 (34.7) |
| Oral | 85 (69.1) | 215 (75.4) |
| Length of stay, mean (SD) | 7.4 (8.4) | 7.7 (7.2) |
Abbreviations: ABSSSI, acute bacterial skin and skin structure infection; ED, emergency department; ICU, intensive care unit; SD, standard deviation; UTI, urinary tract infection.
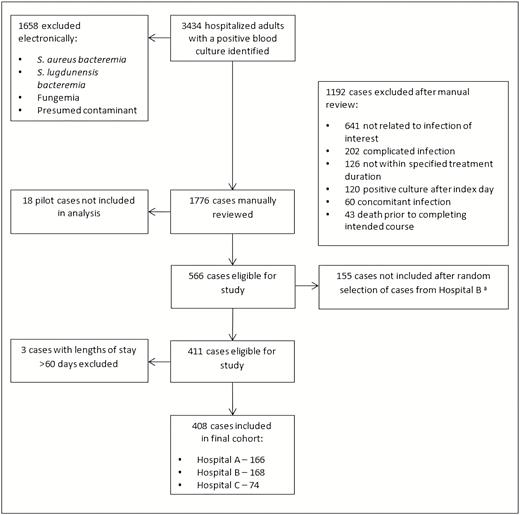
Flowchart detailing process of identifying the study cohort. *Due to Hospital B having a larger number of eligible patients relative to Hospitals A and C, a subset of patients was randomly selected for inclusion in the study. Abbreviation: S. aureus bacteremia, Staphylococcus aureus bacteremia.
Details of the covariate balance between the shorter and longer duration groups before and after propensity weighting are provided in Table 3. A <10% standardized difference between groups was achieved for all covariates. Additionally, the variance ratio was close to 1.0 for all covariates except length of stay. A sensitivity analysis in which length of stay was included in the outcome models did not yield different results.
Covariate Balance Between Shorter- and Longer-duration of Antibiotic Therapy Before and After Propensity Score Weighting
| . | Standardized Difference . | Variance Ratio . | ||
|---|---|---|---|---|
| Covariate . | Raw . | Weighted . | Raw . | Weighted . |
| Hospital | ||||
| Hospital A | 18.8% | −3.0% | 1.06 | 0.99 |
| Hospital B | −6.3% | 5.6% | 0.98 | 1.02 |
| Hospital C | −16.5% | −3.5% | 0.75 | 0.95 |
| Duration of hospitalization | −4.4% | 4.8% | 1.36 | 2.67 |
| Age | 8.5% | 0.8% | 0.86 | 0.89 |
| Female sex | 13.3% | 2.5% | 1.02 | 1.01 |
| Admission to ICU | −4.8% | 0.4% | 0.96 | 1.01 |
| Admitting team | ||||
| Medicine teaching | −4.2% | −3.3% | 1.05 | 1.04 |
| Medicine non-teaching | 5.6% | −6.7% | 1.11 | 0.88 |
| Surgical service | −8.1% | 0.2% | 0.76 | 1.01 |
| Consultation during admission | ||||
| Infectious diseases | −25.8% | −2.4% | 0.66 | 0.97 |
| Surgical service | −16.2% | 2.1% | 0.69 | 1.05 |
| Comorbid conditions | ||||
| Diabetes | −16.4% | 0.0% | 0.89 | 1.00 |
| Obesity | 8.7% | −1.1% | 1.07 | 1.00 |
| Rare comorbiditya | −28.4% | −7.0% | 0.89 | 0.98 |
| Current or former smoker | −15.7% | −1.5% | 1.08 | 1.01 |
| COPD | 7.5% | 0.2% | 1.15 | 1.01 |
| Prior UTI, PNA, or ABSSSI infection requiring treatment | 10.9% | −2.5% | 0.99 | 1.00 |
| Charlson comorbidity index | −9.1% | −5.7% | 0.77 | 0.93 |
| Pitt bacteremia score | −11.5% | −8.8% | 0.79 | 0.91 |
| Source of bacteremia (infection type) | ||||
| UTI | −10.8% | 0.0% | 1.04 | 1.00 |
| Pneumonia | 14.8% | −2.6% | 1.18 | 0.97 |
| Acute bacterial skin and skin structure infection | −3.6% | 2.9% | 0.93 | 1.06 |
| Allergies | ||||
| Penicillin allergy | 13.8% | 3.9% | 1.26 | 1.07 |
| Other allergy | −32.5% | −5.6% | 0.44 | 0.89 |
| Antibiotic class | ||||
| Beta lactam (≥50% of course) | 11.0% | 2.6% | 0.94 | 0.99 |
| Fluoroquinolone (≥50% of course) | 6.6% | −2.0% | 1.06 | 0.99 |
| . | Standardized Difference . | Variance Ratio . | ||
|---|---|---|---|---|
| Covariate . | Raw . | Weighted . | Raw . | Weighted . |
| Hospital | ||||
| Hospital A | 18.8% | −3.0% | 1.06 | 0.99 |
| Hospital B | −6.3% | 5.6% | 0.98 | 1.02 |
| Hospital C | −16.5% | −3.5% | 0.75 | 0.95 |
| Duration of hospitalization | −4.4% | 4.8% | 1.36 | 2.67 |
| Age | 8.5% | 0.8% | 0.86 | 0.89 |
| Female sex | 13.3% | 2.5% | 1.02 | 1.01 |
| Admission to ICU | −4.8% | 0.4% | 0.96 | 1.01 |
| Admitting team | ||||
| Medicine teaching | −4.2% | −3.3% | 1.05 | 1.04 |
| Medicine non-teaching | 5.6% | −6.7% | 1.11 | 0.88 |
| Surgical service | −8.1% | 0.2% | 0.76 | 1.01 |
| Consultation during admission | ||||
| Infectious diseases | −25.8% | −2.4% | 0.66 | 0.97 |
| Surgical service | −16.2% | 2.1% | 0.69 | 1.05 |
| Comorbid conditions | ||||
| Diabetes | −16.4% | 0.0% | 0.89 | 1.00 |
| Obesity | 8.7% | −1.1% | 1.07 | 1.00 |
| Rare comorbiditya | −28.4% | −7.0% | 0.89 | 0.98 |
| Current or former smoker | −15.7% | −1.5% | 1.08 | 1.01 |
| COPD | 7.5% | 0.2% | 1.15 | 1.01 |
| Prior UTI, PNA, or ABSSSI infection requiring treatment | 10.9% | −2.5% | 0.99 | 1.00 |
| Charlson comorbidity index | −9.1% | −5.7% | 0.77 | 0.93 |
| Pitt bacteremia score | −11.5% | −8.8% | 0.79 | 0.91 |
| Source of bacteremia (infection type) | ||||
| UTI | −10.8% | 0.0% | 1.04 | 1.00 |
| Pneumonia | 14.8% | −2.6% | 1.18 | 0.97 |
| Acute bacterial skin and skin structure infection | −3.6% | 2.9% | 0.93 | 1.06 |
| Allergies | ||||
| Penicillin allergy | 13.8% | 3.9% | 1.26 | 1.07 |
| Other allergy | −32.5% | −5.6% | 0.44 | 0.89 |
| Antibiotic class | ||||
| Beta lactam (≥50% of course) | 11.0% | 2.6% | 0.94 | 0.99 |
| Fluoroquinolone (≥50% of course) | 6.6% | −2.0% | 1.06 | 0.99 |
Abbreviations: ABSSSI, acute bacterial skin and skin structure infection; COPD, chronic obstructive pulmonary disease; CTD, connective tissue disease; ESRD, end stage renal disease; HIV, human immunodeficiency virus; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; PAD, peripheral artery disease; PNA, pneumonia; UTI, urinary tract infection.
a Rare comorbidities include any of the following: HIV/AIDS, ESRD, PAD, venous Stasis, prior MRSA, CTD, cirrhosis, any malignancy, chronic urinary catheterization, spinal cord injury, or immunosuppressive therapy
Covariate Balance Between Shorter- and Longer-duration of Antibiotic Therapy Before and After Propensity Score Weighting
| . | Standardized Difference . | Variance Ratio . | ||
|---|---|---|---|---|
| Covariate . | Raw . | Weighted . | Raw . | Weighted . |
| Hospital | ||||
| Hospital A | 18.8% | −3.0% | 1.06 | 0.99 |
| Hospital B | −6.3% | 5.6% | 0.98 | 1.02 |
| Hospital C | −16.5% | −3.5% | 0.75 | 0.95 |
| Duration of hospitalization | −4.4% | 4.8% | 1.36 | 2.67 |
| Age | 8.5% | 0.8% | 0.86 | 0.89 |
| Female sex | 13.3% | 2.5% | 1.02 | 1.01 |
| Admission to ICU | −4.8% | 0.4% | 0.96 | 1.01 |
| Admitting team | ||||
| Medicine teaching | −4.2% | −3.3% | 1.05 | 1.04 |
| Medicine non-teaching | 5.6% | −6.7% | 1.11 | 0.88 |
| Surgical service | −8.1% | 0.2% | 0.76 | 1.01 |
| Consultation during admission | ||||
| Infectious diseases | −25.8% | −2.4% | 0.66 | 0.97 |
| Surgical service | −16.2% | 2.1% | 0.69 | 1.05 |
| Comorbid conditions | ||||
| Diabetes | −16.4% | 0.0% | 0.89 | 1.00 |
| Obesity | 8.7% | −1.1% | 1.07 | 1.00 |
| Rare comorbiditya | −28.4% | −7.0% | 0.89 | 0.98 |
| Current or former smoker | −15.7% | −1.5% | 1.08 | 1.01 |
| COPD | 7.5% | 0.2% | 1.15 | 1.01 |
| Prior UTI, PNA, or ABSSSI infection requiring treatment | 10.9% | −2.5% | 0.99 | 1.00 |
| Charlson comorbidity index | −9.1% | −5.7% | 0.77 | 0.93 |
| Pitt bacteremia score | −11.5% | −8.8% | 0.79 | 0.91 |
| Source of bacteremia (infection type) | ||||
| UTI | −10.8% | 0.0% | 1.04 | 1.00 |
| Pneumonia | 14.8% | −2.6% | 1.18 | 0.97 |
| Acute bacterial skin and skin structure infection | −3.6% | 2.9% | 0.93 | 1.06 |
| Allergies | ||||
| Penicillin allergy | 13.8% | 3.9% | 1.26 | 1.07 |
| Other allergy | −32.5% | −5.6% | 0.44 | 0.89 |
| Antibiotic class | ||||
| Beta lactam (≥50% of course) | 11.0% | 2.6% | 0.94 | 0.99 |
| Fluoroquinolone (≥50% of course) | 6.6% | −2.0% | 1.06 | 0.99 |
| . | Standardized Difference . | Variance Ratio . | ||
|---|---|---|---|---|
| Covariate . | Raw . | Weighted . | Raw . | Weighted . |
| Hospital | ||||
| Hospital A | 18.8% | −3.0% | 1.06 | 0.99 |
| Hospital B | −6.3% | 5.6% | 0.98 | 1.02 |
| Hospital C | −16.5% | −3.5% | 0.75 | 0.95 |
| Duration of hospitalization | −4.4% | 4.8% | 1.36 | 2.67 |
| Age | 8.5% | 0.8% | 0.86 | 0.89 |
| Female sex | 13.3% | 2.5% | 1.02 | 1.01 |
| Admission to ICU | −4.8% | 0.4% | 0.96 | 1.01 |
| Admitting team | ||||
| Medicine teaching | −4.2% | −3.3% | 1.05 | 1.04 |
| Medicine non-teaching | 5.6% | −6.7% | 1.11 | 0.88 |
| Surgical service | −8.1% | 0.2% | 0.76 | 1.01 |
| Consultation during admission | ||||
| Infectious diseases | −25.8% | −2.4% | 0.66 | 0.97 |
| Surgical service | −16.2% | 2.1% | 0.69 | 1.05 |
| Comorbid conditions | ||||
| Diabetes | −16.4% | 0.0% | 0.89 | 1.00 |
| Obesity | 8.7% | −1.1% | 1.07 | 1.00 |
| Rare comorbiditya | −28.4% | −7.0% | 0.89 | 0.98 |
| Current or former smoker | −15.7% | −1.5% | 1.08 | 1.01 |
| COPD | 7.5% | 0.2% | 1.15 | 1.01 |
| Prior UTI, PNA, or ABSSSI infection requiring treatment | 10.9% | −2.5% | 0.99 | 1.00 |
| Charlson comorbidity index | −9.1% | −5.7% | 0.77 | 0.93 |
| Pitt bacteremia score | −11.5% | −8.8% | 0.79 | 0.91 |
| Source of bacteremia (infection type) | ||||
| UTI | −10.8% | 0.0% | 1.04 | 1.00 |
| Pneumonia | 14.8% | −2.6% | 1.18 | 0.97 |
| Acute bacterial skin and skin structure infection | −3.6% | 2.9% | 0.93 | 1.06 |
| Allergies | ||||
| Penicillin allergy | 13.8% | 3.9% | 1.26 | 1.07 |
| Other allergy | −32.5% | −5.6% | 0.44 | 0.89 |
| Antibiotic class | ||||
| Beta lactam (≥50% of course) | 11.0% | 2.6% | 0.94 | 0.99 |
| Fluoroquinolone (≥50% of course) | 6.6% | −2.0% | 1.06 | 0.99 |
Abbreviations: ABSSSI, acute bacterial skin and skin structure infection; COPD, chronic obstructive pulmonary disease; CTD, connective tissue disease; ESRD, end stage renal disease; HIV, human immunodeficiency virus; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; PAD, peripheral artery disease; PNA, pneumonia; UTI, urinary tract infection.
a Rare comorbidities include any of the following: HIV/AIDS, ESRD, PAD, venous Stasis, prior MRSA, CTD, cirrhosis, any malignancy, chronic urinary catheterization, spinal cord injury, or immunosuppressive therapy
The primary outcome of clinical failure occurred in 50 patients, including 15 (12.2%) in the shorter duration group and 35 (12.3%) in the longer group (Table 4). After propensity weighting, the predicted probability of clinical failure was similar: 13.5% in the shorter duration group and 11.1% in the longer group with an average treatment effect difference of 2.4% (odds ratio [OR], 1.25; 95% confidence interval [CI], .65–2.40; P = .505) (Table 5).
Unadjusted Primary and Secondary Outcomes Among Patients Hospitalized With Uncomplicated Bacteremia
| . | Shorter Course N = 123 . | Longer Course N = 285 . | . |
|---|---|---|---|
| . | N (%) . | N (%) . | P value . |
| Composite primary outcome (clinical failure) | 15 (12.2) | 35 (12.3) | .9904 |
| Rehospitalization for the same infection | 2 (1.6) | 13 (4.5) | .2490 |
| Restarted antibiotics for the same infection | 15 (12.2) | 28 (9.7) | .4829 |
| All-cause 30-day mortality | 0 (0) | 6 (2.1) | .1850 |
| Secondary outcomes | |||
| Clostridioides difficile infection | 5 (4.1) | 4 (1.4) | .1347 |
| Antibiotic-related adverse events leading to change in or discontinuation of antibiotic therapy | 0 (0) | 4 (1.4) | .3217 |
| . | Shorter Course N = 123 . | Longer Course N = 285 . | . |
|---|---|---|---|
| . | N (%) . | N (%) . | P value . |
| Composite primary outcome (clinical failure) | 15 (12.2) | 35 (12.3) | .9904 |
| Rehospitalization for the same infection | 2 (1.6) | 13 (4.5) | .2490 |
| Restarted antibiotics for the same infection | 15 (12.2) | 28 (9.7) | .4829 |
| All-cause 30-day mortality | 0 (0) | 6 (2.1) | .1850 |
| Secondary outcomes | |||
| Clostridioides difficile infection | 5 (4.1) | 4 (1.4) | .1347 |
| Antibiotic-related adverse events leading to change in or discontinuation of antibiotic therapy | 0 (0) | 4 (1.4) | .3217 |
Unadjusted Primary and Secondary Outcomes Among Patients Hospitalized With Uncomplicated Bacteremia
| . | Shorter Course N = 123 . | Longer Course N = 285 . | . |
|---|---|---|---|
| . | N (%) . | N (%) . | P value . |
| Composite primary outcome (clinical failure) | 15 (12.2) | 35 (12.3) | .9904 |
| Rehospitalization for the same infection | 2 (1.6) | 13 (4.5) | .2490 |
| Restarted antibiotics for the same infection | 15 (12.2) | 28 (9.7) | .4829 |
| All-cause 30-day mortality | 0 (0) | 6 (2.1) | .1850 |
| Secondary outcomes | |||
| Clostridioides difficile infection | 5 (4.1) | 4 (1.4) | .1347 |
| Antibiotic-related adverse events leading to change in or discontinuation of antibiotic therapy | 0 (0) | 4 (1.4) | .3217 |
| . | Shorter Course N = 123 . | Longer Course N = 285 . | . |
|---|---|---|---|
| . | N (%) . | N (%) . | P value . |
| Composite primary outcome (clinical failure) | 15 (12.2) | 35 (12.3) | .9904 |
| Rehospitalization for the same infection | 2 (1.6) | 13 (4.5) | .2490 |
| Restarted antibiotics for the same infection | 15 (12.2) | 28 (9.7) | .4829 |
| All-cause 30-day mortality | 0 (0) | 6 (2.1) | .1850 |
| Secondary outcomes | |||
| Clostridioides difficile infection | 5 (4.1) | 4 (1.4) | .1347 |
| Antibiotic-related adverse events leading to change in or discontinuation of antibiotic therapy | 0 (0) | 4 (1.4) | .3217 |
Inverse-probability of Treatment Weighted Models for the Duration of Antibiotic Therapy on Patient Outcomes
| . | Predicted Probability of Outcome . | . | . | . | . | . | |
|---|---|---|---|---|---|---|---|
| Outcome . | Shorter Course . | Longer Course . | Average Treatment Effecta . | Odds Ratio . | 95% Confidence Interval . | P value . | |
| Composite primary outcome | 13.5% | 11.1% | 2.4% | 1.25 | .65 | 2.40 | .5050 |
| Rehospitalization for the same infection | 2.0% | 3.8% | −1.9% | 0.51 | .05 | 5.06 | .5630 |
| Restarted antibiotics for the same infection | 13.5% | 8.8% | 4.7% | 1.62 | 1.01 | 2.61 | .0460 |
| Clostridioides difficile infection | 4.4% | 1.1% | 3.3% | 4.01 | 2.12 | 7.59 | <.0001 |
| . | Predicted Probability of Outcome . | . | . | . | . | . | |
|---|---|---|---|---|---|---|---|
| Outcome . | Shorter Course . | Longer Course . | Average Treatment Effecta . | Odds Ratio . | 95% Confidence Interval . | P value . | |
| Composite primary outcome | 13.5% | 11.1% | 2.4% | 1.25 | .65 | 2.40 | .5050 |
| Rehospitalization for the same infection | 2.0% | 3.8% | −1.9% | 0.51 | .05 | 5.06 | .5630 |
| Restarted antibiotics for the same infection | 13.5% | 8.8% | 4.7% | 1.62 | 1.01 | 2.61 | .0460 |
| Clostridioides difficile infection | 4.4% | 1.1% | 3.3% | 4.01 | 2.12 | 7.59 | <.0001 |
All-cause mortality and antibiotic-related adverse events leading to change in antibiotic therapy not included in this analysis due to there being no cases with these outcomes in the shorter duration group.
aInterpreted as an absolute difference of the predicted probabilities.
Inverse-probability of Treatment Weighted Models for the Duration of Antibiotic Therapy on Patient Outcomes
| . | Predicted Probability of Outcome . | . | . | . | . | . | |
|---|---|---|---|---|---|---|---|
| Outcome . | Shorter Course . | Longer Course . | Average Treatment Effecta . | Odds Ratio . | 95% Confidence Interval . | P value . | |
| Composite primary outcome | 13.5% | 11.1% | 2.4% | 1.25 | .65 | 2.40 | .5050 |
| Rehospitalization for the same infection | 2.0% | 3.8% | −1.9% | 0.51 | .05 | 5.06 | .5630 |
| Restarted antibiotics for the same infection | 13.5% | 8.8% | 4.7% | 1.62 | 1.01 | 2.61 | .0460 |
| Clostridioides difficile infection | 4.4% | 1.1% | 3.3% | 4.01 | 2.12 | 7.59 | <.0001 |
| . | Predicted Probability of Outcome . | . | . | . | . | . | |
|---|---|---|---|---|---|---|---|
| Outcome . | Shorter Course . | Longer Course . | Average Treatment Effecta . | Odds Ratio . | 95% Confidence Interval . | P value . | |
| Composite primary outcome | 13.5% | 11.1% | 2.4% | 1.25 | .65 | 2.40 | .5050 |
| Rehospitalization for the same infection | 2.0% | 3.8% | −1.9% | 0.51 | .05 | 5.06 | .5630 |
| Restarted antibiotics for the same infection | 13.5% | 8.8% | 4.7% | 1.62 | 1.01 | 2.61 | .0460 |
| Clostridioides difficile infection | 4.4% | 1.1% | 3.3% | 4.01 | 2.12 | 7.59 | <.0001 |
All-cause mortality and antibiotic-related adverse events leading to change in antibiotic therapy not included in this analysis due to there being no cases with these outcomes in the shorter duration group.
aInterpreted as an absolute difference of the predicted probabilities.
Regarding the secondary analyses, 9 cases of C. difficile infection occurred; 5 in the shorter duration group and 4 in the longer group (Table 4). After propensity weighting, there was a significantly higher predicted probability of C. difficile infection in the shorter group (OR, 4.01; 95% CI, 2.12–7.59; P < .0001) (Table 5). In a post hoc review of these cases, 7 occurred within 9 days of the index positive blood culture and 2 (both in the longer duration group) occurred beyond 9 days from the index culture.
Of the 43 patients for whom antibiotics were restarted for the same infection, 15 were in the shorter duration group and 28 in the longer group (Table 4). After propensity weighting, there was a significantly higher predicted probability of restarting antibiotics in the shorter group (OR, 1.62; 95% CI, 1.01–2.61; P = .046) (Table 5). Propensity-weighted analysis could not be performed for all-cause mortality and adverse drug events leading to antibiotic change or discontinuation due to there being no cases with these outcomes in the shorter duration group.
In the prespecified stratifications of the primary outcome, among patients who received predominantly beta-lactam antibiotic therapy, there was a significantly higher predicted probability of clinical failure in the shorter duration group (OR, 1.72; 95% CI, 1.46–2.03; P < .0001) (Table 6, Figure 2). There were no significant differences in clinical failure between shorter and longer groups when stratified by infection type, organism type, or predominant administration route (Figure 2). A total of 61 cases were not included in the antibiotic class stratification analysis due to not having received at least 50% of their course with either a beta-lactam or fluoroquinolone agent or due to being treated with an antibiotic from both classes for at least 50% of their course (Table 6). Similarly, 63 cases were not included in the route of antibiotic administration analysis due to having received both an intravenous and an oral agent for at least 50% of their course.
Inverse-probability of Treatment Weighted Models for the Duration of Antibiotic Therapy on the Composite Primary Outcome, by Additional Characteristics
| . | . | Predicted Probability of Outcome . | . | . | . | . | . | |
|---|---|---|---|---|---|---|---|---|
| Characteristic . | Sample Sizea . | Shorter Course . | Longer Course . | Average Treatment Effect b . | Odds Ratio . | 95% Confidence Interval . | P-value . | |
| Type of infection | 408 | |||||||
| Pneumonia | 104 | 4.0% | 11.8% | −7.9% | 0.31 | .06 | 1.70 | .1300 |
| Urinary tract infection | 244 | 17.6% | 12.2% | 5.4% | 1.54 | .30 | 8.06 | .6280 |
| ABSSSI | 60 | 12.8% | 6.2% | 6.5% | 2.20 | .11 | 42.74 | .5720 |
| Type of organism | 408 | |||||||
| Gram-negative | 268 | 15.0% | 12.9% | 2.1% | 1.19 | .17 | 8.48 | .8670 |
| Gram-positive | 140 | 10.8% | 7.7% | 3.2% | 1.46 | .09 | 23.34 | .7870 |
| Predominant antibiotic class | 347 | |||||||
| Fluoroquinolone | 107 | 3.9% | 8.5% | −4.6% | 0.44 | .04 | 5.38 | .4640 |
| Beta-lactam | 240 | 18.1% | 11.4% | 6.7% | 1.72 | 1.46 | 2.03 | <.0001 |
| Predominant route of antibiotic administration | 345 | |||||||
| Intravenous | 108 | 12.2% | 12.2% | 0.0% | 1.00 | .40 | 2.48 | .9930 |
| Oral | 237 | 13.8% | 10.9% | 3.0% | 1.32 | .37 | 4.63 | .7060 |
| . | . | Predicted Probability of Outcome . | . | . | . | . | . | |
|---|---|---|---|---|---|---|---|---|
| Characteristic . | Sample Sizea . | Shorter Course . | Longer Course . | Average Treatment Effect b . | Odds Ratio . | 95% Confidence Interval . | P-value . | |
| Type of infection | 408 | |||||||
| Pneumonia | 104 | 4.0% | 11.8% | −7.9% | 0.31 | .06 | 1.70 | .1300 |
| Urinary tract infection | 244 | 17.6% | 12.2% | 5.4% | 1.54 | .30 | 8.06 | .6280 |
| ABSSSI | 60 | 12.8% | 6.2% | 6.5% | 2.20 | .11 | 42.74 | .5720 |
| Type of organism | 408 | |||||||
| Gram-negative | 268 | 15.0% | 12.9% | 2.1% | 1.19 | .17 | 8.48 | .8670 |
| Gram-positive | 140 | 10.8% | 7.7% | 3.2% | 1.46 | .09 | 23.34 | .7870 |
| Predominant antibiotic class | 347 | |||||||
| Fluoroquinolone | 107 | 3.9% | 8.5% | −4.6% | 0.44 | .04 | 5.38 | .4640 |
| Beta-lactam | 240 | 18.1% | 11.4% | 6.7% | 1.72 | 1.46 | 2.03 | <.0001 |
| Predominant route of antibiotic administration | 345 | |||||||
| Intravenous | 108 | 12.2% | 12.2% | 0.0% | 1.00 | .40 | 2.48 | .9930 |
| Oral | 237 | 13.8% | 10.9% | 3.0% | 1.32 | .37 | 4.63 | .7060 |
Abbreviation: ABSSSI, acute bacterial skin and skin structure infection.
aSample size varied depending on characteristic. Three cases with durations of hospitalizations longer than 60 days were excluded from all analyses. Additional cases excluded from the analysis of fluoroquinolone (FQ) and beta lactam (BL) agents included 17 receiving both FQ and BL for more 50 percent or more of the course and 44 cases that did not receive either FQ or BL for 50 percent or more of the course. Additional cases excluded from the analysis of intravenous (IV) and oral antibiotic therapy included 63 cases receiving both IV and oral therapy for 50 percent or more of the course.
bInterpreted as an absolute difference of the predicted probabilities.
Inverse-probability of Treatment Weighted Models for the Duration of Antibiotic Therapy on the Composite Primary Outcome, by Additional Characteristics
| . | . | Predicted Probability of Outcome . | . | . | . | . | . | |
|---|---|---|---|---|---|---|---|---|
| Characteristic . | Sample Sizea . | Shorter Course . | Longer Course . | Average Treatment Effect b . | Odds Ratio . | 95% Confidence Interval . | P-value . | |
| Type of infection | 408 | |||||||
| Pneumonia | 104 | 4.0% | 11.8% | −7.9% | 0.31 | .06 | 1.70 | .1300 |
| Urinary tract infection | 244 | 17.6% | 12.2% | 5.4% | 1.54 | .30 | 8.06 | .6280 |
| ABSSSI | 60 | 12.8% | 6.2% | 6.5% | 2.20 | .11 | 42.74 | .5720 |
| Type of organism | 408 | |||||||
| Gram-negative | 268 | 15.0% | 12.9% | 2.1% | 1.19 | .17 | 8.48 | .8670 |
| Gram-positive | 140 | 10.8% | 7.7% | 3.2% | 1.46 | .09 | 23.34 | .7870 |
| Predominant antibiotic class | 347 | |||||||
| Fluoroquinolone | 107 | 3.9% | 8.5% | −4.6% | 0.44 | .04 | 5.38 | .4640 |
| Beta-lactam | 240 | 18.1% | 11.4% | 6.7% | 1.72 | 1.46 | 2.03 | <.0001 |
| Predominant route of antibiotic administration | 345 | |||||||
| Intravenous | 108 | 12.2% | 12.2% | 0.0% | 1.00 | .40 | 2.48 | .9930 |
| Oral | 237 | 13.8% | 10.9% | 3.0% | 1.32 | .37 | 4.63 | .7060 |
| . | . | Predicted Probability of Outcome . | . | . | . | . | . | |
|---|---|---|---|---|---|---|---|---|
| Characteristic . | Sample Sizea . | Shorter Course . | Longer Course . | Average Treatment Effect b . | Odds Ratio . | 95% Confidence Interval . | P-value . | |
| Type of infection | 408 | |||||||
| Pneumonia | 104 | 4.0% | 11.8% | −7.9% | 0.31 | .06 | 1.70 | .1300 |
| Urinary tract infection | 244 | 17.6% | 12.2% | 5.4% | 1.54 | .30 | 8.06 | .6280 |
| ABSSSI | 60 | 12.8% | 6.2% | 6.5% | 2.20 | .11 | 42.74 | .5720 |
| Type of organism | 408 | |||||||
| Gram-negative | 268 | 15.0% | 12.9% | 2.1% | 1.19 | .17 | 8.48 | .8670 |
| Gram-positive | 140 | 10.8% | 7.7% | 3.2% | 1.46 | .09 | 23.34 | .7870 |
| Predominant antibiotic class | 347 | |||||||
| Fluoroquinolone | 107 | 3.9% | 8.5% | −4.6% | 0.44 | .04 | 5.38 | .4640 |
| Beta-lactam | 240 | 18.1% | 11.4% | 6.7% | 1.72 | 1.46 | 2.03 | <.0001 |
| Predominant route of antibiotic administration | 345 | |||||||
| Intravenous | 108 | 12.2% | 12.2% | 0.0% | 1.00 | .40 | 2.48 | .9930 |
| Oral | 237 | 13.8% | 10.9% | 3.0% | 1.32 | .37 | 4.63 | .7060 |
Abbreviation: ABSSSI, acute bacterial skin and skin structure infection.
aSample size varied depending on characteristic. Three cases with durations of hospitalizations longer than 60 days were excluded from all analyses. Additional cases excluded from the analysis of fluoroquinolone (FQ) and beta lactam (BL) agents included 17 receiving both FQ and BL for more 50 percent or more of the course and 44 cases that did not receive either FQ or BL for 50 percent or more of the course. Additional cases excluded from the analysis of intravenous (IV) and oral antibiotic therapy included 63 cases receiving both IV and oral therapy for 50 percent or more of the course.
bInterpreted as an absolute difference of the predicted probabilities.
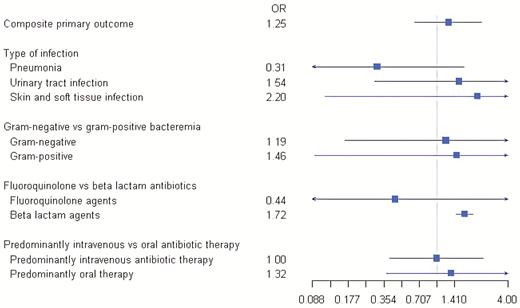
Forest plot depicting the odds ratio of the composite primary outcome stratified by type of infection, type of pathogen, predominant antibiotic class, and predominant route of antibiotic administration. Abbreviation: OR, odds ratio.
A post hoc analysis was performed to explore the observed higher risk of failure in the shorter duration group with beta-lactam predominant therapy. Two patients in the shorter group and one in the longer group were treated with agents inactive by in vitro testing. Of the patients who experienced clinical failure, 6 (40%) patients in the shorter group and 10 (36%) in the longer group completed their treatment with an oral penicillin or cephalosporin. The remaining patients in each group were treated exclusively with an intravenous agent or completed their treatment course with an oral fluoroquinolone. Further details regarding characteristics of these patients are provided in Supplementary Table 1.
DISCUSSION
In this study, we examined the impact of duration of antibiotic therapy on clinical outcomes for patients hospitalized with PNA, UTI, or ABSSSI with associated bacteremia. By both unadjusted and propensity-weighted analyses, we did not identify an increased risk of clinical failure among patients treated with shorter durations compared with those treated with longer durations. We did, however, observe that shorter durations were associated with an increased risk of reinitiation of antibiotics during the 30-day follow-up period and C. difficile infection early in the course of therapy. In addition, among patients treated predominantly with a beta-lactam antibiotic, clinical failure was more common with shorter relative to longer durations of therapy.
This study adds to a growing body of literature evaluating the effectiveness of shorter-course therapy for common infections with bacteremia. Yahav and colleagues randomized patients with uncomplicated gram-negative bacteremia to receive either 7 or 14 days of therapy [24]. The rate of the 90-day composite primary outcome (death, relapse, complications, readmission, or extended hospitalization) in the 7-day group was noninferior to that of the 14-day group. A faster return to baseline activity level in the 7-day group was also observed. In a prospective cohort of patients with gram-negative bacteremia, a propensity-stratified comparison of 7–10 days versus >10 days of antibiotic therapy showed no difference in 30-day mortality or 90-day recurrence [21]. Chotiprasitsakul and colleagues performed a propensity-score matched study of patients with Enterobacteriaceae bacteremia and found shorter and longer courses of antibiotic therapy were associated with similar outcomes [22]. Our study adds to this existing literature in that we included patients with a heterogenous group of underlying infections with diverse microbiology and evaluated several clinically relevant outcomes. Our findings support the notion that, in general, patients with uncomplicated bacteremia due to common infections do not require extension of antibiotics beyond 7–10 days.
Although we did not find a significant difference between groups in the composite endpoint of clinical failure, it is important to note that patients treated with shorter courses were more likely to be restarted on antibiotics during the follow-up period. The clinical relevance of this finding is unclear since in a retrospective study, it is difficult to determine whether reinitiation of antibiotics was truly indicated. Although persistent or recurrent signs or symptoms of infection may indeed warrant antibiotic reinitiation, clinicians also may also unnecessarily extend or restart antibiotics, for example, in cases of cellulitis where slowly resolving inflammation is mistaken for ongoing infection [25]. It is also plausible that the knowledge itself that a patient received a shorter course of antibiotics lowered the clinicians’ threshold to restart treatment.
Another important finding from this work is that among patients treated predominantly with beta-lactam agents, the rate of clinical failure was higher with shorter duration therapy. A study by Kutob and colleagues similarly demonstrated higher failure rates among patients with gram-negative bacteremia treated with antibiotics with low oral bioavailability, although moderate bioavailability fluoroquinolones also had a higher rate of failure [26]. In contrast, Mercuro and colleagues demonstrated that for patients with Enterobacteriaceae bacteremia, oral step-down therapy with beta-lactams was noninferior to fluoroquinolones [27]. In our post hoc analysis, we attempted to evaluate the effectiveness of therapy based on susceptibility results of the infecting pathogen and antibiotic bioavailability. Forty percent of patients in the shorter group versus 36% in the longer group completed their course with a less bioavailable agent, which does not clearly explain our findings. Given the increasing use of beta-lactams to treat infections due to fluoroquinolone-resistant pathogens and increasing recognition of fluoroquinolone-associated adverse events [9, 28, 29], prospective clinical trials addressing the appropriate duration of beta-lactam therapy are warranted.
It was an unexpected and counterintuitive finding that patients in the shorter duration group had a higher rate of C. difficile infection than those in the longer group. It is unlikely that the shorter course was causal, given that only 2 cases occurred beyond 9 days from the index positive culture, both of which were in the longer group. It is possible that the diagnosis of C. difficile infection influenced the provider to choose a shorter course; however, we found no explicit documentation of this.
This study has several limitations. First, due to the retrospective design, there is potential for selection bias in that patients who were less severely ill or responded more rapidly to therapy may have been more likely to be prescribed shorter antibiotic courses, potentially biasing the results toward the null. We mitigated this bias by employing propensity score analysis and ultimately demonstrated well-balanced groups for comparison; however, residual confounding may exist. Second, a true association between shorter course therapy and clinical failure may have been missed due to inadequate sample size, and our secondary analyses were limited by small numbers of outcomes. Third, we had the potential to miss outcome events if patients presented to outside hospitals; however, given most area hospitals share a health information exchange network, this should have been uncommon and unlikely to have affected our results. Fourth, we lacked data on durations of therapy prescribed when antibiotics were restarted; thus, it is unknown whether patients in the shorter group who were restarted on antibiotics ultimately had greater antibiotic exposure. Fifth, generalizability of the study may be limited given the relatively selective eligibility criteria. Furthermore, the heterogeneity in the included types of infections and pathogens may be considered a limitation; however, the study design addresses a pragmatic, clinical question: “Is there benefit to extending the duration of antibiotic therapy in patients with common infections if there is associated bacteremia?” Strengths of the study include the multicenter design, diversity of patient populations from the 3 hospitals, and use of propensity-weighted analysis.
In summary, in this cohort of patients with PNA, UTI, and ABSSSI with bacteremia, we did not find an association between shorter durations of antibiotic therapy and increased risk of clinical failure as compared with longer durations. However, further work is needed to understand the implications of the observed increased rate of reinitiation of antibiotic therapy. Additionally, prospective, randomized data are needed to determine the effectiveness and appropriate duration of therapy of beta-lactam antibiotics in this clinical scenario.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors are grateful to Miranda Kroehl from the Center for Innovative Design and Analysis in the Colorado School of Public Health for her support of this collaborative.
Financial support. This work was not supported by any relevant funding sources.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References




