-
PDF
- Split View
-
Views
-
Cite
Cite
Mahesh C Patel, Lelia H Chaisson, Scott Borgetti, Deborah Burdsall, Rashmi K Chugh, Christopher R Hoff, Elizabeth B Murphy, Emily A Murskyj, Shannon Wilson, Joe Ramos, Lynn Akker, Debra Bryars, Evonda Thomas-Smith, Susan C Bleasdale, Ngozi O Ezike, Asymptomatic SARS-CoV-2 Infection and COVID-19 Mortality During an Outbreak Investigation in a Skilled Nursing Facility, Clinical Infectious Diseases, Volume 71, Issue 11, 1 December 2020, Pages 2920–2926, https://doi.org/10.1093/cid/ciaa763
Close - Share Icon Share
Abstract
Outbreaks of coronavirus disease 2019 (COVID-19) have been reported in nursing homes and assisted living facilities; however, the extent of asymptomatic and presymptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in this high-risk population remains unclear.
We conducted an investigation of the first known outbreak of SARS-CoV-2 at a skilled nursing facility (SNF) in Illinois on 15 March 2020 and followed residents for 30 days. We tested 126/127 residents for SARS-CoV-2 via reverse-transcription polymerase chain reaction and performed symptom assessments. We calculated the point prevalence of SARS-CoV-2 and assessed symptom onset over 30-day follow-up to determine: (1) the proportion of cases who were symptomatic, presymptomatic, and asymptomatic and (2) incidence of symptoms among those who tested negative. We used the Kaplan-Meier method to determine the 30-day probability of death for cases.
Of 126 residents tested, 33 had confirmed SARS-CoV-2 on 15 March. Nineteen (58%) had symptoms at the time of testing, 1 (3%) developed symptoms over follow-up, and 13 (39%) remained asymptomatic. Thirty-five residents who tested negative on 15 March developed symptoms over follow-up; of these, 3 were re-tested and 2 were positive. The 30-day probability of death among cases was 29%.
SNFs are particularly vulnerable to SARS-CoV-2, and residents are at risk of severe outcomes. Attention must be paid to preventing outbreaks in these and other congregate care settings. Widespread testing and infection control are key to help prevent COVID-19 morbidity and mortality in these high-risk populations.
Since the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in December 2019, multiple outbreaks of coronavirus disease 2019 (COVID-19) have occurred in long-term care and assisted living facilities [1–6]. Given the high morbidity and mortality associated with COVID-19 for older individuals [7, 8] and those with comorbidities such as hypertension, cardiovascular disease, and diabetes [9–12], outbreaks in these facilities may be particularly severe. However, much remains unknown about the prevalence of asymptomatic infection in this high-risk population, and outcomes for those who develop COVID-19.
Here we describe the first known cluster of SARS-CoV-2 infections among residents of a skilled nursing facility (SNF) in Illinois. We performed symptom assessments and offered testing to all residents on a single day to determine the point prevalence of SARS-CoV-2 in the facility and followed residents for 30 days to evaluate the extent of presymptomatic and asymptomatic infection, incidence of symptoms among those who tested negative, and patient outcomes.
METHODS
Setting
Facility A is a 150-bed SNF located in DuPage County, Illinois, that offers short- and long-term rehabilitation and hospice care. It is staffed by 1 medical director, 4 clinicians, and 2 nurse practitioners, and approximately 60 nurses, 10 housekeepers, and 35 additional full- and part-time staff. The facility has 2 floors with 3 wings for patient care (short-term rehabilitation, long-term rehabilitation, and memory care for patients with dementia). The majority of patient rooms are double occupancy, with a small number of single occupancy rooms.
Description of the Outbreak
On 9 March 2020, a 67-year-old female resident of Facility A was noted to have fever and cough. She was managed at the facility until she became hypoxic and had increasing shortness of breath necessitating transfer to a local hospital on 11 March. Upon admission, a sample was collected for SARS-CoV-2 testing at the Illinois Department of Public Health (IDPH) via real-time reverse-transcription polymerase chain reaction (RT-PCR). On 13 March, IDPH reported the test as positive; from 13–14 March, IDPH and the DuPage County Health Department (DCHD), together with consultants from the University of Illinois at Chicago (UIC) and the Centers for Disease Control and Prevention (CDC), led an initial response consultation and established an on-site outbreak response team at Facility A to (1) determine the prevalence of SARS-CoV-2 infection among residents and (2) provide guidance on infection prevention and control practices.
Point Prevalence Testing
At the time of the investigation, CDC recommended SARS-CoV-2 testing only for individuals with fever and/or acute respiratory symptoms. However, as this was the first known potential outbreak in a congregate setting in Illinois, and given concerns that the typical presenting feature of fever could be absent due to declining immune systems for residents of advanced age and the risk that other symptoms could be missed due to memory impairments, IDPH made the decision to perform a point prevalence assessment in this highly vulnerable population. Therefore, on 15 March, IDPH offered SARS-CoV-2 testing to all residents of Facility A, regardless of symptoms. In addition, testing was offered to 70 staff members who worked on the ward where the index case lived. A 12-person medical team collected nasopharyngeal swabs from 5pm to 8pm. These were transported to IDPH and tested over the following 2 days via RT-PCR in accordance with CDC emergency use authorization guidelines, utilizing the Roche MagNa Pure LC 2.0 for RNA extraction and ABI 7500DX for amplification. In addition, all residents were assessed for typical (fever, cough, shortness of breath, hypoxia) and atypical (sore throat, nasal congestion, diarrhea, decreased appetite, chills, myalgias, headaches, new-onset confusion) symptoms of COVID-19. Facility A nursing staff interviewed residents and recorded symptoms in residents’ medical charts.
Infection Prevention and Control
Facility A began implementing protocols to reduce the risk of COVID-19 prior to the identification of the first case, with screening of all visitors and staff for typical symptoms of COVID-19 and presence of fever (≥100°F), as well as restricted visiting hours (8am–8pm) starting 6 March. Visitors were completely restricted on 12 March. Universal masking of all staff and residents began on 14 March.
From 15–19 March, the on-site response team assessed facility status and implemented additional strategies to mitigate resident transmission, including symptom monitoring and resident cohorting. Residents testing positive for SARS-CoV-2 or who had respiratory illness consistent with COVID-19 were placed in double occupancy rooms together on a single ward. Residents who were asymptomatic and negative for SARS-CoV-2 who shared a room with a positive or symptomatic patient were considered close household contacts and continued to reside with their symptomatic roommates following testing, in accordance with CDC guidance at the time. Asymptomatic and negative SARS-CoV-2 residents rooming with another asymptomatic and negative resident remained together on a separate ward.
Staff who tested positive were instructed to self-isolate and, in accordance with CDC guidelines, were allowed to return to work 7 days after diagnosis or after 72 hours of symptoms resolving, whichever was longer.
Finally, the on-site response team provided education and training to Facility A staff on SARS-CoV-2 infection control, including hand hygiene, environmental cleaning and disinfecting evaluation, transmission-based precautions, personal protective equipment (PPE) use and supplies, symptom and vital signs (including pulse oximetry) monitoring, and cohorting.
Follow-Up
We followed residents from 15 March to 14 April 2020. Throughout this 30-day period, Facility A staff obtained vital signs and performed symptom assessments for all residents at least once during every 8-hour shift; this information was recorded in residents’ medical records. Residents requiring higher level care were transferred to local hospitals. No additional SARS-CoV-2 testing was performed on-site at Facility A after the 15 March investigation; however, some residents were retested at local hospitals.
Definitions and Statistical Analysis
We defined a symptomatic, confirmed case as any resident who tested positive for SARS-CoV-2 via RT-PCR and had at least 1 new-onset typical or atypical symptom of COVID-19 at the time of testing; and a presymptomatic confirmed case as any resident who tested positive and did not have any new-onset typical or atypical symptoms at the time of testing, but developed at least 1 symptom over the 30-day follow-up period. We defined asymptomatic confirmed cases as those who tested positive and did not develop any symptoms over follow-up. We defined a person under investigation (PUI) as any resident who was asymptomatic and tested negative for SARS-CoV-2 on 15 March but developed at least 1 typical or atypical symptom or died over follow-up.
We evaluated demographic and clinical characteristics of residents with confirmed SARS-CoV-2 infection and calculated the proportions of symptomatic, presymptomatic, and asymptomatic confirmed cases. We calculated the 30-day probability of survival for confirmed cases using the Kaplan-Meier method, following patients from the date they tested positive to death or administrative censoring at 30 days or on 14 April, whichever occurred first. In addition, we calculated the proportion of PUI and median time to symptom onset.
Ethics Approval
Facility A residents or their medical proxies provided consent for SARS-CoV-2 testing and transfer to local hospitals. The institutional review boards of IDPH and UIC approved this analysis as a public health investigation and surveillance and waived the requirement for informed consent.
RESULTS
Outbreak Investigation
A total of 127 persons resided at Facility A (Figure 1), of whom 11 (9%) lived in single occupancy rooms and 116 (91%) in double occupancy rooms. From 11–15 March, 8 symptomatic residents (including the index case) were tested for SARS-CoV-2 offsite at local hospitals. Of these, 6 were positive for SARS-CoV-2. Of the remaining 119 residents, 118 were assessed during the on-site investigation on 15 March. Of these, 27 tested positive for SARS-CoV-2, for a total of 33 (26%) confirmed SARS-CoV-2 infections.
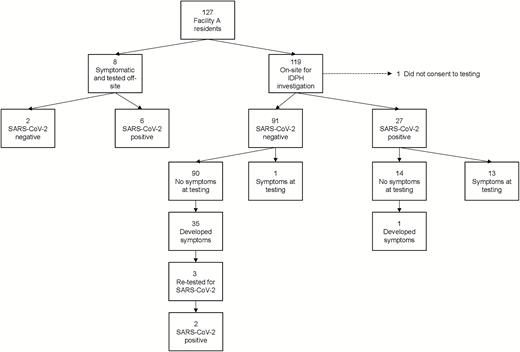
SARS-CoV-2 test results and symptom onset among Facility A residents. Abbreviations: IDPH, Illinois Department of Public Health; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Of the 33 residents with confirmed SARS-CoV-2 on 15 March, 19 (58%) had symptoms at the time of testing (Figures 1 and 2). Of the 14 (42%) positive residents without symptoms at the time of testing, 1 (3%) developed symptoms over the 30-day follow-up period. Seven (21%) confirmed cases lived in single-occupancy rooms, 18 (55%) lived in double-occupancy rooms with another confirmed case, and 8 (24%) lived in double-occupancy rooms with a roommate who tested negative on 15 March.
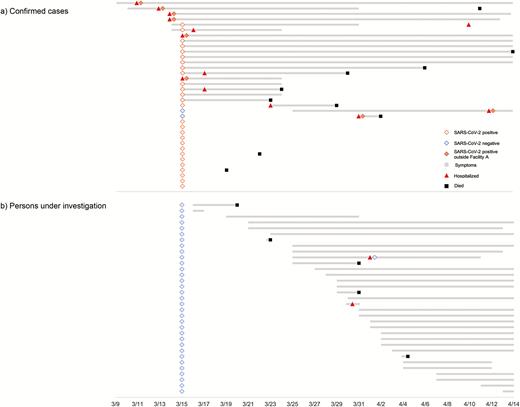
Timeline of testing, symptom onset, hospital admission, and death for (a) confirmed cases and (b) persons under investigation. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Of the 93 residents who tested negative for SARS-CoV-2, 3 (3%) had symptoms at the time of testing (Figures 1 and 2). Of the 90 who tested negative and had no symptoms, 35 (39%) developed symptoms consistent with COVID-19 over the follow-up period (PUI). Among these PUI, symptom onset occurred a median of 15 days (interquartile range [IQR] 10–18) from the time of testing on 15 March. Four PUI were roommates with previously confirmed cases (3 symptomatic confirmed cases and 1 asymptomatic confirmed case). Six PUI in the memory care unit had symptom onset starting 15 days after cohorting was completed on 18 March: none were roommates with a previously confirmed case. Three PUI were admitted to local hospitals and retested for SARS-CoV-2; 2 tested positive (on 31 March and 12 April), for a total of 35 confirmed SARS-CoV-2 infections over the 30-day follow-up period.
Characteristics of Cases and PUI
Among 35 residents with confirmed SARS-CoV-2 infection, median age was 82 years (IQR 75–92). Eight (36%) confirmed cases had typical symptoms only, 4 (11%) had atypical symptoms only, and 10 (29%) had both. The most common symptoms were fever (43%), fatigue (26%), cough (26%), shortness of breath (14%), and loss of appetite (11%; Table 1). Thirteen (37%) confirmed cases remained asymptomatic over follow-up.
| . | Cases b (N = 35) . | PUI c (N = 32) . |
|---|---|---|
| Female | 24 (69%) | 22 (69%) |
| Median age, years (IQR) | 82 (75–92) | 86 (80–90) |
| Symptomsd | ||
| None | 13 (37%) | – |
| Fever | 15 (43%) | 17 (53%) |
| Cough | 9 (26%) | 11 (34%) |
| Fatigue | 9 (26%) | 6 (19%) |
| Shortness of breath | 5 (14%) | 1 (3%) |
| Loss of appetite | 4 (11%) | 5 (16%) |
| Sore throat | 3 (9%) | 3 (9%) |
| Myalgia | 2 (6%) | 0 (0%) |
| Chills | 1 (3%) | 2 (6%) |
| Hypoxia | 1 (3%) | 4 (13%) |
| Seizures | 1 (3%) | 0 (0%) |
| Loss of consciousness or delirium | 1 (3%) | 1 (3%) |
| Nausea/vomiting | 0 (0%) | 2 (6%) |
| . | Cases b (N = 35) . | PUI c (N = 32) . |
|---|---|---|
| Female | 24 (69%) | 22 (69%) |
| Median age, years (IQR) | 82 (75–92) | 86 (80–90) |
| Symptomsd | ||
| None | 13 (37%) | – |
| Fever | 15 (43%) | 17 (53%) |
| Cough | 9 (26%) | 11 (34%) |
| Fatigue | 9 (26%) | 6 (19%) |
| Shortness of breath | 5 (14%) | 1 (3%) |
| Loss of appetite | 4 (11%) | 5 (16%) |
| Sore throat | 3 (9%) | 3 (9%) |
| Myalgia | 2 (6%) | 0 (0%) |
| Chills | 1 (3%) | 2 (6%) |
| Hypoxia | 1 (3%) | 4 (13%) |
| Seizures | 1 (3%) | 0 (0%) |
| Loss of consciousness or delirium | 1 (3%) | 1 (3%) |
| Nausea/vomiting | 0 (0%) | 2 (6%) |
Abbreviations: IQR, interquartile range; PUI, persons under investigation.
a 126/127 facility A residents tested and followed-up.
b Includes 2 persons who tested negative for SARS-CoV-2 on March 15 but developed symptoms and re-tested positive on 13 March and 12 April.
c Persons who were asymptomatic and tested negative for SARS-CoV-2 on 15 March and developed symptoms consistent with COVID-19 over follow-up. One PUI with fever and cough who retested negative for SARS-CoV-2 after symptom onset not included.
d Eight (36%) confirmed cases had typical symptoms only, 4 (11%) had atypical symptoms only, and 10 (29%) had both; 19 (58%) PUI had typical symptoms only, 4 (12%) had atypical symptoms only, and 10 (30%) had both.
| . | Cases b (N = 35) . | PUI c (N = 32) . |
|---|---|---|
| Female | 24 (69%) | 22 (69%) |
| Median age, years (IQR) | 82 (75–92) | 86 (80–90) |
| Symptomsd | ||
| None | 13 (37%) | – |
| Fever | 15 (43%) | 17 (53%) |
| Cough | 9 (26%) | 11 (34%) |
| Fatigue | 9 (26%) | 6 (19%) |
| Shortness of breath | 5 (14%) | 1 (3%) |
| Loss of appetite | 4 (11%) | 5 (16%) |
| Sore throat | 3 (9%) | 3 (9%) |
| Myalgia | 2 (6%) | 0 (0%) |
| Chills | 1 (3%) | 2 (6%) |
| Hypoxia | 1 (3%) | 4 (13%) |
| Seizures | 1 (3%) | 0 (0%) |
| Loss of consciousness or delirium | 1 (3%) | 1 (3%) |
| Nausea/vomiting | 0 (0%) | 2 (6%) |
| . | Cases b (N = 35) . | PUI c (N = 32) . |
|---|---|---|
| Female | 24 (69%) | 22 (69%) |
| Median age, years (IQR) | 82 (75–92) | 86 (80–90) |
| Symptomsd | ||
| None | 13 (37%) | – |
| Fever | 15 (43%) | 17 (53%) |
| Cough | 9 (26%) | 11 (34%) |
| Fatigue | 9 (26%) | 6 (19%) |
| Shortness of breath | 5 (14%) | 1 (3%) |
| Loss of appetite | 4 (11%) | 5 (16%) |
| Sore throat | 3 (9%) | 3 (9%) |
| Myalgia | 2 (6%) | 0 (0%) |
| Chills | 1 (3%) | 2 (6%) |
| Hypoxia | 1 (3%) | 4 (13%) |
| Seizures | 1 (3%) | 0 (0%) |
| Loss of consciousness or delirium | 1 (3%) | 1 (3%) |
| Nausea/vomiting | 0 (0%) | 2 (6%) |
Abbreviations: IQR, interquartile range; PUI, persons under investigation.
a 126/127 facility A residents tested and followed-up.
b Includes 2 persons who tested negative for SARS-CoV-2 on March 15 but developed symptoms and re-tested positive on 13 March and 12 April.
c Persons who were asymptomatic and tested negative for SARS-CoV-2 on 15 March and developed symptoms consistent with COVID-19 over follow-up. One PUI with fever and cough who retested negative for SARS-CoV-2 after symptom onset not included.
d Eight (36%) confirmed cases had typical symptoms only, 4 (11%) had atypical symptoms only, and 10 (29%) had both; 19 (58%) PUI had typical symptoms only, 4 (12%) had atypical symptoms only, and 10 (30%) had both.
Among the 33 PUI, 19 (58%) had typical symptoms only, 4 (12%) had atypical symptoms only, and 10 (30%) had both. Of the 32 PUI who were not retested, the most common symptoms were fever (53%), cough (34%), fatigue (19%), loss of appetite (16%), and hypoxia (13%; Table 1). Symptoms for 1 PUI who retested negative for SARS-CoV-2 included fever and cough.
Hospitalization and Death
Overall, 13/35 (37%) confirmed cases were hospitalized during the follow-up period. A total of 10 confirmed cases died, for a 30-day probability of survival of 71% (95% confidence interval 52%–83%; Figure 3). Median time from testing positive to death was 11.5 days (IQR 7–22 days). Of those who died, 5 (50%) were symptomatic and admitted to hospital, and 3 (33%) were symptomatic but not admitted to hospital (these patients were in hospice care). Two (20%) confirmed cases who died were asymptomatic and were not admitted to hospital: 1 96-year-old female with end-stage dementia who had been in declining health for several months and 1 89-year-old female who died suddenly but had no confirmed cause of death.
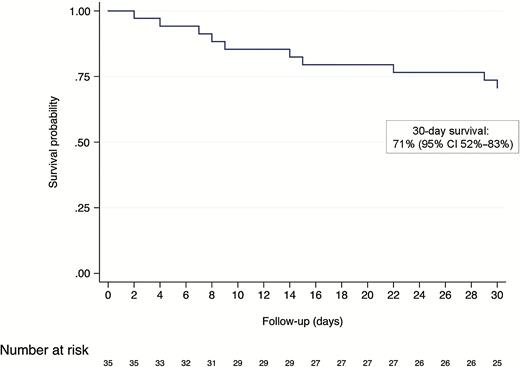
Kaplan-Meier survival curve of 35 residents with confirmed SARS-CoV-2 infection. Cases were followed from the date they tested positive to death or administrative censoring at 30 days or 14 April, whichever occurred first. Abbreviations: CI, confidence interval; SARS-CoV2, severe acute respiratory syndrome coronavirus 2.
Of the 91 residents who tested negative for SARS-CoV-2 and did not retest positive at a local hospital, 4 (4%) were hospitalized, including 1 who was hospitalized and tested offsite on 13 March. In addition, 5 (5%) died: all 5 had end-stage dementia, none were hospitalized, and all developed symptoms after the 15 March investigation. Median time from testing negative to death was 16 days (IQR 8–16).
Staff Testing
Seventy (58%) staff members were offered testing. Of 42 tested, 19 (45%) were SARS-CoV-2-positive. At the time of testing, 11/19 (58%) cases were symptomatic (2 cases did not undergo clinical evaluation). The most common symptoms among staff cases were cough (32%), fever (26%), and sore throat (21%).
DISCUSSION
SARS-CoV-2 poses a significant outbreak risk in long-term care facilities. By early May 2020, an estimated one-third of all COVID-19 deaths in the United States were among nursing home residents or workers [13]. In Illinois, as of 4 June, there have been over 17 000 confirmed cases and 2744 deaths among residents of long-term care facilities, accounting for approximately 13% of all known cases and 50% of COVID-19-related deaths statewide [14]. In this investigation at a SNF in DuPage County, Illinois, we found a high point prevalence of SARS-CoV-2 infection (26%) and substantial asymptomatic infection; furthermore, 37% of confirmed cases required hospitalization and the 30-day probability of death was 29%, confirming the severity of COVID-19 outbreaks for this high-risk population.
This study highlights several important lessons in COVID-19 outbreak response. Since COVID-19 emerged in the United States, lack of adequate testing has hampered the ability of public health responders, clinicians, and local governments to control the epidemic. This testing shortage has particularly affected vulnerable populations, including residents of long-term care facilities [15, 16]. Testing capacity has improved in the United States over the course of the epidemic, and although its continued scale-up is a key strategy for controlling COVID-19, testing of symptomatic individuals alone is insufficient in outbreaks in congregate settings. In our 15 March investigation, a significant proportion of residents that had no symptoms were found to be positive. These findings complement those from an outbreak at a SNF in King County, Washington, in which asymptomatic individuals made up over half of all positive results among residents during point prevalence testing [2, 4]. Given the increasingly apparent importance of asymptomatic and presymptomatic transmission of SARS-CoV-2 [17, 18], testing based on symptom screening alone will likely fail to identify all persons contributing to transmission in these facilities. Importantly, testing of both asymptomatic and symptomatic persons allows for effective cohorting, which may help interrupt SARS-CoV-2 transmission; this strategy is currently being scaled-up in Illinois [19]. In this outbreak, we were able to cohort symptomatic and asymptomatic positive patients into a single unit with dedicated nursing staff. Notably, we did not remove asymptomatic residents who tested negative and who were roommates with positive residents. Several of these residents went on to become PUI, and future outbreak responses may benefit from separating asymptomatic negative residents from their positive roommates to minimize transmission.
Even with implementation of cohorting and strict infection control practices such as universal PPE and masking, outbreak control in congregate settings is challenging. We documented 6 previously negative and asymptomatic residents in facility A’s memory care unit who developed symptoms starting 15 days after SARS-CoV-2 positive or symptomatic patients were appropriately isolated. None of these residents were previously roommates with a confirmed case, suggesting possible ongoing transmission throughout the facility. Implementing infection control practices may be particularly challenging for patients receiving memory and dementia care, as these patients may have more difficulty adhering to guidelines [20]. Furthermore, breaches in infection control practices, such as inattention to frequent environmental cleaning of high touch surfaces, may hinder outbreak control. Despite careful staff training, lapses may occur; and appropriate utilization of PPE poses a significant obstacle in environments where access to supplies is limited. Even with implementation of CDC strategies for optimizing PPE supply with extended use and limited reuse of PPE, cross-contamination may occur. Public health advocacy to prioritize PPE supply allocation to long-term care facilities, particularly to those experiencing an outbreak, may also help limit transmission within facilities. Long-term care facilities should continually evaluate availability of PPE, utilization rates, and environmental and engineering controls to support adherence to infection control practices in these resource-constrained environments. In addition, periodic repeat testing of staff and residents to identify new case clusters may allow for targeted and enhanced infection control practices and re-cohorting of individuals.
Importantly, our point prevalence assessment was conducted just 48 hours after SARS-CoV-2 was confirmed in the index case, and we tested 126/127 residents at facility A, regardless of symptoms. Despite this rapid and comprehensive response, we identified widespread infection among residents, suggesting that transmission had been occurring well before the index case was identified. This finding emphasizes the need for increased vigilance and monitoring of residents and staff as well as the need for rapid testing at the first signs of an outbreak. Furthermore, in addition to testing residents at long-term care facilities, testing all staff, regardless of symptoms, is vital for outbreak control: staff are likely an important vector of SARS-CoV-2 within these settings, and part-time staff who work at multiple facilities may contribute to cross-facility transmission [21]. In this outbreak, we offered testing to approximately 59% of staff members based on availability and risk of exposure. Of the 70 staff offered testing, 42 were tested and 19 were positive. Along with employee testing, long-term care facilities must develop strategies to ensure adequate staffing if cases are identified and subsequently unable to work while in isolation. Efforts to prioritize and expand regional staffing capacity and resources may also help mitigate acute staffing shortages by long-term care facilities experiencing an outbreak and exploring testing practices and protocols.
This study has several limitations. First, we assessed all residents 6 days after the index case developed symptoms. It is therefore possible that some confirmed cases who were asymptomatic during the follow-up period had mild symptoms prior to our investigation that went undetected and were thus post-symptomatic. This may explain the lower prevalence of presymptomatic infection in residents in facility A compared with those of other reported outbreaks [2, 4]. Second, challenges in symptom assessment for residents with advanced dementia or memory impairment may have resulted in an overestimate of asymptomatic infection and underestimate of persons who developed symptoms over follow-up. To minimize this risk, all residents underwent regular, comprehensive physical exams including collection of vital signs with pulse oximetry. To identify potential outbreaks as soon as possible, we recommend that SNF providers caring for patients with memory impairment implement these objective measurements in routine care. Third, we did not perform follow-up testing for patients who developed symptoms, as availability of testing was limited at the time; therefore, we cannot determine the incidence of SARS-CoV-2 over follow-up. It is likely that many PUI were incident cases, and 2 residents who initially tested negative and developed symptoms retested positive at local hospitals 16 and 28 days after the investigation. However, asymptomatic incident cases would have been missed in this investigation. Finally, due to the rapid nature of the outbreak and limited resources during this early point in the SARS-CoV-2 epidemic, we were unable to collect comprehensive data on medical histories for all residents.
The continued rise in COVID-19 cases and deaths poses an unprecedented global health threat. SARS-CoV-2 can spread rapidly in congregate living settings, and asymptomatic infection may contribute to delays in identifying and managing outbreaks. Enhanced clinical monitoring and extensive, strategic testing will be vital to reduce COVID-19 morbidity and mortality. Long-term care facilities are particularly vulnerable given older patients with multiple comorbidities that are cared for in this setting.
Notes
Acknowledgments. The authors thank Theresa Williams, Ron Nunziato, staff and residents of the skilled nursing facility, and members of the on-site response team from Rush University, Srinivas Nanduri (CDC), Matt Charles and the IDPH Laboratory Section team, Craig Conover (IDPH), Tony Serici (DuPage County OHSEM), and Linda Hansen (DCHD).
Funding. This work was supported by the Illinois Department of Public Health Intergovernmental Agency Agreement FY20-DO-1.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
These authors contributed equally.
These authors contributed equally.




