-
PDF
- Split View
-
Views
-
Cite
Cite
Akiko Uchida, Kenji Tanimura, Mayumi Morizane, Kazumichi Fujioka, Ichiro Morioka, Masanobu Oohashi, Toshio Minematsu, Hideto Yamada, Clinical Factors Associated With Congenital Cytomegalovirus Infection: A Cohort Study of Pregnant Women and Newborns, Clinical Infectious Diseases, Volume 71, Issue 11, 1 December 2020, Pages 2833–2839, https://doi.org/10.1093/cid/ciz1156
Close - Share Icon Share
Abstract
The aim of this prospective cohort study was to determine clinical factors associated with the occurrence of congenital cytomegalovirus infection (cCMV) in pregnant women.
Between March 2009 and November 2017, newborns born at a primary maternity hospital received polymerase chain reaction (PCR) analyses for CMV DNA in their urine with informed consent of the mothers at a low risk. Clinical data, including age, gravidity, parity, body mass index, occupation, maternal fever/flulike symptoms, pregnancy complications, gestational weeks at delivery, birth weight, and automated auditory brainstem response, were collected. Logistic regression analyses were performed to determine clinical factors associated with cCMV.
cCMV was diagnosed by positive PCR results of neonatal urine in 9 of 4125 pregnancies. Univariate and multivariable analyses revealed that the presence of fever/flulike symptoms (odds ratio [OR], 17.9; 95% confidence interval [CI], 3.7–86.7; P < .001) and threatened miscarriage/premature labor in the second trimester (OR, 6.0; 95% CI, 1.6–22.8; P < .01) were independent clinical factors associated with cCMV. Maternal fever/flulike symptoms or threatened miscarriage/premature labor in the second trimester had 100% sensitivity, 53.2% specificity, and a maximum Youden index of .85.
This cohort study for the first time demonstrated that these clinical factors of pregnant women and newborns were associated with the occurrence of cCMV. This is useful information for targeted screening to assess risks of cCMV in low-risk mothers, irrespective of primary or nonprimary CMV infection.
Cytomegalovirus (CMV) is the most common cause of congenital infection in humans. The prevalence rate of congenital CMV infection (cCMV) is 0.2%–2.4% in newborns [1], and 10%–15% of infected newborns are symptomatic at birth. The clinical manifestations of cCMV include fetal growth restriction (FGR), low birth weight (LBW), and central nervous system and multiple organ involvement with petechiae, hepatomegaly, splenomegaly, jaundice, pneumonia, and encephalitis. These are very severe and can cause a high perinatal mortality rate and major neurological sequelae in about 90% of surviving infants with symptomatic cCMV [2]. In addition, 10%–15% of infants with asymptomatic cCMV also develop long-term sequelae, which include progressive sensorineural hearing difficulty and mental retardation [2, 3].
Recently, it was reported that early intervention with antiviral drugs can improve neurological outcomes in children with symptomatic cCMV [4–6]. Prenatal detection of newborns at a high risk for cCMV is clinically important where no universal screening of newborns is performed, because it may enable early diagnoses and therapeutic interventions in affected infants. Generally, the risk of maternofetal transmission of CMV is thought to be highest in pregnancies with primary CMV infection. Therefore, maternal serological screening, including blood tests for CMV-specific immunoglobulin G (IgG), immunoglobulin M (IgM), and IgG avidity index, is considered effective for detecting pregnancies at a high risk for cCMV [7, 8]. Recent observational studies, however, demonstrated that the number and severity of symptoms in infants with cCMV from mothers with nonprimary CMV infection during pregnancy were similar to those from mothers with primary infection [9–11].
A prospective study of neonatal CMV screening found that socioeconomic factors such as being a younger, parous mother born in a high-resource country and having a higher income were risk factors for cCMV due to maternal primary CMV infection, whereas younger age and unemployment were found to be risk factors for cCMV due to nonprimary CMV infection [12].
However, no prospective studies have evaluated clinical factors associated with the occurrence of cCMV in pregnant women. The aim of this prospective cohort study was to determine clinical factors predictive of cCMV among low-risk women who delivered at a primary maternity hospital, where pregnant women at a high risk were referred or transferred to regional perinatal medical centers.
METHODS
Study Design and Participants
The institutional review board at Kobe University Hospital and the research ethics committee at Nadeshiko Ladies Hospital approved this prospective cohort study (reference number 923). Written informed consent was obtained from all participants. From March 2009 to November 2017, newborns who were born at Nadeshiko Ladies Hospital, a primary maternity hospital located in Kobe, Japan, underwent universal screening of polymerase chain reaction (PCR) tests for CMV DNA in the urine. Congenital infection was diagnosed with the detection of CMV DNA in newborns’ urine. All newborns who had positive results for CMV DNA in the urine were referred to Kobe University Hospital, and received a workup to identify symptoms of cCMV.
Procedures
Pregnant women were asked whether they had symptoms of fever, flulike illness, genital bleeding, abdominal pain, uterine contraction, or other abnormalities at regular prenatal checkup. The obstetricians (A. U. and K. T.) retrospectively collected the clinical data of pregnant women who visited and gave birth at the maternity hospital, including age, gravidity and parity, body mass index prior to pregnancy, occupation, smoking history, history of assisted reproductive technology therapy, fever or flulike symptoms, maternal and obstetric complications, delivery mode, nonreassuring fetal status (NRFS) during labor, gestational age at delivery, birth weight, sex of newborns, and abnormality of automated auditory brainstem response (AABR) screening test performed at 1–5 days after birth. Maternal and obstetric complications assessed in this study were as follows: hypertensive disorders of pregnancy (HDP), thyroid disease, diabetes mellitus/gestational diabetes mellitus, medical disease requiring immunosuppressive therapy, threatened miscarriage, threatened premature labor, FGR, preterm delivery, light-for-date (LFD), and LBW.
In this study, assisted reproductive technology included in vitro fertilization, intracytoplasmic sperm injection, and embryo transfer. Fever or flulike symptoms were defined as the complaints such as fever, nasal mucus, cough, and/or sore throat. Thyroid disease was defined as hyper- or hypothyroidism that required medication. NRFS during labor was defined as the absence of baseline fetal heart rate variability, the presence of recurrent late deceleration, recurrent variable deceleration, prolonged deceleration, or sinusoidal pattern detected via continuous cardiotocography (CTG) [13]. Threatened miscarriage and threatened premature labor in this study were defined as conditions causing subjective symptoms of uterine pain, contraction, bleeding, and/or shortening of uterine cervical length, and therefore requiring tocolytic agents, including oral administration of β-stimulant or calcium blocker, and intravenous administration of β-stimulant or magnesium sulfate for 1 or more weeks. LFD was defined as a birth weight of <10th percentile for gestational age. LBW was defined as a birth weight <2500 g.
Urine samples were collected from newborns on filter paper within 1 week after birth and the presence of CMV DNA was assessed as described previously [14]. The urine filter–based assay used in the present study and different in vitro diagnostic assays by the regulatory authorities yielded identical results [15]. Liquid urine samples were obtained from CMV-positive newborns, and the CMV DNA copy number was determined by real-time quantitative PCR. The presence of cCMV was confirmed by positive PCR results in the liquid urine samples [16]. All newborns with cCMV received a workup to identify the symptoms of congenital infection. Ophthalmoscopy, cerebral ultrasound, physical and neurological examinations, head computed tomography, head magnetic resonance imaging, and repeated AABR tests were performed.
Statistical Analysis
Clinical characteristics were compared between pregnancies with cCMV and those without it. The differences between the 2 groups were analyzed using the Mann-Whitney U test, Fisher exact test, and the χ 2 test. Statistical significance was considered present at P values < .05.
A stepwise approach was used to evaluate clinical factors associated with the occurrence of cCMV among all pregnant women who delivered at the primary maternity clinic. Variables with P values < .05 in univariate logistic regression analyses were subjected to multivariable logistic regression analyses, and variables with P values < .05 in multivariable logistic regression analyses were determined as clinical factors significantly associated with the occurrence of cCMV. The optimal cutoff value was determined at the maximum Youden index. Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were calculated for the prediction of cCMV. All statistical analyses were performed using SPSS software, version 19 (SPSS Inc, Chicago, Illinois).
RESULTS
A flow diagram of study participants is shown in Figure 1. During the study period, 6443 pregnant women visited the primary maternity hospital. Four hundred fifty-nine pregnancies ended in spontaneous miscarriages, and a total of 1859 pregnant women were referred to other maternity facilities where they delivered. All women with multiple pregnancies were referred to regional perinatal medical centers. A total of 4125 pregnant women gave birth at Nadeshiko Ladies Hospital, and their newborns underwent PCR tests for CMV DNA in the urine. cCMV was diagnosed in 9 newborns (0.22%), including 1 newborn with symptomatic infection and 8 with asymptomatic infection.
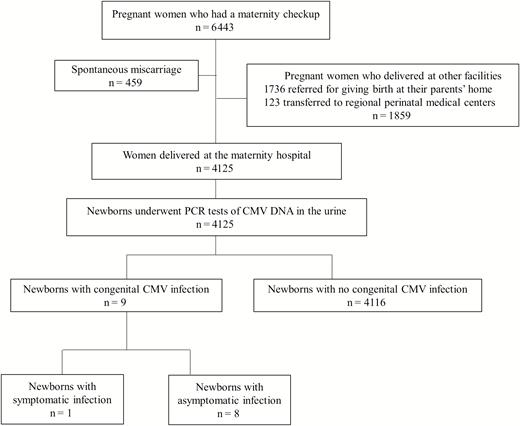
Flow diagram for the study participants and urine cytomegalovirus screening of newborns. A total of 4125 pregnant women were enrolled in this study. Abbreviations: CMV, cytomegalovirus; PCR, polymerase chain reaction.
Table 1 shows clinical characteristics of pregnant women and newborns. The frequencies of the presence of fever/flulike symptoms (P < .05), threatened miscarriage/premature labor in the second trimester (P < .01), and abnormality of AABR screening test for newborns (P < .05) in mothers who had newborns with cCMV were significantly higher than those in mothers who had newborns without cCMV.
Clinical Characteristics of Participants and Results of Logistic Regression Analyses of Clinical Factors Associated With Congenital Cytomegalovirus Infection
| Clinical Findings . | All Women (N = 4125) . | Women Who Had Newborns With Congenital CMV Infection (n = 9) . | Women Who Had Newborns Without Congenital CMV Infection (n = 4116) . | P Value . | Univariate Logistic Regression Analysis . | . | Multivariable Logistic Regression Analysis . | . |
|---|---|---|---|---|---|---|---|---|
| . | Average ± SD or No. (%) . | Median (Range) or No. . | Median (Range) or No. . | . | OR (95% CI) . | P Value . | OR (95% CI) . | P Value . |
| Clinical findings of pregnant women | ||||||||
| Age, y | 30.4 ± 5.1 | 28 (16–35) | 30 (16–46) | NS | … | … | ||
| Gravidity | 1.9 ± 1.1 | 2 (1–5) | 2 (1–14) | NS | … | … | ||
| Parity | 0.7 ± 0.8 | 1 (0–4) | 1 (0–5) | NS | … | … | ||
| Assisted reproductive technology therapy | 362 (8.8) | 0 | 362 | NS | … | … | ||
| BMI prior to pregnancy, kg/m2 | 20.7 ± 2.8 | 19.8 (18.2–27.5) | 20.2 (14.7–41.1) | NS | … | … | ||
| Smoking history | 272 (7.8) | 0 | 272 | NS | … | … | ||
| Fever or flu-like symptoms | 627 (15.2) | 7 | 620 | <.05 | 19.8 (4.1–95.7) | <.001 | 17.9 (3.7–86.7) | <.001 |
| Hypertensive disorders of pregnancy | 74 (1.8) | 1 | 73 | NS | … | … | ||
| Thyroid disease | 33 (0.8) | 0 | 33 | NS | … | … | ||
| DM/gestational DM | 74 (1.8) | 0 | 74 | NS | … | … | ||
| Medical diseases requiring immunosuppressive therapies | 4 (0.1) | 0 | 4 | NS | … | … | ||
| Threatened miscarriage in the first trimester | 255 (6.1) | 0 | 255 | NS | … | … | ||
| Threatened miscarriage or premature labor in the second trimester | 618 (15.0) | 5 | 613 | <.01 | 7.1 (1.9–26.7) | <.005 | 6.0 (1.6–22.8) | <.01 |
| Threatened premature labor in the third trimester | 1101 (26.6) | 4 | 1097 | NS | … | … | ||
| Preterm delivery | 103 (2.4) | 0 | 103 | NS | … | … | ||
| NRFS during labor | 136 (3.4) | 0 | 136 | NS | … | … | ||
| Gestational weeks at delivery | 39.1 ± 1.3 | 39 (38–41) | 39 (33–42) | NS | … | … | ||
| Cesarean delivery | 800 (19.4) | 1 | 799 | NS | … | … | ||
| Clinical findings of newborns | ||||||||
| Birth weight, g | 3051.2 ± 372.5 | 2912 (2232–3840) | 3040 (1936–4676) | NS | … | … | ||
| Light-for-date | 219 (5.3) | 1 | 218 | NS | … | … | ||
| Low birth weight | 248 (6.0) | 1 | 247 | NS | … | … | ||
| Male | 2125 (51.5) | 5 | 2120 | NS | … | … | ||
| Abnormality of AABR screening test | 22 (0.5) | 1 | 21 | <.05 | … | … |
| Clinical Findings . | All Women (N = 4125) . | Women Who Had Newborns With Congenital CMV Infection (n = 9) . | Women Who Had Newborns Without Congenital CMV Infection (n = 4116) . | P Value . | Univariate Logistic Regression Analysis . | . | Multivariable Logistic Regression Analysis . | . |
|---|---|---|---|---|---|---|---|---|
| . | Average ± SD or No. (%) . | Median (Range) or No. . | Median (Range) or No. . | . | OR (95% CI) . | P Value . | OR (95% CI) . | P Value . |
| Clinical findings of pregnant women | ||||||||
| Age, y | 30.4 ± 5.1 | 28 (16–35) | 30 (16–46) | NS | … | … | ||
| Gravidity | 1.9 ± 1.1 | 2 (1–5) | 2 (1–14) | NS | … | … | ||
| Parity | 0.7 ± 0.8 | 1 (0–4) | 1 (0–5) | NS | … | … | ||
| Assisted reproductive technology therapy | 362 (8.8) | 0 | 362 | NS | … | … | ||
| BMI prior to pregnancy, kg/m2 | 20.7 ± 2.8 | 19.8 (18.2–27.5) | 20.2 (14.7–41.1) | NS | … | … | ||
| Smoking history | 272 (7.8) | 0 | 272 | NS | … | … | ||
| Fever or flu-like symptoms | 627 (15.2) | 7 | 620 | <.05 | 19.8 (4.1–95.7) | <.001 | 17.9 (3.7–86.7) | <.001 |
| Hypertensive disorders of pregnancy | 74 (1.8) | 1 | 73 | NS | … | … | ||
| Thyroid disease | 33 (0.8) | 0 | 33 | NS | … | … | ||
| DM/gestational DM | 74 (1.8) | 0 | 74 | NS | … | … | ||
| Medical diseases requiring immunosuppressive therapies | 4 (0.1) | 0 | 4 | NS | … | … | ||
| Threatened miscarriage in the first trimester | 255 (6.1) | 0 | 255 | NS | … | … | ||
| Threatened miscarriage or premature labor in the second trimester | 618 (15.0) | 5 | 613 | <.01 | 7.1 (1.9–26.7) | <.005 | 6.0 (1.6–22.8) | <.01 |
| Threatened premature labor in the third trimester | 1101 (26.6) | 4 | 1097 | NS | … | … | ||
| Preterm delivery | 103 (2.4) | 0 | 103 | NS | … | … | ||
| NRFS during labor | 136 (3.4) | 0 | 136 | NS | … | … | ||
| Gestational weeks at delivery | 39.1 ± 1.3 | 39 (38–41) | 39 (33–42) | NS | … | … | ||
| Cesarean delivery | 800 (19.4) | 1 | 799 | NS | … | … | ||
| Clinical findings of newborns | ||||||||
| Birth weight, g | 3051.2 ± 372.5 | 2912 (2232–3840) | 3040 (1936–4676) | NS | … | … | ||
| Light-for-date | 219 (5.3) | 1 | 218 | NS | … | … | ||
| Low birth weight | 248 (6.0) | 1 | 247 | NS | … | … | ||
| Male | 2125 (51.5) | 5 | 2120 | NS | … | … | ||
| Abnormality of AABR screening test | 22 (0.5) | 1 | 21 | <.05 | … | … |
Abbreviations: AABR, automated auditory brainstem response; BMI, body mass index; CI, confidence interval; CMV, cytomegalovirus; DM, diabetes mellitus; NRFS, nonreassuring fetal status; NS, not significant; OR, odds ratio; SD, standard deviation.
Clinical Characteristics of Participants and Results of Logistic Regression Analyses of Clinical Factors Associated With Congenital Cytomegalovirus Infection
| Clinical Findings . | All Women (N = 4125) . | Women Who Had Newborns With Congenital CMV Infection (n = 9) . | Women Who Had Newborns Without Congenital CMV Infection (n = 4116) . | P Value . | Univariate Logistic Regression Analysis . | . | Multivariable Logistic Regression Analysis . | . |
|---|---|---|---|---|---|---|---|---|
| . | Average ± SD or No. (%) . | Median (Range) or No. . | Median (Range) or No. . | . | OR (95% CI) . | P Value . | OR (95% CI) . | P Value . |
| Clinical findings of pregnant women | ||||||||
| Age, y | 30.4 ± 5.1 | 28 (16–35) | 30 (16–46) | NS | … | … | ||
| Gravidity | 1.9 ± 1.1 | 2 (1–5) | 2 (1–14) | NS | … | … | ||
| Parity | 0.7 ± 0.8 | 1 (0–4) | 1 (0–5) | NS | … | … | ||
| Assisted reproductive technology therapy | 362 (8.8) | 0 | 362 | NS | … | … | ||
| BMI prior to pregnancy, kg/m2 | 20.7 ± 2.8 | 19.8 (18.2–27.5) | 20.2 (14.7–41.1) | NS | … | … | ||
| Smoking history | 272 (7.8) | 0 | 272 | NS | … | … | ||
| Fever or flu-like symptoms | 627 (15.2) | 7 | 620 | <.05 | 19.8 (4.1–95.7) | <.001 | 17.9 (3.7–86.7) | <.001 |
| Hypertensive disorders of pregnancy | 74 (1.8) | 1 | 73 | NS | … | … | ||
| Thyroid disease | 33 (0.8) | 0 | 33 | NS | … | … | ||
| DM/gestational DM | 74 (1.8) | 0 | 74 | NS | … | … | ||
| Medical diseases requiring immunosuppressive therapies | 4 (0.1) | 0 | 4 | NS | … | … | ||
| Threatened miscarriage in the first trimester | 255 (6.1) | 0 | 255 | NS | … | … | ||
| Threatened miscarriage or premature labor in the second trimester | 618 (15.0) | 5 | 613 | <.01 | 7.1 (1.9–26.7) | <.005 | 6.0 (1.6–22.8) | <.01 |
| Threatened premature labor in the third trimester | 1101 (26.6) | 4 | 1097 | NS | … | … | ||
| Preterm delivery | 103 (2.4) | 0 | 103 | NS | … | … | ||
| NRFS during labor | 136 (3.4) | 0 | 136 | NS | … | … | ||
| Gestational weeks at delivery | 39.1 ± 1.3 | 39 (38–41) | 39 (33–42) | NS | … | … | ||
| Cesarean delivery | 800 (19.4) | 1 | 799 | NS | … | … | ||
| Clinical findings of newborns | ||||||||
| Birth weight, g | 3051.2 ± 372.5 | 2912 (2232–3840) | 3040 (1936–4676) | NS | … | … | ||
| Light-for-date | 219 (5.3) | 1 | 218 | NS | … | … | ||
| Low birth weight | 248 (6.0) | 1 | 247 | NS | … | … | ||
| Male | 2125 (51.5) | 5 | 2120 | NS | … | … | ||
| Abnormality of AABR screening test | 22 (0.5) | 1 | 21 | <.05 | … | … |
| Clinical Findings . | All Women (N = 4125) . | Women Who Had Newborns With Congenital CMV Infection (n = 9) . | Women Who Had Newborns Without Congenital CMV Infection (n = 4116) . | P Value . | Univariate Logistic Regression Analysis . | . | Multivariable Logistic Regression Analysis . | . |
|---|---|---|---|---|---|---|---|---|
| . | Average ± SD or No. (%) . | Median (Range) or No. . | Median (Range) or No. . | . | OR (95% CI) . | P Value . | OR (95% CI) . | P Value . |
| Clinical findings of pregnant women | ||||||||
| Age, y | 30.4 ± 5.1 | 28 (16–35) | 30 (16–46) | NS | … | … | ||
| Gravidity | 1.9 ± 1.1 | 2 (1–5) | 2 (1–14) | NS | … | … | ||
| Parity | 0.7 ± 0.8 | 1 (0–4) | 1 (0–5) | NS | … | … | ||
| Assisted reproductive technology therapy | 362 (8.8) | 0 | 362 | NS | … | … | ||
| BMI prior to pregnancy, kg/m2 | 20.7 ± 2.8 | 19.8 (18.2–27.5) | 20.2 (14.7–41.1) | NS | … | … | ||
| Smoking history | 272 (7.8) | 0 | 272 | NS | … | … | ||
| Fever or flu-like symptoms | 627 (15.2) | 7 | 620 | <.05 | 19.8 (4.1–95.7) | <.001 | 17.9 (3.7–86.7) | <.001 |
| Hypertensive disorders of pregnancy | 74 (1.8) | 1 | 73 | NS | … | … | ||
| Thyroid disease | 33 (0.8) | 0 | 33 | NS | … | … | ||
| DM/gestational DM | 74 (1.8) | 0 | 74 | NS | … | … | ||
| Medical diseases requiring immunosuppressive therapies | 4 (0.1) | 0 | 4 | NS | … | … | ||
| Threatened miscarriage in the first trimester | 255 (6.1) | 0 | 255 | NS | … | … | ||
| Threatened miscarriage or premature labor in the second trimester | 618 (15.0) | 5 | 613 | <.01 | 7.1 (1.9–26.7) | <.005 | 6.0 (1.6–22.8) | <.01 |
| Threatened premature labor in the third trimester | 1101 (26.6) | 4 | 1097 | NS | … | … | ||
| Preterm delivery | 103 (2.4) | 0 | 103 | NS | … | … | ||
| NRFS during labor | 136 (3.4) | 0 | 136 | NS | … | … | ||
| Gestational weeks at delivery | 39.1 ± 1.3 | 39 (38–41) | 39 (33–42) | NS | … | … | ||
| Cesarean delivery | 800 (19.4) | 1 | 799 | NS | … | … | ||
| Clinical findings of newborns | ||||||||
| Birth weight, g | 3051.2 ± 372.5 | 2912 (2232–3840) | 3040 (1936–4676) | NS | … | … | ||
| Light-for-date | 219 (5.3) | 1 | 218 | NS | … | … | ||
| Low birth weight | 248 (6.0) | 1 | 247 | NS | … | … | ||
| Male | 2125 (51.5) | 5 | 2120 | NS | … | … | ||
| Abnormality of AABR screening test | 22 (0.5) | 1 | 21 | <.05 | … | … |
Abbreviations: AABR, automated auditory brainstem response; BMI, body mass index; CI, confidence interval; CMV, cytomegalovirus; DM, diabetes mellitus; NRFS, nonreassuring fetal status; NS, not significant; OR, odds ratio; SD, standard deviation.
Two of 9 mothers who had newborns with cCMV and 201 of 4116 mothers who had newborns without cCMV received public assistance (P = .07). None of 9 mothers with cCMV newborns had sexually transmitted infections including hepatitis B virus, hepatitis C virus, human immunodeficiency virus, syphilis, chlamydia, or gonorrhea.
Table 2 shows the clinical characteristics and laboratory findings for 9 pregnant women who had newborns with cCMV. One of the 9 newborns was diagnosed with symptomatic cCMV due to AABR abnormality (case 1), while the remaining 8 newborns were asymptomatic. The newborn of case 1 received anti-CMV treatments including intravenous immunoglobulin infusion 250 mg/kg/day a week 2 times, and valganciclovir 16 mg/kg/day for 6 weeks. At present, he is 8 years and 3 months old without any sequela. The other 8 newborns also had no sequela without anti-CMV treatment. Neurodevelopment of 9 newborns with cCMV was assessed using the Kyoto scale of psychological development. Their developmental quotient (DQ) measured at 2 and/or 3 years old ranged from DQ 81 to DQ 105. Although complement fixation (CF) tests for CMV antibodies were not performed for all participants with informed consent, 3193 of a total 4125 pregnant women received CF tests, and 71.7% (2288/3193) had positive tests. Mothers of the 9 newborns with cCMV also received CF tests. Three mothers tested negative for CF tests, but further antibody tests were not performed.
Nine Pregnant Women Who Had Newborns With Congenital Cytomegalovirus Infection
| Case . | Age, y . | Gravidity/Parity . | BMI, kg/m2 . | Occupation . | Pregnancy Complications (GW) . | GW at Flu-like Symptoms . | CF Tests, Times (GW) . | Delivery Mode . | Gestational Age at Delivery . | Birth Weight, g . | Sex . | Blood Gas pH of Umbilical Artery . | Symptoms of Newborns . | Infant Development, Age . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 | 5/4 | 27.3 | None | None | 8 | 16 (21) | Cesarean | 38 w 4 d | 3160 | Male | 7.354 | AABR abnormality | Normal, 8 y and 3 mo |
| 2 | 16 | 2/0 | 18.2 | None | Threatened miscarriage (19) | 8 | 64 (7) | Vaginal | 41 w 3 d | 3620 | Female | 7.271 | None | Normal, 7 y and 9 mo |
| 3 | 28 | 3/1 | 27.5 | None | Threatened premature labor (24) | None | 32 (8) | Vaginal | 40 w 4 d | 3840 | Male | 7.300 | None | Normal, 7 y and 7 mo |
| 4 | 32 | 2/1 | 21.7 | None | Threatened premature labor (23) | None | 16 (8) | Vaginal | 38 w 5 d | 2606 | Female | 7.392 | None | Normal, 6 y and 5 mo |
| 5 | 35 | 3/2 | 23 | None | Threatened premature labor (34), HDP (36) | 24 | 16 (8) | Vaginal | 39 w 1 d | 2740 | Female | 7.385 | None | Normal, 6 y |
| 6 | 25 | 2/0 | 19.8 | Child carer | Threatened premature labor (23) | 29 | <4 (19) | Vaginal | 39 w 2 d | 2768 | Male | 7.324 | None | Normal, 5 y and 3 mo |
| 7 | 29 | 1/0 | 16.9 | Pharmacist | Threatened miscarriage (18) | 27 | 32 (9) | Vaginal | 39 w 3 d | 2232 | Male | 7.349 | Light-for-date, low birth weight | Normal, 2 y and 9 mo |
| 8 | 28 | 2/1 | 19.8 | Nurse | None | 29 | <4 (10) | Vaginal | 40 w 4 d | 2912 | Male | 7.365 | None | Normal, 2 y and 8 mo |
| 9 | 31 | 2/1 | 19.6 | None | Threatened premature labor (34) | 32 | <4 (9) | Vaginal | 38 w 3 d | 3248 | Female | 7.363 | None | Normal, 2 y and 5 mo |
| Case . | Age, y . | Gravidity/Parity . | BMI, kg/m2 . | Occupation . | Pregnancy Complications (GW) . | GW at Flu-like Symptoms . | CF Tests, Times (GW) . | Delivery Mode . | Gestational Age at Delivery . | Birth Weight, g . | Sex . | Blood Gas pH of Umbilical Artery . | Symptoms of Newborns . | Infant Development, Age . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 | 5/4 | 27.3 | None | None | 8 | 16 (21) | Cesarean | 38 w 4 d | 3160 | Male | 7.354 | AABR abnormality | Normal, 8 y and 3 mo |
| 2 | 16 | 2/0 | 18.2 | None | Threatened miscarriage (19) | 8 | 64 (7) | Vaginal | 41 w 3 d | 3620 | Female | 7.271 | None | Normal, 7 y and 9 mo |
| 3 | 28 | 3/1 | 27.5 | None | Threatened premature labor (24) | None | 32 (8) | Vaginal | 40 w 4 d | 3840 | Male | 7.300 | None | Normal, 7 y and 7 mo |
| 4 | 32 | 2/1 | 21.7 | None | Threatened premature labor (23) | None | 16 (8) | Vaginal | 38 w 5 d | 2606 | Female | 7.392 | None | Normal, 6 y and 5 mo |
| 5 | 35 | 3/2 | 23 | None | Threatened premature labor (34), HDP (36) | 24 | 16 (8) | Vaginal | 39 w 1 d | 2740 | Female | 7.385 | None | Normal, 6 y |
| 6 | 25 | 2/0 | 19.8 | Child carer | Threatened premature labor (23) | 29 | <4 (19) | Vaginal | 39 w 2 d | 2768 | Male | 7.324 | None | Normal, 5 y and 3 mo |
| 7 | 29 | 1/0 | 16.9 | Pharmacist | Threatened miscarriage (18) | 27 | 32 (9) | Vaginal | 39 w 3 d | 2232 | Male | 7.349 | Light-for-date, low birth weight | Normal, 2 y and 9 mo |
| 8 | 28 | 2/1 | 19.8 | Nurse | None | 29 | <4 (10) | Vaginal | 40 w 4 d | 2912 | Male | 7.365 | None | Normal, 2 y and 8 mo |
| 9 | 31 | 2/1 | 19.6 | None | Threatened premature labor (34) | 32 | <4 (9) | Vaginal | 38 w 3 d | 3248 | Female | 7.363 | None | Normal, 2 y and 5 mo |
Abbreviations: AABR, automated auditory brainstem response; BMI, body mass index; CF, complement fixation; CMV, cytomegalovirus; GW, gestational weeks; HDP, hypertensive disorders of pregnancy.
Nine Pregnant Women Who Had Newborns With Congenital Cytomegalovirus Infection
| Case . | Age, y . | Gravidity/Parity . | BMI, kg/m2 . | Occupation . | Pregnancy Complications (GW) . | GW at Flu-like Symptoms . | CF Tests, Times (GW) . | Delivery Mode . | Gestational Age at Delivery . | Birth Weight, g . | Sex . | Blood Gas pH of Umbilical Artery . | Symptoms of Newborns . | Infant Development, Age . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 | 5/4 | 27.3 | None | None | 8 | 16 (21) | Cesarean | 38 w 4 d | 3160 | Male | 7.354 | AABR abnormality | Normal, 8 y and 3 mo |
| 2 | 16 | 2/0 | 18.2 | None | Threatened miscarriage (19) | 8 | 64 (7) | Vaginal | 41 w 3 d | 3620 | Female | 7.271 | None | Normal, 7 y and 9 mo |
| 3 | 28 | 3/1 | 27.5 | None | Threatened premature labor (24) | None | 32 (8) | Vaginal | 40 w 4 d | 3840 | Male | 7.300 | None | Normal, 7 y and 7 mo |
| 4 | 32 | 2/1 | 21.7 | None | Threatened premature labor (23) | None | 16 (8) | Vaginal | 38 w 5 d | 2606 | Female | 7.392 | None | Normal, 6 y and 5 mo |
| 5 | 35 | 3/2 | 23 | None | Threatened premature labor (34), HDP (36) | 24 | 16 (8) | Vaginal | 39 w 1 d | 2740 | Female | 7.385 | None | Normal, 6 y |
| 6 | 25 | 2/0 | 19.8 | Child carer | Threatened premature labor (23) | 29 | <4 (19) | Vaginal | 39 w 2 d | 2768 | Male | 7.324 | None | Normal, 5 y and 3 mo |
| 7 | 29 | 1/0 | 16.9 | Pharmacist | Threatened miscarriage (18) | 27 | 32 (9) | Vaginal | 39 w 3 d | 2232 | Male | 7.349 | Light-for-date, low birth weight | Normal, 2 y and 9 mo |
| 8 | 28 | 2/1 | 19.8 | Nurse | None | 29 | <4 (10) | Vaginal | 40 w 4 d | 2912 | Male | 7.365 | None | Normal, 2 y and 8 mo |
| 9 | 31 | 2/1 | 19.6 | None | Threatened premature labor (34) | 32 | <4 (9) | Vaginal | 38 w 3 d | 3248 | Female | 7.363 | None | Normal, 2 y and 5 mo |
| Case . | Age, y . | Gravidity/Parity . | BMI, kg/m2 . | Occupation . | Pregnancy Complications (GW) . | GW at Flu-like Symptoms . | CF Tests, Times (GW) . | Delivery Mode . | Gestational Age at Delivery . | Birth Weight, g . | Sex . | Blood Gas pH of Umbilical Artery . | Symptoms of Newborns . | Infant Development, Age . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 | 5/4 | 27.3 | None | None | 8 | 16 (21) | Cesarean | 38 w 4 d | 3160 | Male | 7.354 | AABR abnormality | Normal, 8 y and 3 mo |
| 2 | 16 | 2/0 | 18.2 | None | Threatened miscarriage (19) | 8 | 64 (7) | Vaginal | 41 w 3 d | 3620 | Female | 7.271 | None | Normal, 7 y and 9 mo |
| 3 | 28 | 3/1 | 27.5 | None | Threatened premature labor (24) | None | 32 (8) | Vaginal | 40 w 4 d | 3840 | Male | 7.300 | None | Normal, 7 y and 7 mo |
| 4 | 32 | 2/1 | 21.7 | None | Threatened premature labor (23) | None | 16 (8) | Vaginal | 38 w 5 d | 2606 | Female | 7.392 | None | Normal, 6 y and 5 mo |
| 5 | 35 | 3/2 | 23 | None | Threatened premature labor (34), HDP (36) | 24 | 16 (8) | Vaginal | 39 w 1 d | 2740 | Female | 7.385 | None | Normal, 6 y |
| 6 | 25 | 2/0 | 19.8 | Child carer | Threatened premature labor (23) | 29 | <4 (19) | Vaginal | 39 w 2 d | 2768 | Male | 7.324 | None | Normal, 5 y and 3 mo |
| 7 | 29 | 1/0 | 16.9 | Pharmacist | Threatened miscarriage (18) | 27 | 32 (9) | Vaginal | 39 w 3 d | 2232 | Male | 7.349 | Light-for-date, low birth weight | Normal, 2 y and 9 mo |
| 8 | 28 | 2/1 | 19.8 | Nurse | None | 29 | <4 (10) | Vaginal | 40 w 4 d | 2912 | Male | 7.365 | None | Normal, 2 y and 8 mo |
| 9 | 31 | 2/1 | 19.6 | None | Threatened premature labor (34) | 32 | <4 (9) | Vaginal | 38 w 3 d | 3248 | Female | 7.363 | None | Normal, 2 y and 5 mo |
Abbreviations: AABR, automated auditory brainstem response; BMI, body mass index; CF, complement fixation; CMV, cytomegalovirus; GW, gestational weeks; HDP, hypertensive disorders of pregnancy.
Logistic regression analyses of clinical factors associated with the occurrence of cCMV among the pregnant women were performed. Univariate logistic regression analyses for findings shown in Table 1 demonstrated that the presence of maternal fever/flulike symptoms (odds ratio [OR], 19.8; 95% confidence interval [CI], 4.1–95.7; P < .001) and threatened miscarriage/premature labor in the second trimester (OR, 7.1; 95% CI, 1.9–26.7; P < .01) were associated with the occurrence of cCMV. Multivariable logistic regression analyses of the 2 factors revealed that the presence of maternal fever/flulike symptoms (OR, 17.9; 95% CI, 3.7–86.7; P < .001) and threatened miscarriage/premature labor in the second trimester (OR, 6.0; 95% CI, 1.6–22.8; P < .01) were clinical factors associated with the occurrence of cCMV in pregnant women who delivered at the primary maternity hospital.
The optimal predictive factors were estimated using the maximum value of the Youden index, which is defined as “sensitivity + specificity − 1.” As a result, the presence of maternal fever/flulike symptoms alone and threatened miscarriage/premature labor in the second trimester alone yielded sensitivity of 77.8% and 77.8%, specificity of 85.1% and 61.4%, and the Youden index of 0.63 and 0.39, respectively. Furthermore, combination of the presence of maternal fever/flulike symptoms or threatened miscarriage/premature labor in the second trimester were determined as optimal predictive factors, showing sensitivity of 100%, specificity of 53.2%, positive predictive value of 0.5%, negative predictive value of 100%, accuracy of 53.3%, and a maximum Youden index of 0.85.
DISCUSSION
In the present study, 9 (0.22%) of the 4125 women delivered newborns with cCMV. This frequency of cCMV was lower than that of previous reports, which showed 0.31%–0.46% in Japan [14, 17]. The present cohort study found that frequencies of maternal fever/flulike symptoms during pregnancy, threatened miscarriage/premature labor in the second trimester, and abnormality of AABR screening test for newborns were significantly higher in women who had newborns with cCMV. This study demonstrated for the first time that the presence of maternal fever/flulike symptoms during pregnancy and threatened miscarriage/premature labor in the second trimester were clinical factors associated with cCMV among pregnant women who delivered at a primary maternity hospital, where pregnant women at a high risk were referred or transferred to regional perinatal medical centers. The high-risk pregnancies included maternal complications, fetal abnormality, severe FGR, HDP, multiple pregnancy, and preterm delivery before 34 gestational weeks. It was likely that maternal fever/flulike symptoms and threatened miscarriage/premature labor in the second trimester were clinical factors associated with the occurrence of cCMV among women with low-risk pregnancies.
In the present study, not all pregnant women underwent CMV antibody tests, because some did not provide informed consent. However, mothers of the 9 newborns with cCMV coincidentally underwent CF tests once during pregnancy. Seven of the 9 pregnant women with cCMV had fever/flulike symptoms during pregnancy. Two women (case 1 and case 2) had fever/flulike symptoms in the first trimester and the other 2 (case 5 and case 7) in the second trimester. The remaining 3 with negative CF results (cases 6, 8, and 9) had fever/flulike symptoms in the third trimester, suggesting that at least the 3 had primary CMV infection during pregnancy. Case 1 had fever/flulike symptoms at 8 gestational weeks and a positive for CF test at 21 gestational weeks, so she might have had primary CMV infection during pregnancy. Cases 2, 3, 4, 5, and 7 might have had primary infection, reactivation, or reinfection of CMV during pregnancy, because they had positive CF tests in the first trimester. CF tests were known to be less sensitive for detecting CMV IgG antibody than tests using the radioimmunoassay (RIA) or enzyme-linked immunosorbent assay (ELISA) techniques. However, there were few discrepancies (1.5%) of results between the CF tests and tests using RIA or ELISA techniques as the screening tests for CMV antibody [18].
Maternal fever/flulike symptoms may be associated with primary infection or reinfection of CMV, causing CMV transmission to their fetuses. If pregnant women have fever/flulike symptoms during pregnancy, as soon as possible they should receive CMV antibody tests, including CMV IgG/IgM, and IgG avidity measurements, and plasma CMV DNA analysis to determine whether they have acquired CMV infection, because they may have a substantial risk for cCMV. Plasma CMV DNA analyses help to diagnose CMV infection.
Threatened miscarriage/premature labor in the second trimester may be caused by intrauterine infection as an effect of cCMV. Alternatively, threatened miscarriage/premature labor may be associated with local inflammation causing reactivation of latent CMV in the uterus and blood circulation. A cohort study also reported that threatened premature labor was a risk factor for cCMV in 1287 pregnant women with nonprimary CMV infection [19]. This study demonstrated that 5 of a total 7 women with cCMV had threatened premature labor during the second and third trimesters, and none during the first trimester. It is likely that reactivation of latent CMV or reinfection during the second and third trimesters cause cCMV more frequently than during the first trimester in women with nonprimary CMV infection. The present study did not find an association between cCMV and NRFS, although a previous study did [20].
Observational studies of neonatal CMV screening have found that frequencies of cCMV are 1.3% in very LBW newborns, 1.7%–3.7% in those small for gestational age, and 3.0% in preterm delivery [21–23]. Other studies also have shown that frequencies of cCMV are 2.9%–3.3% in multiple pregnancy, 0.8%–2.0% in threatened premature labor, 1.1%–1.4% in maternal fever/flulike symptoms, 0.8%–1.4% in LFD, 1.1%–1.4% in LBW, and 1.2%–1.3% in preterm delivery [17, 19]. A prospective study of 11 715 newborns screened by PCR tests for CMV DNA in saliva found that socioeconomic factors such as younger age (<25 years old), being a parous mother born in a high-resource country, and higher income were risk factors for cCMV due to maternal primary CMV infection. On the other hand, younger age and unemployment were found to be risk factors for cCMV caused by nonprimary CMV infection [12].
Recent observational studies demonstrated that the number and severity of symptoms in infants with cCMV from mothers with nonprimary CMV infection during pregnancy were similar to those from mothers with primary infection [9–11]. Furthermore, a majority of newborns with cCMV were born from mothers with nonprimary CMV infection during pregnancy [24]. A registry-based cohort study also found that a majority of newborns with symptomatic cCMV were from mothers with nonprimary CMV infection during pregnancy [10]. Recently, a prospective cohort study of CMV screening for 2193 pregnant women and their newborns in a perinatal medical center demonstrated that maternal antibody screening using CMV IgG, IgG avidity index, and IgM could identify pregnancies with cCMV due to maternal primary CMV infection accounting for 30% of cases; however, it overlooked those caused by nonprimary CMV infection, which accounted for 70% [17]. However, there have been no cohort studies to assess clinical findings predictive of cCMV in a primary maternity hospital that usually manages low-risk pregnancies. The present cohort study for the first time evaluated whether clinical factors of mothers and newborns in low-risk populations were associated with cCMV, and univariate and multivariable analyses demonstrated that maternal fever/flulike symptoms during pregnancy and threatened miscarriage/premature labor in the second trimester were associated with cCMV. Clinical factors of maternal fever/flulike symptoms or threatened miscarriage/premature labor in the second trimester were selected with a maximum Youden index of .85, showing 100% sensitivity and 100% negative predictive value. If frequency, severity, morbidity, and mortality of newborns with cCMV due to maternal nonprimary CMV infection is not different from those with cCMV caused by maternal primary CMV infection, prenatal risk estimation and prediction of cCMV based on manifestation of clinical symptoms on mothers, fetuses, and newborns in combination with CMV antibody measurements on that occasion may be more effective than universal antibody screening for pregnant women.
It remains controversial whether universal or targeted screening for cCMV based on PCR assays for CMV DNA in the saliva or urine of newborns is cost-effective. In targeted neonatal screening approaches, infants who are referred for AABR undergo PCR testing of the urine, but this strategy may overlook infants with asymptomatic cCMV or cases with a delayed onset of hearing loss [25]. In the present study, abnormality of AABR screening tests was significantly associated with cCMV. However, only 1 of the 21 newborns with AABR abnormality had cCMV, while the other 8 newborns with cCMV showed no AABR abnormality. The targeted neonatal CMV screening that is based on only results of AABR performed during 1–5 days after birth may not be effective for detecting cCMV.
Universal neonatal screening using saliva or urine for CMV DNA can identify almost all newborns with cCMV. If universal neonatal screening cannot be performed, neonatal CMV screening targeting not only neonates with AABR abnormality but also mothers who have maternal fever/flulike symptoms during pregnancy or threatened miscarriage/premature labor in the second trimester may be an effective method to detect cCMV with a high sensitivity. Clinical practitioners have to check CMV antibodies for mothers who have these risk factors. Early diagnoses of cCMV can lead to early commencement of antiviral therapies for infants with symptomatic cCMV to reduce sequelae.
These results will provide useful information for clinical practitioners to assess risks of cCMV in low-risk mothers, irrespective of primary or nonprimary CMV infection. The study of the transmission and potential harm after reactivation may be facilitated applying these clinical markers as well as strain-specific serology and characterization of the cCMV strain. However, the present study had some limitations. Threatened miscarriage or premature labor may be diagnosed somewhat subjectively depending on practitioners. The choice of tocolytic agents used for treatment of threatened miscarriage or premature labor differs between countries. Clinical factors associated with cCMV in high-risk pregnant women who are usually managed in tertiary maternofetal centers might be different. The findings in the present study are valid for low-risk pregnancies in this population, and must be followed by further studies in other population with a higher rate of cCMV. CMV serology, socioeconomic status, and education of pregnant women may influence the results.
Notes
Acknowledgments. The authors acknowledge and thank Shinya Tairaku, Masashi Deguchi, and Yasuhiko Ebina at the Department of Obstetrics and Gynecology, Kobe University Graduate School of Medicine for their advice. The authors are also grateful for the participation of the subjects and care provided by the staff at Kobe University Hospital and the clinical and laboratory personnel who supported this study at Kobe University Hospital.
Financial support. This work was supported by the Ministry of Health, Labour and Welfare of Japan (grant numbers H23-Jisedai-Ippan-001), and Japan Agency for Medical Research and Development (grant numbers AM55708030, JP19gk0110042, and JP19gk0110047).
Potential conflicts of interest. I. M. reports grants from Teijin Pharma Co, Ltd; Mochida Pharmaceutical Co, Ltd; Taisho Toyama Pharmaceutical Co, Ltd; and Air Water Inc; grants and personal fees from Japan Blood Product Organization; Daiichi Sankyo Co, Ltd; Merck Sharp and Dohme Co, Ltd; AbbVie LLC; Pfizer Japan, Inc; Shionogi Co, Ltd; Atom Medical Corporation; Chugai Pharmaceutical Co, Ltd; and JCR Pharmaceuticals Co, Ltd; personal fees from Novo Nordisk Pharma Ltd; Japan Vaccine Co, Ltd; Asahikasei Medical Co, Ltd; Mitsubishi Tanabe Pharma Corporation; Shino-test Corporation; Alexion Pharmaceutical Inc; and Nihon Kohden Corporation; and nonfinancial support from Sanofi K.K.; Atom Medical Corporation; Asahikasei Medical Co, Ltd; and Japan Blood Product Organization. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the Editors consider relevant to the content of the manuscript have been disclosed.




