-
PDF
- Split View
-
Views
-
Cite
Cite
Alain Amstutz, Jennifer Anne Brown, Isaac Ringera, Josephine Muhairwe, Thabo Ishmael Lejone, Thomas Klimkait, Tracy Renée Glass, Niklaus Daniel Labhardt, Engagement in Care, Viral Suppression, Drug Resistance, and Reasons for Nonengagement After Home-Based Same-Day Antiretroviral Therapy Initiation in Lesotho: A Two-Year Follow-up of the CASCADE Trial, Clinical Infectious Diseases, Volume 71, Issue 10, 15 November 2020, Pages 2608–2614, https://doi.org/10.1093/cid/ciz1126
Close - Share Icon Share
Abstract
The CASCADE trial showed that compared with usual care (UC), offering same-day (SD) antiretroviral therapy (ART) during home-based human immunodeficiency virus testing improved engagement in care and viral suppression 12 months after diagnosis. However, questions remain regarding long-term outcomes and the risk of propagating drug resistance.
After completion of the primary endpoint at 12 months, participants not in care in both arms were traced and encouraged to access care. At 24 months, the following outcomes were assessed in both arms: engagement in care, viral suppression, and reasons for nonengagement. Furthermore, we explored the acquisition of drug resistance mutations (DRMs) among SD arm nonlinkers.
At 24 months, 64% (88/137) in the SD arm vs 59% (81/137) in the UC arm were in care (absolute difference [AD], 5%; 95% confidence interval [CI], −6 to16; P = .38) and 57% (78/137) vs 54% (74/137) had documented viral suppression (AD, 3%; 95% CI, −9 to 15; P = .28). Among 36 participants alive and not in care at 24 months with ascertained status, the majority rejected contact with the health system or were unwilling to take ART. Among 8 interviewed SD arm nonlinkers, 6 had not initiated ART upon enrollment, and no acquired DRMs were detected. Two had taken the initial 30-day ART supply and acquired DRMs.
SD ART resulted in higher rates of engagement in care and viral suppression at 12 months but not at 24 months. Leveling off between both arms was driven by linkage beyond 12 months in the UC arm. We did not observe compensatory long-term disengagement in the SD arm. These long-term results endorse SD ART initiation policies.
NCT02692027.
(See the Editorial Commentary by Koenig and Pape on pages 2615–7.)
Human immunodeficiency virus (HIV) care and treatment programs globally adopted the recommendation to initiate lifelong antiretroviral therapy (ART) for all people living with HIV regardless of CD4 cell count [1]. A major challenge in implementing a universal test-and-treat strategy in sub-Saharan Africa is low linkage to care among individuals diagnosed as living with HIV [2–6]. This is particularly pronounced in the context of community-based testing, where less than half of those newly diagnosed link to care [7, 8]. Accelerated ART initiation, including starting ART on the day of confirmed HIV diagnosis, is a promising strategy to close the gap between testing and start of treatment. Despite 1 trial showing a trend toward higher loss to follow-up after rapid ART initiation [9], several recent randomized clinical trials from resource-limited settings [10–12] and 2 systematic reviews [13, 14] have concluded that this strategy can improve patient and program outcomes by increasing linkage to care, engagement in care, and sustained viral suppression. As a result, the World Health Organization (WHO) currently recommends offering immediate ART initiation [15].
The CASCADE trial, conducted in Lesotho, southern Africa, was the first of its kind to demonstrate the feasibility and effectiveness of home-based same-day (SD) ART initiation during a door-to-door HIV testing campaign. Offering SD ART start to individuals found to be living with HIV resulted in a significantly higher proportion linked to care within 3 months, as well as engaged in care and virally suppressed 12 months after the home-based HIV diagnosis [16].
However, knowledge gaps still remain relating to SD ART initiation. These include the long-term outcome, emergence of drug resistance among those who subsequently do not link to care, and data on reasons for nonlinkage to care despite the offer of SD ART. In this follow-up study among participants of the CASCADE trial, we aim to shed light on these knowledge gaps.
METHODS
Study Design and Participants
The CASCADE trial was a parallel-group, open-label, randomized clinical trial that assigned individuals who tested positive for HIV during a home-based HIV testing campaign to either the SD or usual care (UC) treatment arm (1:1 allocation). Study participants in the SD arm were offered home-based ART initiation on the day of HIV diagnosis after point-of-care baseline tests, a counseling session, and a readiness assessment. In the SD arm, 98% were considered ready, accepted SD ART start, and received a 30-day ART supply according to the national ART guidelines. In the UC arm, participants underwent a minimum of 2 pre-ART counseling sessions at the health facility with the subsequent offer to start ART, which was the standard of care in Lesotho at the time of enrollment. Consenting adults living with HIV who were ART-naive (aged ≥18 years) were eligible. The trial was conducted in the catchment area of 6 health facilities in the Butha-Buthe district in rural northern Lesotho. The detailed study protocol and the 12-month outcomes have been published [16, 17]. After assessment of the primary endpoint at 12 months, those not in care were contacted and encouraged to (re-) engage in care at the clinic. First contact attempts were made through phone (if available) and, if unsuccessful, were followed by physical tracing through village health workers, health facility staff, and/or the study nurse.
The original study protocol was approved by a Swiss Ethics Committee and the National Health and Research Ethics Committee of Lesotho. The study protocol was subsequently amended to conduct a 24-month follow-up and approved by the National Health and Research Committee of Lesotho. Participants provided written informed consent for a blood draw. Illiterate participants provided a thumb print, and a witness (independent to the trial and aged >21 years), chosen by the participant, cosigned the form. Informed consent was provided in the local language, Sesotho, and the participant received a copy of the consent form.
Procedures
At 24 months, the status of all participants was systematically assessed by the local study nurse responsible for the main trial. The following information sources were searched: the patient files at each health facility in the study district, the laboratory information system of the Ministry of Health, and the district-wide viral load (VL) database of the research consortium. Participants who were not in care (more than 2 months late for ART refill) were traced by the study nurse by phone (if available) or through home visits in collaboration with the clinic staff and the village health workers. Self-reported transfers to another clinic were followed up with the corresponding health facility to confirm the participants’ status. Participants from both arms who had disengaged from care by 24 months and who could be contacted were interviewed about reasons for leaving care.
SD arm participants who had never linked to care and were successfully traced were interviewed using a structured questionnaire to assess their reasons for not linking to care, their adherence to the initial 30-day ART supply, and their history of ART exposure before or since enrollment. Furthermore, they underwent venous blood draw to perform drug resistance testing. The EDTA blood samples were transported within 1 day to the hospital laboratory of the study district (Butha-Buthe Government Hospital) where plasma was separated and frozen at −80°C. Thereafter, plasma aliquots were shipped to a reference laboratory in Switzerland. Drug resistance was assessed using next-generation sequencing (NGS). HIV RNA was extracted using the Maxwell Viral Total Nucleic Acid Purification Kit (PROMEGA Corporation, Fitchburg, WI). Workup and NGS were conducted according to the protocol established by Mbunkah et al [18]. NGS data were processed using MinVar version 2.2.2 [19]. Drug resistance mutations (DRMs) were identified according to the Stanford HIV drug resistance database (www.hivdb.stanford.edu). In order to minimize the risk of false-negative results when assessing the potential harm of transient exposure to ART without subsequent linkage to care, an NGS cutoff of 1% was used.
Outcomes
In this study, we aimed to assess at 24 months (i) among all study participants: engagement in care, reasons for nonengagement, and viral suppression and (ii) among SD arm nonlinkers or late linkers: the acquisition of DRMs. Engagement in care was defined as at least 1 clinic visit in the 24-month follow-up window (range, 22–28 months) and included participants who transferred to any other health facility with a known outcome (documented proof of visit or laboratory report). Viral suppression was defined as <100 copies/mL, which is in line with the definition used in the CASCADE trial [16, 17].
Statistical Analyses
Participants were analyzed according to their randomization arm. The proportions with viral suppression and engagement in care were compared using the Pearson χ 2 or Fisher exact test and presented as absolute differences (ADs) with 95% confidence intervals (CIs) estimated using the Newcombe-Wilson score method [20]. Baseline characteristics potentially associated with engagement in care at 24 months were analyzed using a multivariate logistic regression model, followed by backward stepwise selection based on the Wald test (P value cutoff point of .15) and are presented as odds ratios (ORs) with their respective 95% CIs. As a sensitivity analysis, forward stepwise selection was performed with the same selection criteria. For all tests, complete case analysis and 2-sided P values with the significance level set at 0.05 were used. All analyses were performed in Stata (version 14, StataCorp, Austin, TX). The CASCADE trial has been registered on clinicaltrials.gov (NCT02692027).
RESULTS
From 22 February 2016 to 17 July 2016, 274 participants (137 per arm) were recruited. Baseline characteristics have been published [16]. The 24-month follow-up window started on 23 December 2017 and closed on 11 November 2018. Figure 1 displays the care cascade from enrollment until the 24-month follow-up. Of the 274 study participants, 64% (88/137) in the SD arm vs 59% (81/137) in the UC arm were in care 24 months after enrollment (AD, 5%; 95% CI, −6 to 16; P = .38), and 57% (78/137) vs 54% (74/137) had documented viral suppression (AD, 3%; 95% CI, −9 to 15; P = .28).
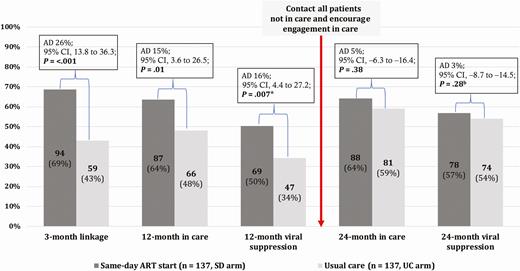
Care cascade in the CASCADE trial until 24-month follow-up. *In each arm, 10.2% (14/137) of participants had no documented viral load result despite having attended the health facility within the predefined outcome window. bFive participants in the UC arm and 4 participants in the SD arm had no documented viral load result despite having attended the health facility within the predefined outcome window. Abbreviations: AD, absolute difference; ART, antiretroviral therapy; CI, confidence interval; SD, same-day; UC, usual care.
Table 1 shows the detailed status of all study participants at 24 months. Figure 2 shows the dynamics of engagement in and disengagement from care during the 24-month follow-up period. Between month 12 and month 24, disengagement from care occurred at a similar rate in the SD (11%; 10 of 87) and the UC (9%; 6 of 66) arms (AD, 2%; 95% CI, −8 to 12, P = .63). New engagement in care during this period was higher in the UC arm (30%, 21/71) than the SD arm (22%, 11/50) (AD, 8%; 95% CI, −22.2 to 8.6; P = .35), though this difference was not statistically significant. In the logistic regression model, older age and known to be living with HIV before the home-based HIV testing campaign at enrollment were associated with higher engagement in care at 24 months (Table 2). Forward stepwise selection as a sensitivity analysis yielded the same results.
| . | Same-Day Arm (N = 137), . | Usual Care Arm (N = 137), . | Total (N = 274), . |
|---|---|---|---|
| 24-Month Status . | n (%) . | n (%) . | n (%) . |
| In care at 24 months, n (%) | 88 (64) | 81 (59) | 169 (62) |
| In care with suppressed VLa | 78 (57) | 74 (54) | 152 (56) |
| In care with unsuppressed VLb | 6 (4) | 2 (2) | 8 (3) |
| In care but without VL in 24-month windowc | 4 (3) | 5 (4) | 9 (3) |
| Not in care at 24 months, n (%) | 49 (36) | 56 (41) | 105 (38) |
| Dead | 3 (2) | 1 (1) | 4 (2) |
| Lost to follow-up | 14 (10) | 18 (13) | 32 (12) |
| Unconfirmed transfer out | 17 (12) | 16 (12) | 33 (12) |
| Traced, alive, no transfer out reportedd | 15 (11) | 21 (15) | 36 (13) |
| . | Same-Day Arm (N = 137), . | Usual Care Arm (N = 137), . | Total (N = 274), . |
|---|---|---|---|
| 24-Month Status . | n (%) . | n (%) . | n (%) . |
| In care at 24 months, n (%) | 88 (64) | 81 (59) | 169 (62) |
| In care with suppressed VLa | 78 (57) | 74 (54) | 152 (56) |
| In care with unsuppressed VLb | 6 (4) | 2 (2) | 8 (3) |
| In care but without VL in 24-month windowc | 4 (3) | 5 (4) | 9 (3) |
| Not in care at 24 months, n (%) | 49 (36) | 56 (41) | 105 (38) |
| Dead | 3 (2) | 1 (1) | 4 (2) |
| Lost to follow-up | 14 (10) | 18 (13) | 32 (12) |
| Unconfirmed transfer out | 17 (12) | 16 (12) | 33 (12) |
| Traced, alive, no transfer out reportedd | 15 (11) | 21 (15) | 36 (13) |
Abbreviation: VL, viral load.
aIncluding 11 confirmed transfers out.
bIncluding 1 confirmed transfer out.
cIncluding 2 confirmed transfers out.
dContact with participant in person or by telephone, or status confirmed by village health worker or relative. For details, see Supplement Table 2.
| . | Same-Day Arm (N = 137), . | Usual Care Arm (N = 137), . | Total (N = 274), . |
|---|---|---|---|
| 24-Month Status . | n (%) . | n (%) . | n (%) . |
| In care at 24 months, n (%) | 88 (64) | 81 (59) | 169 (62) |
| In care with suppressed VLa | 78 (57) | 74 (54) | 152 (56) |
| In care with unsuppressed VLb | 6 (4) | 2 (2) | 8 (3) |
| In care but without VL in 24-month windowc | 4 (3) | 5 (4) | 9 (3) |
| Not in care at 24 months, n (%) | 49 (36) | 56 (41) | 105 (38) |
| Dead | 3 (2) | 1 (1) | 4 (2) |
| Lost to follow-up | 14 (10) | 18 (13) | 32 (12) |
| Unconfirmed transfer out | 17 (12) | 16 (12) | 33 (12) |
| Traced, alive, no transfer out reportedd | 15 (11) | 21 (15) | 36 (13) |
| . | Same-Day Arm (N = 137), . | Usual Care Arm (N = 137), . | Total (N = 274), . |
|---|---|---|---|
| 24-Month Status . | n (%) . | n (%) . | n (%) . |
| In care at 24 months, n (%) | 88 (64) | 81 (59) | 169 (62) |
| In care with suppressed VLa | 78 (57) | 74 (54) | 152 (56) |
| In care with unsuppressed VLb | 6 (4) | 2 (2) | 8 (3) |
| In care but without VL in 24-month windowc | 4 (3) | 5 (4) | 9 (3) |
| Not in care at 24 months, n (%) | 49 (36) | 56 (41) | 105 (38) |
| Dead | 3 (2) | 1 (1) | 4 (2) |
| Lost to follow-up | 14 (10) | 18 (13) | 32 (12) |
| Unconfirmed transfer out | 17 (12) | 16 (12) | 33 (12) |
| Traced, alive, no transfer out reportedd | 15 (11) | 21 (15) | 36 (13) |
Abbreviation: VL, viral load.
aIncluding 11 confirmed transfers out.
bIncluding 1 confirmed transfer out.
cIncluding 2 confirmed transfers out.
dContact with participant in person or by telephone, or status confirmed by village health worker or relative. For details, see Supplement Table 2.
Association Between Baseline Characteristics and Engagement in Care at 24 Months
| . | . | Multivariate Logistic Regression (LR χ2 = 32.92, P = .01) . | . | . | Backward Selection (P Value Cutoff = .15) . | . | . |
|---|---|---|---|---|---|---|---|
| Baseline Characteristic . | n (%) . | aOR (95% CI) . | β Coefficient . | P Value . | aOR (95% CI) . | β Coefficient . | P Value . |
| Same-day arm vs usual care arm | 137 (50) | 1.28 (.74–2.23) | 0.25 | .379 | … | … | |
| Age (per year), median (interquartile range) | 39 (28–52) | 1.04 (1.02–1.07) | 0.04 | .001 | 1.02 (1.00–1.04) | 0.02 | .017 |
| Female vs male | 180 (66) | 1.48 (.77–2.87) | 0.39 | .241 | … | … | |
| Marital statusa | |||||||
| Single | 35 (13) | 1 | – | – | … | … | … |
| Married/lives with partner | 177 (65) | 0.73 (.31–1.75) | −0.31 | .484 | … | … | |
| Widowed | 60 (22) | 0.21 (.06–.67) | −1.57 | .008 | … | … | |
| Completed years of school | |||||||
| Primary not completed | 132 (48) | 1 | – | – | … | … | |
| Primary completed | 120 (44) | 1.45 (.76–2.77) | 0.37 | .262 | … | … | |
| Secondary completed | 18 (7) | 0.61 (.19–1.98) | –0.49 | .412 | … | … | |
| Tertiary completed | 4 (1) | 0.69 (.08–5.93) | –0.37 | .734 | … | … | |
| Employment | |||||||
| In Lesotho with regular income | 54 (20) | 1 | – | – | … | … | |
| Outside Lesotho | 9 (3) | 0.29 (.05–1.52) | −1.25 | .142 | … | … | |
| No regular income | 211 (77) | 0.61 (.29–1.27) | −0.50 | .183 | … | … | |
| Known living with HIV vs newly diagnosed living with HIV | 71 (26) | 2.55 (1.26–5.18) | 0.94 | .009 | 2.43 (1.31–4.51) | 0.89 | .05 |
| Plan to disclose to someoneb | |||||||
| Yes | 235 (86) | 1 | – | – | … | … | |
| No, not for the moment | 19 (7) | 0.73 (.25–2.09) | −0.32 | .557 | … | … | |
| I don’t know yet | 13 (5) | 1.76 (.46–6.74) | 0.56 | .412 | … | … | |
| World Health Organization stagec | |||||||
| I (asymptomatic) | 211 (77) | 1 | – | – | … | … | |
| II (oligosymptomatic) | 48 (18) | 1.36 (.64–2.90) | 0.31 | .419 | … | … | |
| III (advanced) | 11 (4) | 1.96 (.48–8.07) | 0.67 | .351 | … | … | |
| CD4 cell count levels, cells/μLc | |||||||
| <200 | 44 (16) | 1 | – | – | … | … | |
| 200–349 | 76 (28) | 2.22 (.94–5.26) | 0.80 | .070 | … | … | |
| ≥350 | 150 (55) | 1.55 (.69–3.48) | 0.44 | .284 | … | … |
| . | . | Multivariate Logistic Regression (LR χ2 = 32.92, P = .01) . | . | . | Backward Selection (P Value Cutoff = .15) . | . | . |
|---|---|---|---|---|---|---|---|
| Baseline Characteristic . | n (%) . | aOR (95% CI) . | β Coefficient . | P Value . | aOR (95% CI) . | β Coefficient . | P Value . |
| Same-day arm vs usual care arm | 137 (50) | 1.28 (.74–2.23) | 0.25 | .379 | … | … | |
| Age (per year), median (interquartile range) | 39 (28–52) | 1.04 (1.02–1.07) | 0.04 | .001 | 1.02 (1.00–1.04) | 0.02 | .017 |
| Female vs male | 180 (66) | 1.48 (.77–2.87) | 0.39 | .241 | … | … | |
| Marital statusa | |||||||
| Single | 35 (13) | 1 | – | – | … | … | … |
| Married/lives with partner | 177 (65) | 0.73 (.31–1.75) | −0.31 | .484 | … | … | |
| Widowed | 60 (22) | 0.21 (.06–.67) | −1.57 | .008 | … | … | |
| Completed years of school | |||||||
| Primary not completed | 132 (48) | 1 | – | – | … | … | |
| Primary completed | 120 (44) | 1.45 (.76–2.77) | 0.37 | .262 | … | … | |
| Secondary completed | 18 (7) | 0.61 (.19–1.98) | –0.49 | .412 | … | … | |
| Tertiary completed | 4 (1) | 0.69 (.08–5.93) | –0.37 | .734 | … | … | |
| Employment | |||||||
| In Lesotho with regular income | 54 (20) | 1 | – | – | … | … | |
| Outside Lesotho | 9 (3) | 0.29 (.05–1.52) | −1.25 | .142 | … | … | |
| No regular income | 211 (77) | 0.61 (.29–1.27) | −0.50 | .183 | … | … | |
| Known living with HIV vs newly diagnosed living with HIV | 71 (26) | 2.55 (1.26–5.18) | 0.94 | .009 | 2.43 (1.31–4.51) | 0.89 | .05 |
| Plan to disclose to someoneb | |||||||
| Yes | 235 (86) | 1 | – | – | … | … | |
| No, not for the moment | 19 (7) | 0.73 (.25–2.09) | −0.32 | .557 | … | … | |
| I don’t know yet | 13 (5) | 1.76 (.46–6.74) | 0.56 | .412 | … | … | |
| World Health Organization stagec | |||||||
| I (asymptomatic) | 211 (77) | 1 | – | – | … | … | |
| II (oligosymptomatic) | 48 (18) | 1.36 (.64–2.90) | 0.31 | .419 | … | … | |
| III (advanced) | 11 (4) | 1.96 (.48–8.07) | 0.67 | .351 | … | … | |
| CD4 cell count levels, cells/μLc | |||||||
| <200 | 44 (16) | 1 | – | – | … | … | |
| 200–349 | 76 (28) | 2.22 (.94–5.26) | 0.80 | .070 | … | … | |
| ≥350 | 150 (55) | 1.55 (.69–3.48) | 0.44 | .284 | … | … |
Complete-case regression analysis (N = 257).
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; HIV, human immunodeficiency virus; LR, likelihood ratio; (–)N/A, freestanding dashes.
aData from 2 participants missing.
bData from 7 participants missing.
cData from 4 participants missing.
Association Between Baseline Characteristics and Engagement in Care at 24 Months
| . | . | Multivariate Logistic Regression (LR χ2 = 32.92, P = .01) . | . | . | Backward Selection (P Value Cutoff = .15) . | . | . |
|---|---|---|---|---|---|---|---|
| Baseline Characteristic . | n (%) . | aOR (95% CI) . | β Coefficient . | P Value . | aOR (95% CI) . | β Coefficient . | P Value . |
| Same-day arm vs usual care arm | 137 (50) | 1.28 (.74–2.23) | 0.25 | .379 | … | … | |
| Age (per year), median (interquartile range) | 39 (28–52) | 1.04 (1.02–1.07) | 0.04 | .001 | 1.02 (1.00–1.04) | 0.02 | .017 |
| Female vs male | 180 (66) | 1.48 (.77–2.87) | 0.39 | .241 | … | … | |
| Marital statusa | |||||||
| Single | 35 (13) | 1 | – | – | … | … | … |
| Married/lives with partner | 177 (65) | 0.73 (.31–1.75) | −0.31 | .484 | … | … | |
| Widowed | 60 (22) | 0.21 (.06–.67) | −1.57 | .008 | … | … | |
| Completed years of school | |||||||
| Primary not completed | 132 (48) | 1 | – | – | … | … | |
| Primary completed | 120 (44) | 1.45 (.76–2.77) | 0.37 | .262 | … | … | |
| Secondary completed | 18 (7) | 0.61 (.19–1.98) | –0.49 | .412 | … | … | |
| Tertiary completed | 4 (1) | 0.69 (.08–5.93) | –0.37 | .734 | … | … | |
| Employment | |||||||
| In Lesotho with regular income | 54 (20) | 1 | – | – | … | … | |
| Outside Lesotho | 9 (3) | 0.29 (.05–1.52) | −1.25 | .142 | … | … | |
| No regular income | 211 (77) | 0.61 (.29–1.27) | −0.50 | .183 | … | … | |
| Known living with HIV vs newly diagnosed living with HIV | 71 (26) | 2.55 (1.26–5.18) | 0.94 | .009 | 2.43 (1.31–4.51) | 0.89 | .05 |
| Plan to disclose to someoneb | |||||||
| Yes | 235 (86) | 1 | – | – | … | … | |
| No, not for the moment | 19 (7) | 0.73 (.25–2.09) | −0.32 | .557 | … | … | |
| I don’t know yet | 13 (5) | 1.76 (.46–6.74) | 0.56 | .412 | … | … | |
| World Health Organization stagec | |||||||
| I (asymptomatic) | 211 (77) | 1 | – | – | … | … | |
| II (oligosymptomatic) | 48 (18) | 1.36 (.64–2.90) | 0.31 | .419 | … | … | |
| III (advanced) | 11 (4) | 1.96 (.48–8.07) | 0.67 | .351 | … | … | |
| CD4 cell count levels, cells/μLc | |||||||
| <200 | 44 (16) | 1 | – | – | … | … | |
| 200–349 | 76 (28) | 2.22 (.94–5.26) | 0.80 | .070 | … | … | |
| ≥350 | 150 (55) | 1.55 (.69–3.48) | 0.44 | .284 | … | … |
| . | . | Multivariate Logistic Regression (LR χ2 = 32.92, P = .01) . | . | . | Backward Selection (P Value Cutoff = .15) . | . | . |
|---|---|---|---|---|---|---|---|
| Baseline Characteristic . | n (%) . | aOR (95% CI) . | β Coefficient . | P Value . | aOR (95% CI) . | β Coefficient . | P Value . |
| Same-day arm vs usual care arm | 137 (50) | 1.28 (.74–2.23) | 0.25 | .379 | … | … | |
| Age (per year), median (interquartile range) | 39 (28–52) | 1.04 (1.02–1.07) | 0.04 | .001 | 1.02 (1.00–1.04) | 0.02 | .017 |
| Female vs male | 180 (66) | 1.48 (.77–2.87) | 0.39 | .241 | … | … | |
| Marital statusa | |||||||
| Single | 35 (13) | 1 | – | – | … | … | … |
| Married/lives with partner | 177 (65) | 0.73 (.31–1.75) | −0.31 | .484 | … | … | |
| Widowed | 60 (22) | 0.21 (.06–.67) | −1.57 | .008 | … | … | |
| Completed years of school | |||||||
| Primary not completed | 132 (48) | 1 | – | – | … | … | |
| Primary completed | 120 (44) | 1.45 (.76–2.77) | 0.37 | .262 | … | … | |
| Secondary completed | 18 (7) | 0.61 (.19–1.98) | –0.49 | .412 | … | … | |
| Tertiary completed | 4 (1) | 0.69 (.08–5.93) | –0.37 | .734 | … | … | |
| Employment | |||||||
| In Lesotho with regular income | 54 (20) | 1 | – | – | … | … | |
| Outside Lesotho | 9 (3) | 0.29 (.05–1.52) | −1.25 | .142 | … | … | |
| No regular income | 211 (77) | 0.61 (.29–1.27) | −0.50 | .183 | … | … | |
| Known living with HIV vs newly diagnosed living with HIV | 71 (26) | 2.55 (1.26–5.18) | 0.94 | .009 | 2.43 (1.31–4.51) | 0.89 | .05 |
| Plan to disclose to someoneb | |||||||
| Yes | 235 (86) | 1 | – | – | … | … | |
| No, not for the moment | 19 (7) | 0.73 (.25–2.09) | −0.32 | .557 | … | … | |
| I don’t know yet | 13 (5) | 1.76 (.46–6.74) | 0.56 | .412 | … | … | |
| World Health Organization stagec | |||||||
| I (asymptomatic) | 211 (77) | 1 | – | – | … | … | |
| II (oligosymptomatic) | 48 (18) | 1.36 (.64–2.90) | 0.31 | .419 | … | … | |
| III (advanced) | 11 (4) | 1.96 (.48–8.07) | 0.67 | .351 | … | … | |
| CD4 cell count levels, cells/μLc | |||||||
| <200 | 44 (16) | 1 | – | – | … | … | |
| 200–349 | 76 (28) | 2.22 (.94–5.26) | 0.80 | .070 | … | … | |
| ≥350 | 150 (55) | 1.55 (.69–3.48) | 0.44 | .284 | … | … |
Complete-case regression analysis (N = 257).
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; HIV, human immunodeficiency virus; LR, likelihood ratio; (–)N/A, freestanding dashes.
aData from 2 participants missing.
bData from 7 participants missing.
cData from 4 participants missing.
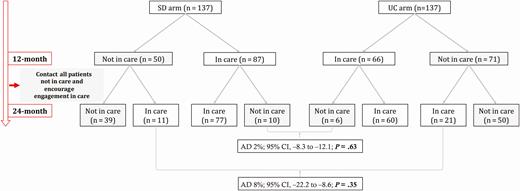
Dynamics of engagement in and disengagement from care in the CASCADE trial. Abbreviations: AD, absolute difference; CI, confidence interval; SD, same-day; UC, usual care.
Reasons for nonengagement in care for those alive and not in care at 24 months who could be traced are listed in Supplementary Table 1. The main reasons cited included rejection of contact with the healthcare system, rejection of ART due to skepticism related to the HIV diagnosis or ART, and unwillingness/unreadiness to take ART; only a minority of reasons were structural.
In the SD arm, 43 participants had not linked to care within 3 months and were thus potentially exposed to transient ART. Their 24-month outcomes are shown in Table 3. Ten (23%) linked to care after 3 months and remained in care at 24 months. Among these 10, 9 had VL measurement within the 24-month window and 7 of these had a suppressed VL. The other 2 had a VL of 144 and 3180 copies/mL.
| Outcome . | Total (N = 43), n (%) . |
|---|---|
| Late linkers: linked to care >3 months after enrollment . | Subtotal: 13 (30) . |
| In care at 24 monthsa | 10 (23) |
| In care with suppressed VL | 7 |
| In care with unsuppressed VL | 2 |
| In care but without VL in 24-month window | 1 |
| Dead | 1 (2) |
| Lost to follow-up | 2 (5) |
| Unconfirmed transfer out | 0 (0) |
| Nonlinkers: never linked to care | Subtotal: 30 (70) |
| Dead | 2 (5) |
| Lost to follow-up | 6 (14) |
| Unconfirmed transfer out | 9 (21) |
| Traced, alive, no transfer out reportedb | 13 (30) |
| Only village health worker reached or participant reached but did not agree to interview and phlebotomy | 5 |
| Reached and agreed to interview and phlebotomy (see Table 4) | 8 |
| Outcome . | Total (N = 43), n (%) . |
|---|---|
| Late linkers: linked to care >3 months after enrollment . | Subtotal: 13 (30) . |
| In care at 24 monthsa | 10 (23) |
| In care with suppressed VL | 7 |
| In care with unsuppressed VL | 2 |
| In care but without VL in 24-month window | 1 |
| Dead | 1 (2) |
| Lost to follow-up | 2 (5) |
| Unconfirmed transfer out | 0 (0) |
| Nonlinkers: never linked to care | Subtotal: 30 (70) |
| Dead | 2 (5) |
| Lost to follow-up | 6 (14) |
| Unconfirmed transfer out | 9 (21) |
| Traced, alive, no transfer out reportedb | 13 (30) |
| Only village health worker reached or participant reached but did not agree to interview and phlebotomy | 5 |
| Reached and agreed to interview and phlebotomy (see Table 4) | 8 |
Abbreviations: VL, viral load.
aIncluding confirmed transfer out.
bContact with participant in person or by telephone, or status confirmed by village health worker.
| Outcome . | Total (N = 43), n (%) . |
|---|---|
| Late linkers: linked to care >3 months after enrollment . | Subtotal: 13 (30) . |
| In care at 24 monthsa | 10 (23) |
| In care with suppressed VL | 7 |
| In care with unsuppressed VL | 2 |
| In care but without VL in 24-month window | 1 |
| Dead | 1 (2) |
| Lost to follow-up | 2 (5) |
| Unconfirmed transfer out | 0 (0) |
| Nonlinkers: never linked to care | Subtotal: 30 (70) |
| Dead | 2 (5) |
| Lost to follow-up | 6 (14) |
| Unconfirmed transfer out | 9 (21) |
| Traced, alive, no transfer out reportedb | 13 (30) |
| Only village health worker reached or participant reached but did not agree to interview and phlebotomy | 5 |
| Reached and agreed to interview and phlebotomy (see Table 4) | 8 |
| Outcome . | Total (N = 43), n (%) . |
|---|---|
| Late linkers: linked to care >3 months after enrollment . | Subtotal: 13 (30) . |
| In care at 24 monthsa | 10 (23) |
| In care with suppressed VL | 7 |
| In care with unsuppressed VL | 2 |
| In care but without VL in 24-month window | 1 |
| Dead | 1 (2) |
| Lost to follow-up | 2 (5) |
| Unconfirmed transfer out | 0 (0) |
| Nonlinkers: never linked to care | Subtotal: 30 (70) |
| Dead | 2 (5) |
| Lost to follow-up | 6 (14) |
| Unconfirmed transfer out | 9 (21) |
| Traced, alive, no transfer out reportedb | 13 (30) |
| Only village health worker reached or participant reached but did not agree to interview and phlebotomy | 5 |
| Reached and agreed to interview and phlebotomy (see Table 4) | 8 |
Abbreviations: VL, viral load.
aIncluding confirmed transfer out.
bContact with participant in person or by telephone, or status confirmed by village health worker.
Among those in the SD arm who never linked to care, 8 could be reached and agreed to an interview and phlebotomy for drug resistance testing. Their self-reported adherence to the initial 30-day supply of ART, reasons for not linking, as well as baseline and 24-month NGS drug resistance results are listed in Table 4. Among those 8, 2 reported initial adherence to the 30-day ART supply. In comparison to baseline, new DRMs were detectable in both (participant CA194: V106M; participant CA336: K103N, P225H) at 24 months. The remaining 6 stated that they never started ART; in those participants, no new DRMs were detected at 24 months.
Individual 24-Month Outcomes of a Subsample of Same-Day Arm Participants Who Never Linked to Care
| Patient . | Self-reported Adherence to Initial 30-Day ART Supply . | Reasons for Nonlinkage to Care After Same-Day ART Offer . | Resistance-Associated Mutations (Prevalence in %) at Baselinea . | Resistance-Associated Mutations (Prevalence in %) at 24 Monthsa . |
|---|---|---|---|---|
| 1 | All pills taken; full adherence (once daily) | Cost of transport to clinic | K103N (23) | K103N (84) V106M (18) |
| 2 | All pills taken; likely mistakenly taken twice daily | Perceived poor treatment from healthcare professionals | None | K103N (100) P225H (14) |
| 3 | No pills taken | Reliance on traditional medicine | n.d. | None |
| 4 | No pills taken | Fear of being on medication; not feeling ready for lifelong treatment | n.d. | n.d. |
| 5 | No pills takenb | Fear of being on medication; not feeling well-informed about ART regimen | K103N (100) | K103N (100) |
| 6 | No pills taken | Fear of being on medication; not feeling certain that he/she would be able to take medication correctly | None | None |
| 7 | No pills taken | Reliance on traditional medicine; travel to South Africa for work | None | None |
| 8 | No pills taken | Not believing in human immunodeficiency virus diagnosis | n.d. | n.d. |
| Patient . | Self-reported Adherence to Initial 30-Day ART Supply . | Reasons for Nonlinkage to Care After Same-Day ART Offer . | Resistance-Associated Mutations (Prevalence in %) at Baselinea . | Resistance-Associated Mutations (Prevalence in %) at 24 Monthsa . |
|---|---|---|---|---|
| 1 | All pills taken; full adherence (once daily) | Cost of transport to clinic | K103N (23) | K103N (84) V106M (18) |
| 2 | All pills taken; likely mistakenly taken twice daily | Perceived poor treatment from healthcare professionals | None | K103N (100) P225H (14) |
| 3 | No pills taken | Reliance on traditional medicine | n.d. | None |
| 4 | No pills taken | Fear of being on medication; not feeling ready for lifelong treatment | n.d. | n.d. |
| 5 | No pills takenb | Fear of being on medication; not feeling well-informed about ART regimen | K103N (100) | K103N (100) |
| 6 | No pills taken | Fear of being on medication; not feeling certain that he/she would be able to take medication correctly | None | None |
| 7 | No pills taken | Reliance on traditional medicine; travel to South Africa for work | None | None |
| 8 | No pills taken | Not believing in human immunodeficiency virus diagnosis | n.d. | n.d. |
Abbreviations: ART, antiretroviral therapy; n.d., no data.
aExcluding polymorphic resistance-associated mutations that are prevalent at similar frequencies (within 5 percentage points) at baseline and 24 months or, if baseline data are not available, could not be selected by the given drug regimen (ie, polymorphic protease or integrase mutations).
bParticipant indicated prior exposure to antiretroviral drugs in the context of prevention of mother-to-child transmission strategies.
Individual 24-Month Outcomes of a Subsample of Same-Day Arm Participants Who Never Linked to Care
| Patient . | Self-reported Adherence to Initial 30-Day ART Supply . | Reasons for Nonlinkage to Care After Same-Day ART Offer . | Resistance-Associated Mutations (Prevalence in %) at Baselinea . | Resistance-Associated Mutations (Prevalence in %) at 24 Monthsa . |
|---|---|---|---|---|
| 1 | All pills taken; full adherence (once daily) | Cost of transport to clinic | K103N (23) | K103N (84) V106M (18) |
| 2 | All pills taken; likely mistakenly taken twice daily | Perceived poor treatment from healthcare professionals | None | K103N (100) P225H (14) |
| 3 | No pills taken | Reliance on traditional medicine | n.d. | None |
| 4 | No pills taken | Fear of being on medication; not feeling ready for lifelong treatment | n.d. | n.d. |
| 5 | No pills takenb | Fear of being on medication; not feeling well-informed about ART regimen | K103N (100) | K103N (100) |
| 6 | No pills taken | Fear of being on medication; not feeling certain that he/she would be able to take medication correctly | None | None |
| 7 | No pills taken | Reliance on traditional medicine; travel to South Africa for work | None | None |
| 8 | No pills taken | Not believing in human immunodeficiency virus diagnosis | n.d. | n.d. |
| Patient . | Self-reported Adherence to Initial 30-Day ART Supply . | Reasons for Nonlinkage to Care After Same-Day ART Offer . | Resistance-Associated Mutations (Prevalence in %) at Baselinea . | Resistance-Associated Mutations (Prevalence in %) at 24 Monthsa . |
|---|---|---|---|---|
| 1 | All pills taken; full adherence (once daily) | Cost of transport to clinic | K103N (23) | K103N (84) V106M (18) |
| 2 | All pills taken; likely mistakenly taken twice daily | Perceived poor treatment from healthcare professionals | None | K103N (100) P225H (14) |
| 3 | No pills taken | Reliance on traditional medicine | n.d. | None |
| 4 | No pills taken | Fear of being on medication; not feeling ready for lifelong treatment | n.d. | n.d. |
| 5 | No pills takenb | Fear of being on medication; not feeling well-informed about ART regimen | K103N (100) | K103N (100) |
| 6 | No pills taken | Fear of being on medication; not feeling certain that he/she would be able to take medication correctly | None | None |
| 7 | No pills taken | Reliance on traditional medicine; travel to South Africa for work | None | None |
| 8 | No pills taken | Not believing in human immunodeficiency virus diagnosis | n.d. | n.d. |
Abbreviations: ART, antiretroviral therapy; n.d., no data.
aExcluding polymorphic resistance-associated mutations that are prevalent at similar frequencies (within 5 percentage points) at baseline and 24 months or, if baseline data are not available, could not be selected by the given drug regimen (ie, polymorphic protease or integrase mutations).
bParticipant indicated prior exposure to antiretroviral drugs in the context of prevention of mother-to-child transmission strategies.
DISCUSSION
The CASCADE trial has shown that offering SD ART initiation after home-based HIV testing significantly increases the proportion of patients engaged in care with viral suppression 12 months after diagnosis [16]. In this follow-up study, we assessed the 24-month status of care among all participants of the CASCADE trial, the emergence of drug resistance among those receiving home-based SD ART who subsequently did not link to care, and their reasons for not linking to care. To our knowledge, we are the first to report on 24-month outcomes after SD ART initiation in resource-limited settings.
Two years after testing positive for HIV, a significant difference in engagement in care and viral suppression was no longer observed between the SD and UC arms. Equalization between both arms appears to be mainly driven by higher rates of later linkage in the UC arm, as we did not observe higher rates of disengagement from care in the SD arm. Previous studies in pregnant women report higher mid- and long-term disengagement from care upon SD ART initiation [21]. Our findings do not endorse the fear of a compensatory higher attrition from care after SD ART initiation. The reason for the increased late linkage in the UC arm is likely multifactorial. Participants in either arm who were not in care at 12 months were traced and encouraged to (re-) engage in care, which might have had a greater effect in the UC arm. On the other hand, the passing of time without any additional intervention likely had an equalizing effect given the natural cycling of patients in and out of care. Overall rates of 24-month engagement in care and viral suppression observed in our study are in line with previous reports in which we analyzed data from before the test-and-treat era, thus likely missing the pre-ART disengagement from care [22–24].
To our knowledge, there are no reports assessing the potential for rapid community-based ART initiation to cause harm in recipients of SD ART who are transiently exposed to ART but do not link to care. It is encouraging to note that 7 of 9 SD arm late linkers to care for whom VL results were available achieved viral suppression (Table 3). Furthermore, self-reported accounts from the 8 interviewed nonlinkers in the SD arm indicate that 6 did not take any of the initial ART supply and thus had no risk of developing drug resistance, which was confirmed by NGS. The remaining 2 declared full initial adherence; both had acquired therapy-related DRMs still detectable at 24 months that had not existed at enrollment. However, this risk also exists upon attrition from care after UC ART initiation. More importantly, the risk of acquiring DRMs has to be viewed in light of the overall benefit that home-based SD ART decreases the time to linkage to care and successful therapy (Figure 1). This is an essential factor not only for individual health but also to prevent further HIV transmission.
The systematic assessment of the status of all study participants, including verifying self-reported transfer out of care as well as collecting valuable but hard-to-obtain data about participants who never linked after SD ART start, are among the strengths of this study. Our study has, however, several limitations. The study was conducted in the catchment areas of 6 facilities in 1 rural district in Lesotho, and generalizability may be limited. Furthermore, the assessment of the risk of developing therapy resistance as a consequence of unstructured treatment stop was based on very few individuals. Reasons given for nonlinkage to or disengagement from care should be considered exploratory. Finally, it is not clear if the similar outcomes in both study arms are due to the active tracing of participants not engaged in care at 12 months (after completion of the primary endpoint) or simply due to the additional time available to link to care.
Although both arms of the CASCADE trial had higher rates of linkage to care than reported in previous studies [7, 8], almost one-third of SD arm participants did not link to care. More research is needed to explore strategies to increase engagement in care directly after home-based SD ART. The 2 SD arm participants who did not link to care after taking their first 30-day supply of ART noted transport costs and perceived poor treatment by healthcare professionals, respectively, as their reasons. One promising approach for such participants may be decentralized, community-based ART refills following the SD ART initiation, whereby participants are directly linked to a nearby village health worker for subsequent ART refills [25–29].
In conclusion, our findings endorse the current WHO recommendation to offer rapid or even SD ART initiation to individuals diagnosed as living with HIV. Our findings do not indicate that higher initial linkage to care through SD ART would result in substantially higher attrition from care beyond 12 months. Furthermore, the risk of developing drug resistance through SD ART in those who do not link to care exists but appears to be low. However, offering SD ART during home-based HIV testing campaigns alone does not lead to sufficient linkage to care nor sufficient engagement in care. Additional strategies are needed to address the remaining individual challenges in linking to and remaining in care.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. N. D. L. was the principal investigator of the CASCADE trial. A. A., J. A. B., and N. D. L. were responsible for the design of the follow-up study. J. M., I. R., and T. I. L. coordinated the local project implementation. A. A. and I. R. were responsible for data and biological sample collection. A. A., J. A. B., and N. D. L. analyzed the data. T. R. G. contributed to the statistical analyses. J. A. B. and T. K. were responsible for the virologic analyses conducted in Switzerland. J. A. B. performed next-generation sequencing and analyzed the laboratory data. A. A. and J. A. B. wrote the first draft of the manuscript with input from N. D. L. All authors commented on the manuscript and read and approved the final manuscript for publication.
Acknowledgments. Sample preparation for next-generation sequencing (NGS) was conducted by T. K.’s group as well as Prof Karin Metzner’s group at the University Hospital Zurich and was supported by Christine Leemann. NGS result analysis was supported by Nadine Bachmann in Prof Roger Kouyos’ group at the University Hospital Zurich. The authors thank all participants in the study. The authors also thank the staff at the clinics who supported the study.
Disclaimer. The funders had no role in study design, data collection, data analysis, data interpretation, writing of the manuscript, or the decision to submit the manuscript for publication. Data from this study, including deidentified participant data, study protocol, and informed consent documents, will be made available to researchers. To access data, researchers should contact the corresponding author. Researchers will need to present a concept sheet for their proposed analysis. This will have to be reviewed and approved by all coauthors. The coauthors will consider overlap of the proposed project with active or planned analyses and the appropriateness of study data for the proposed analysis.
Financial support. The study was supported by 2 grants from the Swiss National Science Foundation (grants IZ07Z0_160876/1 and PCEFP3_181355), obtained by N. D. L. A. A. receives his salary through a grant from the MD-PhD program of the Swiss National Science Foundation (grant 323530_177576). This study was embedded in the SolidarMed country program and thus benefited from logistics and human resources provided by SolidarMed Lesotho.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
Author notes
A. A. and J. A. B. contributed equally to this article and share first authorship.




