-
PDF
- Split View
-
Views
-
Cite
Cite
Dongmei Yan, Dongyan Wang, Shuangli Zhu, Yong Zhang, Xiaolei Li, Haishu Tang, Jing Guan, Wenbo Xu, Immunogenicity of Oral Polio Vaccine and Salk Inactive Polio Vaccine Against Xinjiang Imported Type 1 Wild Poliovirus, Clinical Infectious Diseases, Volume 70, Issue 9, 1 May 2020, Pages 1980–1984, https://doi.org/10.1093/cid/ciz549
Close - Share Icon Share
Abstract
An outbreak of an imported Type 1 wild poliovirus from Pakistan occurred in the Xinjiang Uygur Autonomous Region of China in 2011, although the local immunity status of the oral polio vaccine (OPV) was relatively satisfied.
Neutralizing antibody titers against the Xinjiang strain and Sabin 1 strain were measured in 237 sera from 3 groups of fully OPV-vaccinated persons and 1 group of infants fully vaccinated with the inactive polio vaccine (IPV). Additionally, 17 sera collected from 1 Xinjiang poliomyelitis case and his 16 contacts were also tested. Genomic sequencing was conducted the Xinjiang strain.
The antibody titers against the Xinjiang strain in each of 237 sera were significantly lower than those against the Sabin 1 strain. Notably, 40.0% of children in Group 1 were seronegative against the Xinjiang strain, which indicated that they might play an important role in wild poliovirus transmission, although their antibody titers against the Sabin 1 strain varied between 1:8 and 1:512. Meanwhile, serological results of the Xinjiang poliomyelitis case and his contacts also provided evidence that a proportion of OPV-vaccinated children had indeed been involved in the transmission chain of the Xinjiang outbreak. Genomic sequencing indicated that the Xinjiang strain was greatly distinguishable from the Sabin 1 strain in neutralizing antigenic sites.
The lack of neutralizing antibodies against the Xinjiang strain in persons vaccinated by OPV may be associated with the transmission of Type 1 wild poliovirus in Xinjiang. Using Salk IPV along with OPV might be considered in a wild poliovirus outbreak response, especially in the countries which continued to have persistent wild poliovirus circulation.
A standard 4-dose trivalent oral polio vaccine (OPV; tOPV) vaccination schedule for children at 2, 4, and 6 months and a booster dose at 4 years old was introduced in the Expanded Program on Immunization in 1978 in China, which has led to the elimination of indigenous cases of poliomyelitis since 1994 [1]. In 2000, China was certified as a poliomyelitis-free region [2]. However, after a more than 10-year absence of poliomyelitis in China, an outbreak caused by an imported Type 1 wild poliovirus from Pakistan occurred in the Xinjiang Uygur Autonomous Region of China in July 2011 [3]. A rapid coverage survey of OPV showed that coverage rates for the first, second, and third doses of tOPV routine immunization in the Hotan prefecture of Xinjiang, where the majority of wild poliovirus cases occurred, were 93.2%, 90.3%, and 86.0%, respectively [4]. Polio neutralizing antibody testing of 2611 serum samples collected from the Hotan prefecture indicated that the positive rate for antibodies against the Sabin 1 strain was 90.4% [3]. Although the above 2 indicators showed that the local immunity status of OPV was relatively satisfied, the Type 1 wild poliovirus broke the OPV immunity barrier and caused 21 wild poliovirus cases. Therefore, we aimed to investigate whether the OPV-induced immunity provided adequate protection against the Xinjiang Type 1 wild poliovirus transmission in local populations.
In this study, we compared the neutralizing antibody titers against the Xinjiang strain and Sabin 1 strain in 237 serum samples of 3 groups of fully OPV-vaccinated persons and 1 group of infants fully vaccinated with inactive polio vaccine (IPV). Additionally, 17 serum samples collected from 1 Xinjiang wild poliovirus case patient, and 16 of his healthy, close contacts were tested for the neutralizing antibody titers against the Xinjiang strain to roughly estimate the asymptomatic infection rate of Xinjiang poliovirus. To explain the antigenic difference between the Xinjiang strain and Sabin 1 strain, genome sequencing was conducted on the Xinjiang strain (CHN15115/XJ/2011) used in the neutralization test.
MATERIALS AND METHODS
Collection of Serum Samples
A total of 237 serum samples used in this study were collected from 4 groups of persons. Group 1 was from the Hotan prefecture of Xinjiang, with the highest poliomyelitis attack rate in the outbreak. Groups 2, 3, and 4 were from Province Heilongjiang, Beijing, and Province Henan, respectively, where no wild poliovirus cases were found. As shown in Table 1, Group 1 included 45 healthy children aged 0–13 years. Single serum samples were collected from these children in August 2011, soon after the wild poliovirus outbreak was confirmed. Group 2 consisted of 64 healthy children aged 1–3 years. They received a booster dose of OPV in October 2011. The paired sera were collected immediately prior to the OPV booster and on Day 14 after the booster. Group 3 consisted of 12 adults aged 25–35 years. The adults received a Salk-IPV booster in October 2011. The paired sera were collected before and at Day 14 after the Salk-IPV booster. Group 4 consisted of 40 infants aged 5 months. The infants had no OPV immunization history and received 3 doses of Salk-IPV immunization at ages 2, 3, and 4 months. The single serum was collected 30 days after the last dose of Salk-IPV immunization. Additionally, serum samples were collected from 1 Xinjiang wild poliovirus case patient and his 16 healthy close contacts in August 2011. Their immunization histories indicated that all the persons mentioned above were fully vaccinated with tOPV. Written informed consent for the use of their serum samples was obtained from all persons (or their parents) involved in this study.
Neutralization Antibody Seropositivity and Geometric Mean Titers Against Xinjiang Strain and Sabin1 in 237 Sera
| Group . | Province . | Age, years . | Vaccination Booster . | Sera Samples, n . | Seropositivity (%) Against . | . | GMT Against . | . |
|---|---|---|---|---|---|---|---|---|
| XJ Strain | Sabin 1 | XJ Strain | Sabin 1 | |||||
| G1 | Xinjiang | 0–13 | No vaccine booster | 45 | 60.0 | 97.8 | 27.0 | 222.9 |
| G2 | Heilongjiang | 1–3 | Before OPV booster | 64 | 84.4 | 96.9 | 22.9 | 164.2 |
| After OPV booster | 64 | 100 | 100 | 73.7 | 474.6 | |||
| G3 | Beijing | 25–35 | Before IPV booster | 12 | 33.3 | 83.3 | 7.1 | 38.0 |
| After IPV booster | 12 | 100 | 100 | 271.2 | 1824.6 | |||
| G4 | Henan | 0.4 | No vaccine booster | 40 | 95 | 100 | 221.3 | 371.2 |
| Group . | Province . | Age, years . | Vaccination Booster . | Sera Samples, n . | Seropositivity (%) Against . | . | GMT Against . | . |
|---|---|---|---|---|---|---|---|---|
| XJ Strain | Sabin 1 | XJ Strain | Sabin 1 | |||||
| G1 | Xinjiang | 0–13 | No vaccine booster | 45 | 60.0 | 97.8 | 27.0 | 222.9 |
| G2 | Heilongjiang | 1–3 | Before OPV booster | 64 | 84.4 | 96.9 | 22.9 | 164.2 |
| After OPV booster | 64 | 100 | 100 | 73.7 | 474.6 | |||
| G3 | Beijing | 25–35 | Before IPV booster | 12 | 33.3 | 83.3 | 7.1 | 38.0 |
| After IPV booster | 12 | 100 | 100 | 271.2 | 1824.6 | |||
| G4 | Henan | 0.4 | No vaccine booster | 40 | 95 | 100 | 221.3 | 371.2 |
Abbreviations: GMT, geometric mean titers; IPV, inactive polio vaccine; OPV, oral polio vaccine; XJ, Xinjiang.
Neutralization Antibody Seropositivity and Geometric Mean Titers Against Xinjiang Strain and Sabin1 in 237 Sera
| Group . | Province . | Age, years . | Vaccination Booster . | Sera Samples, n . | Seropositivity (%) Against . | . | GMT Against . | . |
|---|---|---|---|---|---|---|---|---|
| XJ Strain | Sabin 1 | XJ Strain | Sabin 1 | |||||
| G1 | Xinjiang | 0–13 | No vaccine booster | 45 | 60.0 | 97.8 | 27.0 | 222.9 |
| G2 | Heilongjiang | 1–3 | Before OPV booster | 64 | 84.4 | 96.9 | 22.9 | 164.2 |
| After OPV booster | 64 | 100 | 100 | 73.7 | 474.6 | |||
| G3 | Beijing | 25–35 | Before IPV booster | 12 | 33.3 | 83.3 | 7.1 | 38.0 |
| After IPV booster | 12 | 100 | 100 | 271.2 | 1824.6 | |||
| G4 | Henan | 0.4 | No vaccine booster | 40 | 95 | 100 | 221.3 | 371.2 |
| Group . | Province . | Age, years . | Vaccination Booster . | Sera Samples, n . | Seropositivity (%) Against . | . | GMT Against . | . |
|---|---|---|---|---|---|---|---|---|
| XJ Strain | Sabin 1 | XJ Strain | Sabin 1 | |||||
| G1 | Xinjiang | 0–13 | No vaccine booster | 45 | 60.0 | 97.8 | 27.0 | 222.9 |
| G2 | Heilongjiang | 1–3 | Before OPV booster | 64 | 84.4 | 96.9 | 22.9 | 164.2 |
| After OPV booster | 64 | 100 | 100 | 73.7 | 474.6 | |||
| G3 | Beijing | 25–35 | Before IPV booster | 12 | 33.3 | 83.3 | 7.1 | 38.0 |
| After IPV booster | 12 | 100 | 100 | 271.2 | 1824.6 | |||
| G4 | Henan | 0.4 | No vaccine booster | 40 | 95 | 100 | 221.3 | 371.2 |
Abbreviations: GMT, geometric mean titers; IPV, inactive polio vaccine; OPV, oral polio vaccine; XJ, Xinjiang.
Neutralizing Antibody Detection
Neutralizing antibody titers against poliovirus in sera were determined with a microneutralization test, according to World Health Organization guidelines [5]. In brief, all serum samples were inactivated at 56°C for 30 min before use, serially 2-fold diluted from 1:8 to 1:1024, and then incubated with 25 μl of 100 times of 50% tissue culture infective doses (TCID50) challenge virus in duplicate wells for 2 hours in a CO2 incubator. After the addition of 0.5 ml of cell suspension, the plates were incubated in a CO2 incubator at 36°C. For the next 6 days, the appearance of a cytopathic effect, which measured cell death, was examined by a standard microscope. Neutralizing antibody titers ≥1:8 in a neutralization test are considered as positive and protective according to World Health Organization guidelines. The titers <1:8 and >1:1024 were indicted as 1:4 and 1:2048 in the results, respectively.
Genome of Xinjiang Strain
Xinjiang strain CHN15115/XJ/2011, used in this study, was isolated from a wild poliovirus case patient in Hotan Prefecture, Xinjiang. The complete genome of CHN15115/XJ/2011 was sequenced [6] and deposited in the GenBank database under accession number MH750912.
RESULTS
Neutralizing Antibody Titers Against Sabin 1 Strain and Xinjiang Strain in 237 Serum Samples From 4 Groups
The neutralizing antibody titers of individual serum samples against the Xinjiang strain and Sabin 1 strain, regardless of the individual’s grouping and pre- or postvaccine booster status, were plotted in Figure 1. The antibody titers against the Xinjiang strain were significantly lower than those against the Sabin 1 strain in each serum sample in the OPV immunization group (Group 1), OPV booster group (Group 2), IPV booster group (Group 3), and IPV immunization group (Group 4). A difference of 4-fold or higher was found between the anti–Sabin 1 and anti–Xinjiang strain antibody titers in 164 of 197 sera (69.2%). A titer difference of 8-fold or more was observed in 113 of 197 sera (47.7%) between the 2 strains. Notably, following receipt of the OPV booster in Group 2, both anti-Sabin 1 and anti-Xinjiang strain antibody titers in each participant’s serum samples increased significantly, but the antibody titers against the Xinjiang strain were still significantly lower than those against the Sabin 1 strain. A similar result was observed following receipt of the Salk-IPV booster in Group 3. The above results imply that both OPV-induced and IPV-induced antibodies neutralized the Sabin 1 strain better than the Xinjiang strain.
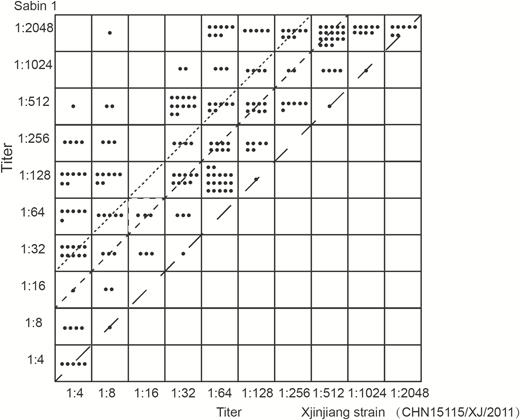
Plots of neutralizing antibody titers against Xinjiang strain and Sabin 1 in all 237 sera from 4 groups of persons. The line with long dashes indicates the same titer between Xinjiang strain and Sabin 1. The line with short dashes indicates a 4-fold titer difference. The dotted line indicates an 8-fold titer difference.
Antibody Seropositivity Rates and Geometric Mean Titers Against Sabin 1 Strain and Xinjiang Strain in 237 Serum Samples
The seropositivity rates and geometric mean titers (GMTs) of antibodies against the Xinjiang strain and Sabin 1 strain from 4 groups of serum samples are summarized in Table 1. The seropositivity rates against the Sabin 1 strain in Group 1, Group 2 (prior to the OPV booster), and Group 3 (prior to the IPV booster) were 97.8%, 96.9%, and 83.3%, respectively, suggesting that the immunity levels conferred by OPV in the 3 groups were satisfactory. In contrast, the seropositivity rates against the Xinjiang strain were significantly lower, at 60.0%, 84.4%, and 33.3%, respectively, implying that 40.0%, 15.6%, and 66.7%, respectively, of persons in the 3 groups were not protected by OPV-induced immunity and had an increased risk of infection with Xinjiang Type 1 wild poliovirus. Compared with Group 1, Group 2—from Province Heilongjiang, where no Type 1 wild poliovirus case was detected—showed a higher seropositivity rate against the Xinjiang strain (84.4% vs 60.0%). Compared with the 2 children’s groups (Groups 1 and 2), the adult group (Group 3) had the lowest antibody seropositivity rate and GMT against both the Xinjiang strain and Sabin 1 strain, which could be explained by the waning of antibody titers with age. Nearly 66.7% of adults were seronegative for the Xinjiang strain, and the GMT was only 7.1. Compared with the first 3 groups, Group 4 had the highest antibody seropositivity rates and GMTs against both the Xinjiang strain and Sabin 1 strain, due to no waning over time.
Following 1 booster dose with OPV in Group 2 and with Salk-IPV in Group 3, the seropositivity rates against both the Sabin and Xinjiang strains reached 100% in both groups, indicating that both Salk-IPV and OPV provided good protection efficiency against both the Sabin 1 and Xinjiang strains. In Group 2, GMTs against the Xinjiang strain and the Sabin 1 strain increased from 22.9 to 73.7 and from 164.2 to 474.6, respectively, and increased by about 2-fold concurrently. In contrast, GMTs against the Xinjiang strain and the Sabin 1 strain in Group 3 increased from 7.1 to 271.2 and from 38.0 to 1824.6, respectively, and increased by about 40-fold, indicating that Salk-IPV probably has superior immune efficiency than OPV.
Serology of 1 Xinjiang Wild Poliovirus Case Patient and 16 Healthy Close Contacts
The neutralization antibody titers against the Xinjiang strain and Sabin 1 strain of the Xinjiang case patient and his 16 healthy contacts are shown in Table 2. The neutralization antibody titers of the Xinjiang case patient against the Xinjiang strain and Sabin 1 strain were 1:128 and 1:256, respectively. Of his 16 healthy contacts, 2 (C12 and C13) had high titers against the Xinjiang strain and low titers against the Sabin 1 strain, indicating an asymptomatic wild poliovirus infection rate of at least 12.5%.
| Neutralization Antibody Titer Against . | Xinjiang Case Patient . | C1 . | C2 . | C3 . | C4 . | C5 . | C6 . | C7 . | C8 . | C9 . | C10 . | C11 . | C12 . | C13 . | C14 . | C15 . | C16 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xinjiang strain (1:) | 128 | 16 | 2048 | 128 | 256 | 512 | 4 | 4 | 256 | 512 | 512 | 4 | 512 | 2048 | 4 | 8 | 2048 |
| Sabin 1 (1:) | 256 | 2048 | 2048 | 128 | 256 | 1024 | 2048 | 4 | 2048 | 1024 | 1048 | 2048 | 32 | 128 | 32 | 128 | 2048 |
| Neutralization Antibody Titer Against . | Xinjiang Case Patient . | C1 . | C2 . | C3 . | C4 . | C5 . | C6 . | C7 . | C8 . | C9 . | C10 . | C11 . | C12 . | C13 . | C14 . | C15 . | C16 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xinjiang strain (1:) | 128 | 16 | 2048 | 128 | 256 | 512 | 4 | 4 | 256 | 512 | 512 | 4 | 512 | 2048 | 4 | 8 | 2048 |
| Sabin 1 (1:) | 256 | 2048 | 2048 | 128 | 256 | 1024 | 2048 | 4 | 2048 | 1024 | 1048 | 2048 | 32 | 128 | 32 | 128 | 2048 |
Data are from the Xinjiang case patient and 16 healthy contacts.
Abbreviation: C, contact.
| Neutralization Antibody Titer Against . | Xinjiang Case Patient . | C1 . | C2 . | C3 . | C4 . | C5 . | C6 . | C7 . | C8 . | C9 . | C10 . | C11 . | C12 . | C13 . | C14 . | C15 . | C16 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xinjiang strain (1:) | 128 | 16 | 2048 | 128 | 256 | 512 | 4 | 4 | 256 | 512 | 512 | 4 | 512 | 2048 | 4 | 8 | 2048 |
| Sabin 1 (1:) | 256 | 2048 | 2048 | 128 | 256 | 1024 | 2048 | 4 | 2048 | 1024 | 1048 | 2048 | 32 | 128 | 32 | 128 | 2048 |
| Neutralization Antibody Titer Against . | Xinjiang Case Patient . | C1 . | C2 . | C3 . | C4 . | C5 . | C6 . | C7 . | C8 . | C9 . | C10 . | C11 . | C12 . | C13 . | C14 . | C15 . | C16 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xinjiang strain (1:) | 128 | 16 | 2048 | 128 | 256 | 512 | 4 | 4 | 256 | 512 | 512 | 4 | 512 | 2048 | 4 | 8 | 2048 |
| Sabin 1 (1:) | 256 | 2048 | 2048 | 128 | 256 | 1024 | 2048 | 4 | 2048 | 1024 | 1048 | 2048 | 32 | 128 | 32 | 128 | 2048 |
Data are from the Xinjiang case patient and 16 healthy contacts.
Abbreviation: C, contact.
Changes in Neutralizing Antigenic Sites of Xinjiang Strain
In order to characterize the antigen differences among poliovirus strains, the amino acid sequences within or near the predicted neutralizing antigen (NAg) sites of the Xinjiang strain (CHN15115/XJ/2011) were aligned with those of the Sabin 1 strain and Mahoney strain (prototype strain of Salk-IPV; Figure 2). The Xinjiang strain had 9 and 6 amino acids different from the Sabin 1 and Mahoney strains, respectively.

Alignment of amino acid residues of NAg sites 1 (viral capsid protein (VP): 95–106), 2 (VP2: 164–173; VP2: 269–271; and VP1: 221–226), 3a (VP3: 56–62; VP3: 70–74; and VP1: 287–292), and 3b (VP2: 71–73 and VP3: 75–80) for Sabin 1, the Xinjiang strain (CHN15115/XJ/2011), and the Mahoney strain. Abbreviation: NAg, neutralizing antigenic.
DISCUSSION
In this study, we assessed differences in neutralizing antibody titers against the Sabin 1 and Xinjiang strains (CHN15115/XJ/2011) in 237 serum samples of 4 groups of persons, comprised of 45 children from Hotan, 64 children with an OPV booster from Heilongjiang, 12 adults with a Salk-IPV booster from Beijing, and 40 infants vaccinated by only Salk-IPV. The neutralizing antibody titers against the Xinjiang strain were lower than those against the Sabin 1 strain in each serum sample, implying that both OPV-induced and IPV-induced antibodies neutralized the Sabin 1 strain better than the Xinjiang strain. The results can be explained by the antigenic differences between the 2 strains, resulting from amino acid differences in 4 known NAg sites. The amino acid sequence alignments within or near the predicted NAg sites showed that the Xinjiang strain had 9 and 6 amino acids different from the Sabin 1 and Mahoney strains, respectively.
Notably, 40% of children in Group 1, 15.6 % of children in Group 2 (prior to the OPV booster), and 66.7% of adults in Group 3 (prior to the IPV booster) were seronegative against the Xinjiang strain, although their antibody titers against the Sabin 1 strain were positive and varied between 1:8 and 1:512. Our findings raise the question of whether these OPV immunized persons with negative antibody titers against the Xinjiang strain could be infected with the Xinjiang Type 1 wild poliovirus. In the early years of polio vaccine development, many studies were performed that indicated that persons with undetected, serum-neutralizing antibody titers could be reinfected with wild viruses, as well with vaccine viruses, and excrete the viruses, although the durations and titers of viral excretion among these persons were reduced compared to those among unvaccinated persons [7–11]. Fortunately, seronegative persons with OPV immunization histories, though susceptible to reinfection with poliovirus, are probably not in danger of developing clinical poliomyelitis because they possess memory immunity (an accelerated immune response after a challenge) [7, 9, 12]. The secondary response to infection was postulated to be rapid enough to result in a rapid and strong increase of antibody levels and, therefore, protect against paralytic disease. However, the immune memory does not protect against virus excretion [13].
We presumed that the lack of neutralizing antibodies against the Xinjiang Type 1 wild poliovirus in persons vaccinated by OPV likely played a part in establishing the Type 1 wild poliovirus outbreak in Xinjiang, despite relatively satisfactory OPV coverage in the local population. A portion of immunized persons with negative antibody titers against the Xinjiang strain may get infected with Type 1 wild poliovirus, excrete the virus to others who have not been vaccinated, and participate in wild poliovirus circulation, although in a Xinjiang wild poliovirus outbreak they may not develop poliomyelitis due to memory immunity. The asymptomatic, wild-type poliovirus infection among persons with previous OPV vaccination has been reported as a probable reason for the Type 1 wild poliovirus outbreaks in highly vaccinated populations in Oman during 1988 [14] and in India during 2003–2008 [15, 16]. Serological results of 1 Xinjiang case patient and his 16 contacts in this study provided evidence that there was indeed an asymptomatic infection of wild poliovirus in the Xinjiang outbreak: we roughly estimated the asymptomatic infection rate to be 12.5%. However, this study did not collect more serum samples from poliomyelitis patients and their contacts, so it is not possible to accurately estimate the asymptomatic infection rate in the local area, and additional studies are required to determine the extent to which such OPV-vaccinated persons participated in the transmission of wild-type poliovirus in Xinjiang.
In our study, the seropositive rates against both the Xinjiang strain and Sabin 1 strain increased up to 100% in Groups 2 and 3 following vaccine boosters, which indicated that both OPV and Salk-IPV provide good protection efficiency against Xinjiang wild poliovirus, as well as against the Sabin 1 strain, at least at the start phase of immunization. Immunization with OPV mimics a natural infection and induces both humoral and gut mucosal immunity that protects not just against paralytic disease, but also against infection with and excretion of poliovirus. However, both the humoral and mucosal immunity induced by OPV are imperfect and may wane over time. Salk-IPV has long been thought to effectively induce humoral immunity but does not induce mucosal immunity. However, recent studies showed that Salk-IPV may boost mucosal immunity among persons previously immunized with OPV. This effect may exceed the boost in mucosal immunity induced by additional doses of OPV [17, 18]. In our study, the comparison of the increasing multiples of GMTs against the Xinjiang strain and Sabin 1 strain between Group 2 and Group 3 after vaccine boosters indicated that Salk-IPV has superior immune efficiency to OPV. Salk-IPV might be considered for use alongside OPV in wild poliovirus outbreak responses for greater compensation effects on both the humoral and mucosal immunity waning that occurs over time in persons previously immunized with OPV, as compared to the use of OPV alone.
In conclusion, the lack of neutralizing antibodies against Xinjiang Type 1 wild poliovirus in persons immunized with OPV may be associated with the Type 1 wild poliovirus outbreak in Xinjiang. Although the individuals infected might not necessarily develop poliomyelitis due to immune memory, they may be a source of infection to others who have not been vaccinated and could increase the risk of the wild poliovirus transmission in the local population. In the polio posteradication era, 2 countries (Afghanistan and Pakistan) continued to have persistent indigenous wild poliovirus circulation; exported the poliovirus to Africa, Asia, and Europe; and caused large outbreaks in the past decade, even after multiple rounds of OPV supplementary immunizations. The interruption of the endemic wild poliovirus transmission in the 2 countries and the response to these imported outbreaks became a stubborn challenge for the Global Poliomyelitis Eradication Initiative. Can we assume that the asymptomatic wild poliovirus infections among persons with previous OPV vaccinations are a probable reason for the Type 1 wild poliovirus outbreaks in Pakistan? Using Salk-IPV along with OPV might be considered in wild poliovirus outbreak responses—especially in the countries which continue to have persistent indigenous wild poliovirus circulation—at the last stage of the Global Poliomyelitis Eradication Initiative for a greater effect on both humoral and mucosal protection than the use of OPV alone. This study provided insight into a possible cause and a response measure for the persistent wild poliovirus circulation in the polio posteradication era.
Notes
Author contributions. D. Y. prepared the manuscript. W. X. and Y. Z. contributed to the study design. H. T. and J. G. contributed to the sample collection. D. Y., D. W., S. Z., and X. L. contributed to the neutralization test and genomic sequencing. All authors reviewed and approved the manuscript.
Financial support. This work was supported by the National Key Technology R&D Program of China Key Technologies R&D Program of the National Ministry of Science (grant numbers 2017ZX10104001 and 2018ZX10713002).
Potential conflicts of interest. The authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




