-
PDF
- Split View
-
Views
-
Cite
Cite
Huan V Dong, Loc Q Pham, Hoa T Nguyen, Minh X B Nguyen, Trung V Nguyen, Folasade May, Giang M Le, Jeffrey D Klausner, Decreased Cephalosporin Susceptibility of Oropharyngeal Neisseria Species in Antibiotic-using Men Who Have Sex With Men in Hanoi, Vietnam, Clinical Infectious Diseases, Volume 70, Issue 6, 15 March 2020, Pages 1169–1175, https://doi.org/10.1093/cid/ciz365
Close - Share Icon Share
Abstract
Neisseria gonorrhoeae (NG) infections are a global health burden. NG resistance to cephalosporins, which is increasingly reported, is an imminent threat to public health. Many hypothesize that commensal Neisseria species are an important reservoir for genetic material conferring antimicrobial resistance in NG; however, clinical data are lacking.
Men who have sex with men (MSM) in Hanoi, Vietnam, completed a questionnaire regarding antibiotic use. We collected pharyngeal specimens, cultured Neisseria species, and measured minimum inhibitory concentrations (MICs) to ciprofloxacin, cefixime, ceftriaxone, and cefpodoxime. Using MIC criteria for antimicrobial susceptibility in NG, we categorized the Neisseria species and compared mean MIC levels between different antibiotic user groups.
Of 207 participants, 38% used at least 1 antibiotic in the past 6 months; 52% without a prescription. A median of 1 Neisseria species was cultured from each participant (range, 1–4) with 10 different Neisseria species identified overall. The proportion of Neisseria with reduced susceptibility to ciprofloxacin was 93%, cefpodoxime 84%, cefixime 31%, and ceftriaxone 28%. Antibiotic use within the past month was strongly associated with Neisseria species having increased MICs to cefixime, ceftriaxone, and cefpodoxime (mean MIC ratios of 6.27, 4.11, and 7.70, respectively), compared with those who used antibiotics between 1 and 6 months prior (P < .05, all comparisons).
MSM in our study often used antibiotics without a prescription. At least 1 commensal Neisseria species colonized all men. Recent use of any antibiotics may select for oropharyngeal Neisseria species with antimicrobial resistance. The normal flora of the oropharynx may be an important source of antimicrobial resistance in Neisseria gonorrhoeae.
Effective antibiotic treatment options are dwindling for Neisseria gonorrhoeae (NG) infections [1]. The World Health Organization (WHO) estimates nearly 80 million new NG infections each year worldwide [2]. Since the 1930s, cases of treatment failure and antimicrobial resistance to all classes of antibiotics used for the treatment of NG have emerged [3]. Despite increasing media and public health awareness of antimicrobial resistance to both common and rare pathogens, global antibiotic use continues to increase, especially in developing nations [4].
In Vietnam, as with many nations globally, studies have indicated decreased susceptibility of pathogenic bacteria such as Escherichia coli and Streptococcus pneumoniae to a broad range of antimicrobials including cephalosporins as a result of human antibiotic use [5, 6]. As a developing nation, Vietnam has striven to bring health services to rural communities; however, civilian access to antimicrobials has outpaced antimicrobial stewardship [7]. In a township near the capital city of Hanoi, oral cephalosporin use was reported in up to 35% of all acute respiratory illnesses, regardless of whether antimicrobials were indicated [8]. In fact, an average of 96 different generic brands of antibiotics were found to be stored, for anticipation of future illness, in approximately 27% of all rural households surveyed in Vietnam in 2002 [9].
Previous studies on antimicrobial resistance have mainly focused on pathogenic organisms; however, nonpathogenic organisms within the Neisseria genus are particularly apt in horizontal gene transfer, which can occur within their genus, as well as with other bacteria such as Haemophilus influenzae and E. coli [10–14]. Genomic analyses have shown widespread genetic exchange of multiple virulence factors between pathogenic and nonpathogenic Neisseria [15]. Studies of penicillin-binding protein 2 (PBP2), the target of β-lactam and cephalosporin antibiotics, uncovered mosaicism of PBP2 with genetic fragments analogous to those found in Neisseria cinerea and Neisseria perflava, which are commensal nonpathogenic species found in the human oropharynx [14, 16–18].
The oropharynx has been hypothesized as the site for a “commensal reservoir of resistance” due to complex pharyngeal microbiota interactions, prolonged duration of untreated asymptomatic NG infections allowing time for genetic exchange, and suboptimal antibiotic penetration leading to incomplete eradication of targeted microbes [19]. That environment likely facilitates the acquisition of genetic material conferring antimicrobial resistance by pathogenic organisms. Further study of the nonpathogenic Neisseria species within the microbiome of the oropharynx—for which antimicrobial susceptibility data are lacking—is warranted. Therefore, we aimed to describe the prevalence and antimicrobial susceptibility of commensal Neisseria from the oropharynx of a population with frequent antibiotic exposure.
METHODS
From November 2016 through April 2017, we recruited self-identified men who have sex with men (MSM) of at least 18 years of age living around Hanoi, Vietnam. MSM were defined as men having oral or anal sex with another male within the past year. Community health workers recruited men to the Impact of Cephalosporins on Oropharyngeal Neisseria (ICON) study via flyers, through social media (Facebook, Zalo), and in the Sexual Health Promotion (Sống Hạnh Phúc) clinic at Hanoi Medical University (Hanoi, Vietnam). We provided participants with a standardized questionnaire via a tablet to answer questions in the Vietnamese language regarding sociodemographic information, sexual behaviors, health-seeking behaviors, and antibiotic use. We piloted the questionnaire with 33 local Vietnamese MSM to assess for language clarity and sociocultural appropriateness. Participants completed the questionnaire in a private setting with a research assistant available nearby for assistance. We collected and managed the study data using REDCap (Research Electronic Data Capture) software [20]. The University of California, Los Angeles (UCLA) hosted the data on secure servers. The Ethics Review Board at Hanoi Medical University and the UCLA Institutional Review Board approved the study protocol.
Following completion of the questionnaire, we collected 1 pharyngeal swab of both tonsillar pillars and the posterior oropharynx per participant using an ESwab with Liquid Amies Collection and Transport System (Copan Diagnostics, Murietta, California). We stored the swabs at room temperature never exceeding 30°C, and delivered the swabs daily to the laboratory at the National Hospital for Tropical Diseases (NHTD) in Hanoi, Vietnam. At NHTD, trained laboratory staff inoculated swabs onto a Gélose Chocolat PolyViteX VCAT3 (bioMérieux, Marcy-l’Étoile, France) and chocolate agar (Oxoid, United Kingdom) plate. Plates were incubated at 35°C–37°C in a 5% carbon dioxide (CO2) environment. Laboratory staff inspected plates for colonies at 24 and 48 hours. Colonies with a diameter between 0.4 mm and 1.0 mm, which were clear or opaque, smooth or rough, and yellow, gray, or white, were confirmed as Neisseria species using Gram staining and oxidase testing. Staff conducted bacterial speciation via a matrix-assisted laser desorption/ionization–time of flight mass spectrometry (MALDI-TOF) system on the Autoflex III Mass Spectrometer with Biotyper 3.0 software (Bruker Daltonics, Germany). The Bruker database DB 4613 was used in conjunction with the MicrobeNet MALDI-TOF reference library (US Centers for Disease Control and Prevention, Atlanta, Georgia).
Staff performed antimicrobial susceptibility testing by suspending Neisseria colonies in 0.9% phosphate-buffered saline (pH = 7) from an overnight culture of chocolate agar adjusted to an optical density equal to that of a 0.5 McFarland standard using DensiCHEKplus (bioMérieux, Marcy-l’Étoile, France). Mueller-Hinton agar with 5% sheep blood (Oxoid) was used for Neisseria meningitidis and GC agar base medium supplemented with 1% Iso-VitaleX TM (BD BBL Sensi-Disc, Becton Dickinson) for NG and other Neisseria species. Organisms were evenly spread on a 90-mm agar plate using a sterile cotton swab and allowed to dry for 10 minutes before Etest strips (bioMérieux) were applied to the plate. The plates were incubated at 35°C in 5% CO2 for 20–24 hours and the zone of inhibition indicating minimum inhibitory concentration (MIC) values to ciprofloxacin, cefixime, ceftriaxone, and cefpodoxime for each isolate was recorded.
Microbiology staff were blinded to 5 negative controls swabs, 1 of each was submitted for processing at random time points throughout the study to serve as negative control for potential environmental sources of Neisseria or other contaminants.
Data Analysis
We described sociodemographic characteristics of the participants by mean and standard deviation for continuous variables and by frequency with percentages for categorical variables. We used the most inclusive MIC criteria for suspected reduced susceptibility and resistance in the current literature for NG to screen for resistant phenotypes among cultured Neisseria. For all Neisseria (excluding N. meningitidis), we defined resistance as having MIC ≥1.0 µg/mL for ciprofloxacin [21]. We defined reduced susceptibility as MIC >0.6 µg/mL for ciprofloxacin, MIC ≥0.12 µg/mL for cefixime [22], MIC ≥0.125 µg/mL for ceftriaxone [23], and MIC ≥0.25 µg/mL for cefpodoxime [24]. For N. meningitidis, we defined resistance as MIC >0.03 µg/mL for ciprofloxacin and MIC >0.125 µg/mL for ceftriaxone [25].
As multiple Neisseria species could be cultured from a single swab, the highest MIC found for each antibiotic tested for each isolate was used to assess for any correlations with reported antibiotic use. Prevalence ratios (PRs) and 95% confidence intervals (CIs) were estimated using a log-linear model previously described [26]. To assess for the magnitude of effect on MICs for each reported pattern of antibiotic use, a ratio of mean MICs was calculated as an index of antibiotic use impact. To calculate the 95% CI of the ratios, we applied a resampling with replacement technique known as the bootstrap method [27]. For t test analysis between the difference of mean MICs, we logarithmically transformed MIC values to reduce the impact of the skewed distribution of the data. A P value <.05 was selected to indicate statistically significant difference between mean MICs. We performed all analyses using SAS version 9.4 software (SAS Institute, Cary, North Carolina).
RESULTS
From November 2016 through April 2017, we enrolled 218 participants in the study. Of the 218, 3 participants did not complete the questionnaire, 5 experienced technical errors with questionnaire data transfer, and 3 had contaminated laboratory samples; thus, 207 participants were included in the final analysis. Table 1 shows participant characteristics and reported antibiotic use by various periods.
Sociodemographic Characteristics and Reported Antibiotic Use Among 207 Men Who Have Sex With Men—Hanoi, Vietnam, 2016–2017
| Characteristic . | No. (%) . |
|---|---|
| Age, y, mean (SD) | 23.9 (4.3) |
| Education completed | |
| High school or less | 72 (34.8) |
| Any college, university, or graduate school | 135 (65.2) |
| Sex partners, lifetime | |
| Male only | 135 (65.2) |
| Both male and female | 67 (32.4) |
| Transgender men/transgender women | 5 (2.4) |
| No. of sex partners, past 6 mos, median (IQR) | 2 (1–4) |
| Ever exchanged sex to receive food, money, or work | |
| No | 173 (83.6) |
| Yes | 34 (16.4) |
| Testing frequency for any STI or HIV | |
| Never | 49 (23.7) |
| Every 1 year or less frequently | 43 (20.8) |
| Every 6 mos or more frequently | 115 (55.6) |
| Ever tested positive for gonorrhea | |
| No | 176 (85.0) |
| Yes | 21 (10.2) |
| Yes, with pharyngeal infection | 10 (4.8) |
| HIV infection status (n = 205) | |
| Never tested | 52 (25.4) |
| Negative | 146 (71.2) |
| Positive | 7 (3.4) |
| Any antibiotic use in the past 6 mos | |
| No | 129 (62.3) |
| Yes, but not in the past mo | 44 (21.3) |
| Yes, including within the past mo | 34 (16.4) |
| Any cefixime use in the past 6 mos | |
| No | 196 (94.7) |
| Yes | 11 (5.3) |
| Any ceftriaxone use in the past 6 mos (n = 161) | |
| No | 156 (96.9) |
| Yes | 5 (3.1) |
| Any cephalosporin use in the past 6 mos | |
| No | 183 (88.4) |
| Yes | 24 (11.6) |
| Reason for antibiotic use (n = 71) | |
| Sore throat | 30 (42.2) |
| Cough | 4 (5.6) |
| Fever | 16 (22.5) |
| Diarrhea | 4 (5.6) |
| Muscle aches | 4 (5.6) |
| Other | 13 (18.3) |
| Frequency of obtaining a prescription from a medical doctor for antibiotic use, lifetime | |
| Never | 84 (40.6) |
| Sometimes | 93 (44.9) |
| Often | 25 (12.1) |
| Always | 5 (2.4) |
| Characteristic . | No. (%) . |
|---|---|
| Age, y, mean (SD) | 23.9 (4.3) |
| Education completed | |
| High school or less | 72 (34.8) |
| Any college, university, or graduate school | 135 (65.2) |
| Sex partners, lifetime | |
| Male only | 135 (65.2) |
| Both male and female | 67 (32.4) |
| Transgender men/transgender women | 5 (2.4) |
| No. of sex partners, past 6 mos, median (IQR) | 2 (1–4) |
| Ever exchanged sex to receive food, money, or work | |
| No | 173 (83.6) |
| Yes | 34 (16.4) |
| Testing frequency for any STI or HIV | |
| Never | 49 (23.7) |
| Every 1 year or less frequently | 43 (20.8) |
| Every 6 mos or more frequently | 115 (55.6) |
| Ever tested positive for gonorrhea | |
| No | 176 (85.0) |
| Yes | 21 (10.2) |
| Yes, with pharyngeal infection | 10 (4.8) |
| HIV infection status (n = 205) | |
| Never tested | 52 (25.4) |
| Negative | 146 (71.2) |
| Positive | 7 (3.4) |
| Any antibiotic use in the past 6 mos | |
| No | 129 (62.3) |
| Yes, but not in the past mo | 44 (21.3) |
| Yes, including within the past mo | 34 (16.4) |
| Any cefixime use in the past 6 mos | |
| No | 196 (94.7) |
| Yes | 11 (5.3) |
| Any ceftriaxone use in the past 6 mos (n = 161) | |
| No | 156 (96.9) |
| Yes | 5 (3.1) |
| Any cephalosporin use in the past 6 mos | |
| No | 183 (88.4) |
| Yes | 24 (11.6) |
| Reason for antibiotic use (n = 71) | |
| Sore throat | 30 (42.2) |
| Cough | 4 (5.6) |
| Fever | 16 (22.5) |
| Diarrhea | 4 (5.6) |
| Muscle aches | 4 (5.6) |
| Other | 13 (18.3) |
| Frequency of obtaining a prescription from a medical doctor for antibiotic use, lifetime | |
| Never | 84 (40.6) |
| Sometimes | 93 (44.9) |
| Often | 25 (12.1) |
| Always | 5 (2.4) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; SD, standard deviation; STI, sexually transmitted infection.
Sociodemographic Characteristics and Reported Antibiotic Use Among 207 Men Who Have Sex With Men—Hanoi, Vietnam, 2016–2017
| Characteristic . | No. (%) . |
|---|---|
| Age, y, mean (SD) | 23.9 (4.3) |
| Education completed | |
| High school or less | 72 (34.8) |
| Any college, university, or graduate school | 135 (65.2) |
| Sex partners, lifetime | |
| Male only | 135 (65.2) |
| Both male and female | 67 (32.4) |
| Transgender men/transgender women | 5 (2.4) |
| No. of sex partners, past 6 mos, median (IQR) | 2 (1–4) |
| Ever exchanged sex to receive food, money, or work | |
| No | 173 (83.6) |
| Yes | 34 (16.4) |
| Testing frequency for any STI or HIV | |
| Never | 49 (23.7) |
| Every 1 year or less frequently | 43 (20.8) |
| Every 6 mos or more frequently | 115 (55.6) |
| Ever tested positive for gonorrhea | |
| No | 176 (85.0) |
| Yes | 21 (10.2) |
| Yes, with pharyngeal infection | 10 (4.8) |
| HIV infection status (n = 205) | |
| Never tested | 52 (25.4) |
| Negative | 146 (71.2) |
| Positive | 7 (3.4) |
| Any antibiotic use in the past 6 mos | |
| No | 129 (62.3) |
| Yes, but not in the past mo | 44 (21.3) |
| Yes, including within the past mo | 34 (16.4) |
| Any cefixime use in the past 6 mos | |
| No | 196 (94.7) |
| Yes | 11 (5.3) |
| Any ceftriaxone use in the past 6 mos (n = 161) | |
| No | 156 (96.9) |
| Yes | 5 (3.1) |
| Any cephalosporin use in the past 6 mos | |
| No | 183 (88.4) |
| Yes | 24 (11.6) |
| Reason for antibiotic use (n = 71) | |
| Sore throat | 30 (42.2) |
| Cough | 4 (5.6) |
| Fever | 16 (22.5) |
| Diarrhea | 4 (5.6) |
| Muscle aches | 4 (5.6) |
| Other | 13 (18.3) |
| Frequency of obtaining a prescription from a medical doctor for antibiotic use, lifetime | |
| Never | 84 (40.6) |
| Sometimes | 93 (44.9) |
| Often | 25 (12.1) |
| Always | 5 (2.4) |
| Characteristic . | No. (%) . |
|---|---|
| Age, y, mean (SD) | 23.9 (4.3) |
| Education completed | |
| High school or less | 72 (34.8) |
| Any college, university, or graduate school | 135 (65.2) |
| Sex partners, lifetime | |
| Male only | 135 (65.2) |
| Both male and female | 67 (32.4) |
| Transgender men/transgender women | 5 (2.4) |
| No. of sex partners, past 6 mos, median (IQR) | 2 (1–4) |
| Ever exchanged sex to receive food, money, or work | |
| No | 173 (83.6) |
| Yes | 34 (16.4) |
| Testing frequency for any STI or HIV | |
| Never | 49 (23.7) |
| Every 1 year or less frequently | 43 (20.8) |
| Every 6 mos or more frequently | 115 (55.6) |
| Ever tested positive for gonorrhea | |
| No | 176 (85.0) |
| Yes | 21 (10.2) |
| Yes, with pharyngeal infection | 10 (4.8) |
| HIV infection status (n = 205) | |
| Never tested | 52 (25.4) |
| Negative | 146 (71.2) |
| Positive | 7 (3.4) |
| Any antibiotic use in the past 6 mos | |
| No | 129 (62.3) |
| Yes, but not in the past mo | 44 (21.3) |
| Yes, including within the past mo | 34 (16.4) |
| Any cefixime use in the past 6 mos | |
| No | 196 (94.7) |
| Yes | 11 (5.3) |
| Any ceftriaxone use in the past 6 mos (n = 161) | |
| No | 156 (96.9) |
| Yes | 5 (3.1) |
| Any cephalosporin use in the past 6 mos | |
| No | 183 (88.4) |
| Yes | 24 (11.6) |
| Reason for antibiotic use (n = 71) | |
| Sore throat | 30 (42.2) |
| Cough | 4 (5.6) |
| Fever | 16 (22.5) |
| Diarrhea | 4 (5.6) |
| Muscle aches | 4 (5.6) |
| Other | 13 (18.3) |
| Frequency of obtaining a prescription from a medical doctor for antibiotic use, lifetime | |
| Never | 84 (40.6) |
| Sometimes | 93 (44.9) |
| Often | 25 (12.1) |
| Always | 5 (2.4) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; SD, standard deviation; STI, sexually transmitted infection.
All 207 participants (100%) had at least 1 Neisseria species cultured from an oropharyngeal swab, 49 participants (23.6%) had 2 species, 3 (1.5%) had 3 species, and 1 person (0.5%) had 4 species. From the total of 265 isolates cultured, 10 different Neisseria species were identified with varying frequency summarized in Table 2. All 5 negative control swabs showed no growth at 48 hours. Figure 1 shows the antimicrobial susceptibility profiles of all cultured Neisseria species. We found a total of 9 NG isolates; 9 of 9 were resistant to ciprofloxacin, 8 of 9 had reduced susceptibility to cefpodoxime, and 0 of 9 had reduced susceptibility to ceftriaxone or cefixime.
Type and Proportion of Neisseria Species Isolated From the Oropharynx of Men Who Have Sex With Men—Hanoi, Vietnam, 2016–2017 (N = 265 Total Isolates)
| Neisseria Species . | No. (%) . |
|---|---|
| N. cinerea | 7 (2.6) |
| N. flavescens | 125 (47.2) |
| N. gonorrhoeae | 9 (3.4) |
| N. lactamica | 1 (0.4) |
| N. macacae | 12 (4.5) |
| N. meningitidis | 13 (4.9) |
| N. mucosa | 7 (2.6) |
| N. oralis | 4 (1.5) |
| N. perflava | 30 (11.3) |
| N. subflava | 57 (21.5) |
| Neisseria Species . | No. (%) . |
|---|---|
| N. cinerea | 7 (2.6) |
| N. flavescens | 125 (47.2) |
| N. gonorrhoeae | 9 (3.4) |
| N. lactamica | 1 (0.4) |
| N. macacae | 12 (4.5) |
| N. meningitidis | 13 (4.9) |
| N. mucosa | 7 (2.6) |
| N. oralis | 4 (1.5) |
| N. perflava | 30 (11.3) |
| N. subflava | 57 (21.5) |
Type and Proportion of Neisseria Species Isolated From the Oropharynx of Men Who Have Sex With Men—Hanoi, Vietnam, 2016–2017 (N = 265 Total Isolates)
| Neisseria Species . | No. (%) . |
|---|---|
| N. cinerea | 7 (2.6) |
| N. flavescens | 125 (47.2) |
| N. gonorrhoeae | 9 (3.4) |
| N. lactamica | 1 (0.4) |
| N. macacae | 12 (4.5) |
| N. meningitidis | 13 (4.9) |
| N. mucosa | 7 (2.6) |
| N. oralis | 4 (1.5) |
| N. perflava | 30 (11.3) |
| N. subflava | 57 (21.5) |
| Neisseria Species . | No. (%) . |
|---|---|
| N. cinerea | 7 (2.6) |
| N. flavescens | 125 (47.2) |
| N. gonorrhoeae | 9 (3.4) |
| N. lactamica | 1 (0.4) |
| N. macacae | 12 (4.5) |
| N. meningitidis | 13 (4.9) |
| N. mucosa | 7 (2.6) |
| N. oralis | 4 (1.5) |
| N. perflava | 30 (11.3) |
| N. subflava | 57 (21.5) |
![Antimicrobial susceptibility profiles of oropharyngeal Neisseria species among men who have sex with men, Hanoi, Vietnam, 2016–2017 (N = 265). For all Neisseria (excluding N. meningitidis), resistance was defined as a minimum inhibitory concentration (MIC) ≥1.0 for ciprofloxacin [21]; reduced susceptibility was defined as MIC >0.6 for ciprofloxacin, MIC ≥0.12 for cefixime [22], MIC ≥0.125 for ceftriaxone [23], and MIC ≥0.25 for cefpodoxime [24]. For N. meningitidis, suspected resistance was defined as MIC >0.03 for ciprofloxacin and MIC >0.125 for ceftriaxone [25].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/70/6/10.1093_cid_ciz365/4/m_ciz365f0001.jpeg?Expires=1750837884&Signature=A9mVuMGrcfevzw3-FDUurmx-JBeoM6OcwDm-te-qdo7FflHnmCTQCtnfwwaSjqxyTbH6ZzrTdw0GbU4VdKNSO3sbIalw~33n3vlHV~K55CKXZTWuqW8IT55ocWvXSdgDNqcMrpUSH9De4ZoDyigHheLk3sPLJJmHZeymSO9XfjBzfcjCRLUwemP~XVuH7S6WZjkp5PMOzOSGaLS5XhHpz7ECdWuPC1kWUn1nT~ufYsp3I08b-AU8jXdnjZTKiK~3sO2is4i9YWfujndzOTvQT7A1haFzBo7kP1HLVOSkysxWrPbAR78AQG2328cOzcV8xL-OnRh--TKlnzJA1Lwe5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Antimicrobial susceptibility profiles of oropharyngeal Neisseria species among men who have sex with men, Hanoi, Vietnam, 2016–2017 (N = 265). For all Neisseria (excluding N. meningitidis), resistance was defined as a minimum inhibitory concentration (MIC) ≥1.0 for ciprofloxacin [21]; reduced susceptibility was defined as MIC >0.6 for ciprofloxacin, MIC ≥0.12 for cefixime [22], MIC ≥0.125 for ceftriaxone [23], and MIC ≥0.25 for cefpodoxime [24]. For N. meningitidis, suspected resistance was defined as MIC >0.03 for ciprofloxacin and MIC >0.125 for ceftriaxone [25].
For the 4 antibiotics we evaluated, the strength of the association between antibiotic use in the past 6 months and colonization with a Neisseria species with reduced susceptibility or resistance varied by antibiotic: ciprofloxacin (PR, 1.05 [95% CI, 1.00–1.11]; P = .051), cefixime (PR, 1.10 [95% CI, .73–1.66]; P = .640), cefpodoxime (PR, 1.10 [95% CI, .98–1.23]; P = .119), and ceftriaxone (PR, 1.49 [95% CI, .96–2.31]; P = .075).
Figure 2 shows comparisons of mean MIC values of Neisseria isolates, indicating the magnitude of difference in MIC value by different antibiotic use patterns. Of note, when compared to those who had used antibiotics between 1 and 6 months ago, those who used antibiotics within the past 1 month had a 7.70 (95% CI, 2.77–14.55]) increased MIC ratio in MIC values for cefpodoxime, 6.27 (95% CI, 1.92–13.48]) for cefixime, 4.11 (95% CI, 1.44–7.79] for ceftriaxone, and 1.08 (95% CI, .66–1.76]) for ciprofloxacin.
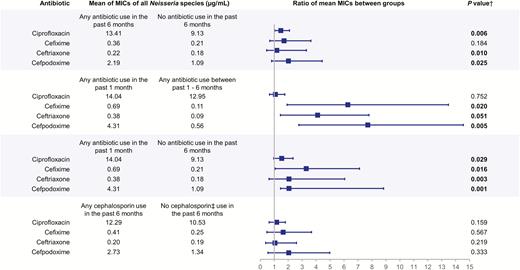
Ratio of mean minimum inhibitory concentrations of commensal Neisseria species by antibiotic use subgroups among men who have sex with men, Hanoi, Vietnam (2016–2017). Boxes indicate value of ratio of 2 means; bars indicate 95% confidence interval of ratio of means. †Two-sided P value of t test for the difference in log-transformed mean of minimum inhibitory concentrations (MICs). ‡Cephalosporins include self-reported use of ceftriaxone, cefixime, cefdinir, cefuroxime, or cephalexin.
DISCUSSION
We completed a cross-sectional assessment of antibiotic use and antimicrobial susceptibility of commensal Neisseria species in MSM of Hanoi, Vietnam. In our study population of young MSM, we cultured at least 1 Neisseria species from the oropharynx of every study participant. When compared to participants who did not use any antibiotics, those who reported antibiotic use within the past month had higher MIC values to extended-spectrum cephalosporins such as cefixime, ceftriaxone, and cefpodoxime. Our findings support the key components of the commensal reservoir hypothesis as shown in Figure 3.
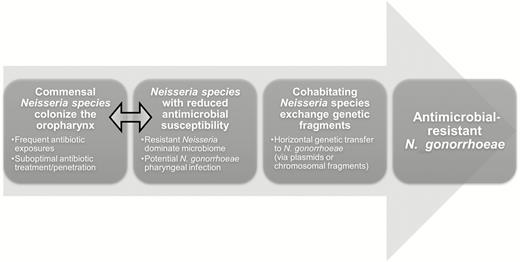
Conceptual model for the “resistant commensal reservoir” hypothesis in antibiotic-resistant Neisseria gonorrhoeae research. Double-headed arrow indicates the area of research relevant to current findings.
Increasing global antibiotic consumption continues to be a challenge, particularly its dual role of decreasing the burden of infectious diseases in medically underserved regions, but also its impact on public health by increasing the risk of antimicrobial resistant infections. A recent evaluation of antibiotics used in children showed that Vietnam, like China and India, has a WHO “access-to-watch” index of <1, indicating that <1 unit of a broad-spectrum antibiotic was used for a more narrow-spectrum antibiotic [28]. The reported antibiotic use in our study of approximately 38% in the past 6 months is similar to the 40%–60% previously reported in the region [9]. Specific antibiotic usage patterns among MSM are generally lacking in the literature, yet mistrust in confidentiality of healthcare providers due to the stigma of sexual orientation and a congested public healthcare system may be contributing to decreased rates of consultation from medical doctors before antibiotic use [29].
Commensal Neisseria are often clinically irrelevant and thus infrequently studied. Our finding of at least 1 Neisseria species in 100% of our participants was similar to prior results in Seattle, Washington and Madrid, Spain, which found Neisseria species carriage frequencies of 97% and 100%, respectively [30, 31]. Though those previous studies were conducted in both men and women, the survey from Seattle showed equivalent carriage frequency of different Neisseria species among heterosexual men, homosexual men, and women [30]. That is in stark contrast to the 10.2% Neisseria species carriage frequency from a survey of both men and women from 7 nations in the African meningitidis belt [32].
The diversity and prevalence of specific Neisseria species also differed among the prior studies and ours. We were able to identify 10 different species compared to the 8 found in Spain, and 5 found in the Seattle and African surveys [30–32]. We likely benefited from utilization of molecular-based identification methods to characterize our isolates compared to the studies in Seattle and Madrid, which used biochemical assays to speciate their isolates [30, 31]. Interestingly, each study appeared to have one dominant Neisseria species with much higher prevalence than the other commensal Neisseria species in the sample. We detected Neisseria flavescens in >47% of our study population, which was only found in 5% of the Madrid survey [31]. Neisseria perflava was most frequent among participants from Seattle and Madrid, at about 96% and 92.5%, respectively [30, 31]. A recent study from Tunisia also found N. perflava in 76% of all their oropharyngeal specimens, with the next most common species being Neisseria mucosa at only 13%; however, all these patients were neutropenic [33]. At 3% and 2%, N. meningitidis and Neisseria lactamica, respectively, were the most frequent species from the African meningitis belt participants [32].
For both overall Neisseria carriage frequency and distinct species found in the study from the African meningitis belt, differences were likely attributed to the selective nature of the modified Thayer-Martin culture media used for primary bacterial culture, whereas the other studies used chocolate agar [30–32]. Geographic variations may also impact the prevalence and number of species, though a temporal component cannot be excluded as the studies from Seattle and Spain were conducted in the 1980s and 1990s, respectively. We are reassured that our microbiology results were unlikely affected by technical error or environmental sources of Neisseria, as our 5 negative control swabs were culture negative.
To capture suspected phenotypes of reduced antimicrobial susceptibility, we initially used the lowest MIC thresholds and “alert” values for NG from the Clinical and Laboratory Standards Institute, the European Committee on Antimicrobial Susceptibility Testing, and the current literature [21–25]. With the MIC values established for NG, we found high frequencies of reduced susceptibility to ciprofloxacin and cefpodoxime (Figure 1). However, it is important to note that MIC thresholds have never been established for commensal Neisseria species and may not accurately indicate reduced susceptibility.
Therefore, our main analysis focused on the relative difference of MIC values between different reported antibiotic use patterns, which we displayed as a ratio to represent the magnitude of difference in MIC values between groups (Figure 2). Our results indicate that antibiotic use within the most recent 30 days can have a profound impact on the MICs of commensal Neisseria species to cefixime, ceftriaxone, and cefpodoxime. The finding of greater reduced susceptibility associated with antibiotic use within 30 days prior to specimen collection, in comparison with longer time frames, was also previously demonstrated with E. coli infections in Vietnam [5]. This finding suggests that the effect is transient and potentially related to fitness costs. Yet, NG with resistance to ceftriaxone and cefixime can have fitness-enhancing mutations as well [34]. The high prevalence of increased MICs in Neisseria commensals is of concern as we continue to learn more about the interspecies exchange of genetic material, among species within the Neisseria genus [14, 35]. The most recent survey of NG in Vietnam revealed resistance to ceftriaxone and cefixime at 5% and 1%, respectively [36]. We did not find significant associations between recent antibiotic use and ciprofloxacin resistance, perhaps due to the already high frequency of ciprofloxacin resistance in Neisseria in Southeast Asia [37].
Our study was limited by the cross-sectional nature of the study, in which we are unable to make specific causal inferences. We recognize that though MSM have a disproportionate prevalence of oropharyngeal sexually transmitted infections, our results may not be generalizable to other populations. We are also limited by reporting bias due to the self-reporting nature of data collection; however, when antibiotics or specific medications were mentioned, locally sourced images were provided to aid in recall. For sensitive information regarding sexual behaviors, substance use, and health-seeking behaviors, we sought to reduce stigma through the private nature of the tablet-based questionnaire as well as using a study identification number instead of any personally identifying information.
CONCLUSIONS
Our results contribute more evidence to the “commensal reservoir” model of antimicrobial resistance by demonstrating the impact of antibiotic consumption on the antimicrobial susceptibility of commensal oropharyngeal Neisseria [3, 38]. The combination of an environment frequently exposed to antibiotics and organisms with high capacity for genetic exchange makes the human oropharynx an optimal site for NG to acquire genetic mechanisms of resistance from commensal Neisseria species.
Our data provide further support to the importance of global antimicrobial stewardship to curb the emergence of resistant organisms. Our data could also suggest that testing for sexually transmitted infections with routine inclusion of pharyngeal specimens might eventually help reduce the development of NG resistance by shortening the duration of NG infections and opportunity for NG diversification. That is particularly important for MSM, who are disproportionately affected by NG infection and may not be as forthcoming in reporting specific sexual activities to health providers to guide screening of infections at extragenital sites. Furthermore, if future genetic analyses of our specimens find known genetic components conferring clinical resistance in NG in the Neisseria commensals, this would further support the “resistant reservoir hypothesis.” Perhaps more interestingly, the discovery of any novel genetic factors of resistance not yet described in clinical NG infections could potentially lead to a mode of surveillance of antimicrobial susceptibility in Neisseria commensals before it even reaches pathogenic Neisseria species. More robust understanding of how antibiotic resistance is acquired in NG could guide further research to combat the threat of untreatable NG infections.
Notes
Acknowledgments. The authors give special acknowledgement to staff at Hanoi Medical University: Mai Quang Anh, Nguyễn Thùy Anh, Trần Quốc Hưởng, Lương Anh Ngọc, Đậu Sỹ Nguyên, Nguyễn Văn Triển, Phạm Văn Trường, Hoàng Quốc Hiệp, Nguyễn Qúy Tú, Văn Định Hòa, and Trần Tuyết Trinh.
Financial support. This work was supported by the GloCal Global Health Fellowship, a Partnership of the Fogarty International Center and the University of California Global Health Institute (National Institutes of Health [NIH] Research Training Grant number R25 TW009343); the National Institute of Allergy and Infectious Diseases (grant number R21 AI117256); and the National Center for Advancing Translational Sciences and NIH for use of REDCap software (grant number UL1TR001881). This work was also supported in part by Team Klausner Saving Lives, an educational and research training program of the Division of Infectious Diseases, University of California, Los Angeles.
Potential conflicts of interest. J. D. K. has received personal fees from Click Diagnostics, Shield Diagnostics, and SpeedX Diagnostics. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




