-
PDF
- Split View
-
Views
-
Cite
Cite
Cédric Dananché, Gláucia Paranhos-Baccalà, Mélina Messaoudi, Mariam Sylla, Shally Awasthi, Ashish Bavdekar, Sonali Sanghavi, Souleymane Diallo, Jean-William Pape, Vanessa Rouzier, Monidarin Chou, Tekchheng Eap, Mala Rakoto-Andrianarivelo, Muriel Maeder, Jianwei Wang, Lili Ren, Budragchaagiin Dash-Yandag, Pagbajabyn Nymadawa, Rosa Guillen, Graciela Russomando, Hubert Endtz, Florence Komurian-Pradel, Philippe Vanhems, Valentina Sánchez Picot, for the Pneumonia GABRIEL (Global Approach for Biological Research on Infectious Epidemics in Low Income Countries) Network, Serotypes of Streptococcus pneumoniae in Children Aged <5 Years Hospitalized With or Without Pneumonia in Developing and Emerging Countries: A Descriptive, Multicenter Study, Clinical Infectious Diseases, Volume 70, Issue 5, 1 March 2020, Pages 875–883, https://doi.org/10.1093/cid/ciz277
Close - Share Icon Share
Abstract
Improving knowledge regarding Streptococcus pneumoniae distribution in pneumonia cases is important to better target preventive and curative measures. The objective was to describe S. pneumoniae serotypes in children with or without pneumonia
It was a case-control study carried out in 8 developing and emerging countries between 2010 and 2014. Cases were children aged <5 years admitted to the hospital for pneumonia. Controls were children admitted for surgery or routine outpatient care.
In nasopharyngeal samples, S. pneumoniae were detected in 68.2% of the cases and 47.5% of the controls (P < .001). Nasopharyngeal carriage was associated with a higher risk of being a case in 6/8 study sites (adjusted odds ratio ranged from 0.71 [95% confidence interval [CI], .39–1.29; P = .26] in India [Pune/Vadu] to 11.86 [95% CI, 5.77–24.41; P < .001] in Mongolia). The 13-valent pneumococcal conjugate vaccine (PCV13) serotypes were more frequently detected in cases with nasopharyngeal carriage (67.1%) than in controls with nasopharyngeal carriage (54.6%), P < .001. Streptococcus pneumoniae was detected in blood by polymerase chain reaction in 8.3% of the cases. Of 34 cases with an S. pneumoniae serotype detected in blood, 27 (79%) had the same serotype in the nasopharyngeal sample.
The results confirm the assumption that the isolate carrying or causing disease in an individual is of the same serotype. Most serotypes independently associated with nasopharyngeal carriage or pneumonia are covered by PCV13, suggesting that increased PCV coverage would reduce the burden of S. pneumoniae–related pneumonia.
Streptococcus pneumoniae, or pneumococcus, remains a major cause of pneumonia in children aged <5 years. Recent studies have reported that the overall mortality rates in children aged <5 years decreased by more than 30% between 2000 and 2015 due to vaccine introduction. Nevertheless, uneven decreases have been observed for pneumonia. In sub-Saharan Africa, the leading cause of death in children aged <5 years changed from malaria in 2000 (0.699 million deaths; 95% confidence interval [CI], .612–.960) to pneumonia in 2015 (0.49 million deaths; 95% CI, .417–.631 in 2015) [1].
The nasopharynx is the major ecological niche for S. pneumoniae, and nasopharyngeal (NP) carriage plays a key role in S. pneumoniae–related pneumonia. The NP carriage rate is highest in children aged <5 years [2]. Invasive pneumococcal disease (IPD) may be caused by a transition from the nasopharynx to the lower respiratory tract [3]. However, few studies have compared the serotypes detected in the nasopharynx and blood of patients with S. pneumoniae–related pneumonia in order to determine the identity of serotypes [4–6]. The role of antibiotic intake on NP carriage is also not well known.
The circulation dynamics of S. pneumoniae are not well known in low-income and low- to middle-income countries since studies are rare in these regions. Knowledge of carriage rates and the serotypes circulating in the community and particularly in children aged <5 years is a cornerstone for prevention. Indeed, it provides useful information for targeting populations for vaccination and future preventive strategies [7].
In order to provide additional epidemiological results on the proportion of NP carriage and serotype distribution of S. pneumoniae in children aged <5 years with or without pneumonia in low-income and low- to middle-income countries, a multicenter, case-control study was implemented by the GABRIEL (Global Approach for Biological Research on Infectious Epidemics in Low Income Countries) Network [8]. The secondary aims were to study the proportion and serotypes of S. pneumoniae detected in the blood of pneumonia cases and to compare them with those detected in NP samples.
METHODS
Study Sites and Design
This descriptive investigation was based on pneumonia cases provided by a large, multicenter, prospective, case-control study carried out between 2010 and 2014 in 9 settings in the following 8 developing and emerging countries: Cambodia, China, Haiti, India (Pune/Vadu and Lucknow), Madagascar, Mali, Mongolia, and Paraguay. The study protocol and sites have been described in detail elsewhere [9, 10]. Supplementary Table 1 presents country-stratified data of S. pneumoniae–related burden and year of implementation of a pneumococcal conjugate vaccine (PCV) vaccination policy.
Definitions of Cases and Controls
The eligible cases were children aged 2–60 months admitted for suspected pneumonia. Eligible cases were identified on admission by study clinicians at each participating site. Incident cases were included if they complied with protocol definitions and met inclusion criteria. Eligible cases presented the clinical features of pneumonia according to World Health Organization criteria [11]. Controls were children hospitalized for surgery or attending a routine outpatient appointment at the hospital site (see Supplementary Materials). For both cases and controls, the parents or legal guardians provided informed consent prior to inclusion. Originally, we planned to include 1 control per case with matching by age and date of admission. Because this condition was not achieved in some countries, frequency-matched pooled analysis [12] was performed by age and period of admission.
Data Sources and Quality Control
Demographic characteristics, medical history, vital signs, clinical symptoms, and biological parameters were recorded prospectively for each patient on a standardized data collection form on admission. Antibiotic usage history was assessed for the current illness for cases and at the admission for controls and was based on declarative data. Data quality was monitored and evaluated by the Fondation Mérieux study conduct team including the Emerging Pathogens Laboratory (Lyon, France).
Biological Samples
Specimens were collected in the first 48 hours of patient hospitalization. NP specimens were collected from all cases and controls. Whole blood was collected from cases only. NP samples enabled the identification of viruses and bacteria by multiplex real-time polymerase chain reaction (RT-PCR) assay with a panel of 19 viruses and 5 bacteria (Fast-track Diagnostic Respiratory Pathogens 21 PLUS, Luxembourg) [13]. Streptococcus pneumoniae–positive specimens were serotyped with a multiplex RT-PCR method that detects 29 serotypes [14]. A centralized, blinded PCR respiratory quality control panel was provided to all sites to ensure on-site procedure validation before the specimens were processed locally. For cases, whole blood was used to obtain the complete blood count, culture, and RT-PCR assay for the identification of Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae type B [15, 16]. Streptococcus pneumoniae–positive specimens were serotyped with the multiplex RT-PCR described previously [14]. For cases, antibiotic substances were detected in urine samples to ascertain antibiotic usage history.
Statistical Analyses
Age was stratified into 3 groups: 2–11 months, 12–23 months, and 24–60 months. Continuous variables were reported as median and interquartile range (IQR) with comparisons using the Mann-Whitney U test. Categorical variables were computed as number of individuals and proportions using the χ2 or Fisher exact test as appropriate for comparison. Calculated proportions of S. pneumoniae–related pneumonia and proportions of serotypes represented the mean occurrence over the study period. They were reported per 100 patients with their 95% CI.
Univariate and multivariate logistic regression modeling was undertaken to calculate the odds ratio (OR) of pneumonia for S. pneumoniae NP carriers compared with noncarriers. Models were adjusted for gender, age, time period, and, in a complementary analysis, antibiotic usage history. No analysis was performed on the overall population because of the amount of data missing on antibiotic usage history and the heterogeneity of antibiotic guidelines in the countries in question [17]. Stratified analyses were performed by study site. Interactions between nasal carriage and covariates were tested. All tests were 2-tailed, with P < .05 considered statistically significant. Statistical analysis was performed with Stata, version 13.0 (College Station, TX).
Inclusion Process and Participant Characteristics
The inclusion process is described in Supplementary Figure 1. A total of 2247 patients were enrolled. Of those, 489 (21.8%, n = 280 [24.0%] cases, n = 171 [16.4%] controls) were excluded owing to missing data, refusal to participate, or not meeting inclusion criteria. By study site, the percentage of excluded patients ranged from 0.5% of all assessed patients in Paraguay to 46.4% in Madagascar. A description of the excluded patients is provided in Supplementary Table 2. In all, 1758 patients were included, with 888 (50.5%) cases and 870 (49.5%) controls; 1024 (58.4%) were male; and the median age was 16 months (IQR, 9–30 months). Participant characteristics are listed in Supplementary Tables 3 and 4 [9].
All study documents were submitted to and approved by the institutional research ethics committee of each site.
RESULTS
Streptococcus pneumoniae Detection in NP Samples From Cases and Controls and Odds Ratio of NP Carriage
In all, S. pneumoniae was detected in 605/888 cases (68.1%; 95% CI, 65.0–71.1) and 415/870 controls (47.5%; 95% CI, 44.4–51.0; P < .001). Among NP carriers, the number of different serotypes detected was not different between cases (median: 1 [min 1–max 6]) and controls (median: 1 [min 0–max 5]), P = .94. The proportions of NP carriage in cases and controls by study center are displayed in Figure 1. The proportions of NP carriers among cases and controls were not significantly different between age groups except for controls in China and Paraguay (P = .012 and 0.013, respectively).
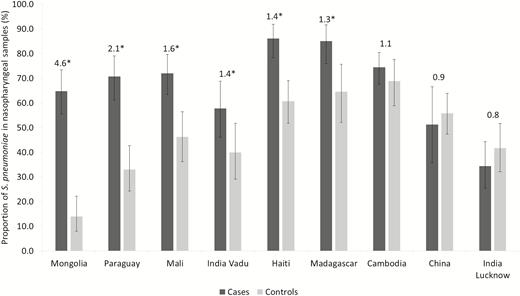
Proportions of Streptococcus pneumoniae detected in nasopharyngeal samples, by group and study site. The Global Approach for Biological Research on Infectious Epidemics in Low Income Countries (GABRIEL) Network, 2010 through 2014. Data labels represent the ratio between the proportions of cases and controls with ≥1 S. pneumoniae detected in nasopharyngeal samples. *P < .05.
Proportions of patients with declared antibiotic usage history and with urinary detection of antibiotics are listed in Supplementary Table 4. In cases, the proportion of urinary detection of antibiotics was higher than the proportion of declared antibiotic usage history (70.5% vs 63.2%, P = .004). Streptococcus pneumoniae detection in NP samples from cases was associated with a lower proportion of declared antibiotic usage history (57.1% of cases with compared with 81.8% without, P < .001; 54.8% of controls with compared with 45.6% without, P = .027).
Table 1 shows the adjusted OR of NP carriage of S. pneumoniae in the pneumonia cases compared with the controls. The pneumonia cases were more frequently NP carriers of S. pneumoniae in Haiti, India (Pune/Vadu), Madagascar, Mali, Mongolia, and Paraguay. Adjusted ORs ranged from 0.71 (95% CI, .39–1.29; P = .26) in India (Lucknow) to 11.86 (95% CI, 5.77–24.41; P < .001) in Mongolia. Supplementary Table 5 shows results after adjustment for antibiotic usage history.
Adjusted Odds Ratios of Nasopharyngeal Carriage of Streptococcus pneumoniae in Children Aged <5 Years With Pneumonia Compared to Controls
| Stratified analysis by study Site . | Adjusted Odds Ratio (95% Confidence Interval)a . | P Value . |
|---|---|---|
| Cambodia | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 1.55 (.89–2.73)b | .12 |
| China | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 1.06 (.44–2.53) | .9 |
| Haiti | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 3.88 (1.96–7.68) | <.001 |
| India Lucknow | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 0.71 (.39–1.29) | .26 |
| India, Vadu | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 2.12 (1.06–4.26) | .034 |
| Madagascar | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 3.35 (1.43–7.86)c | .005 |
| Mali | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 2.90 (1.52–5.53) | .001 |
| Mongolia | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 11.86 (5.77–24.41) | <.001 |
| Paraguay | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 5.87 (3.06–11.27) | <.001 |
| Stratified analysis by study Site . | Adjusted Odds Ratio (95% Confidence Interval)a . | P Value . |
|---|---|---|
| Cambodia | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 1.55 (.89–2.73)b | .12 |
| China | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 1.06 (.44–2.53) | .9 |
| Haiti | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 3.88 (1.96–7.68) | <.001 |
| India Lucknow | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 0.71 (.39–1.29) | .26 |
| India, Vadu | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 2.12 (1.06–4.26) | .034 |
| Madagascar | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 3.35 (1.43–7.86)c | .005 |
| Mali | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 2.90 (1.52–5.53) | .001 |
| Mongolia | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 11.86 (5.77–24.41) | <.001 |
| Paraguay | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 5.87 (3.06–11.27) | <.001 |
The Global Approach for Biological Research on Infectious Epidemics in Low Income Countries (GABRIEL) Network, 2010 Through 2014.
aAdjusted on age group, gender, and time period in a multivariate logistic regression.
bNot adjusted on time period (no controls included in quarters 2 and 3).
cNot adjusted on gender.
Adjusted Odds Ratios of Nasopharyngeal Carriage of Streptococcus pneumoniae in Children Aged <5 Years With Pneumonia Compared to Controls
| Stratified analysis by study Site . | Adjusted Odds Ratio (95% Confidence Interval)a . | P Value . |
|---|---|---|
| Cambodia | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 1.55 (.89–2.73)b | .12 |
| China | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 1.06 (.44–2.53) | .9 |
| Haiti | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 3.88 (1.96–7.68) | <.001 |
| India Lucknow | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 0.71 (.39–1.29) | .26 |
| India, Vadu | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 2.12 (1.06–4.26) | .034 |
| Madagascar | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 3.35 (1.43–7.86)c | .005 |
| Mali | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 2.90 (1.52–5.53) | .001 |
| Mongolia | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 11.86 (5.77–24.41) | <.001 |
| Paraguay | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 5.87 (3.06–11.27) | <.001 |
| Stratified analysis by study Site . | Adjusted Odds Ratio (95% Confidence Interval)a . | P Value . |
|---|---|---|
| Cambodia | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 1.55 (.89–2.73)b | .12 |
| China | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 1.06 (.44–2.53) | .9 |
| Haiti | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 3.88 (1.96–7.68) | <.001 |
| India Lucknow | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 0.71 (.39–1.29) | .26 |
| India, Vadu | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 2.12 (1.06–4.26) | .034 |
| Madagascar | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 3.35 (1.43–7.86)c | .005 |
| Mali | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 2.90 (1.52–5.53) | .001 |
| Mongolia | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 11.86 (5.77–24.41) | <.001 |
| Paraguay | ||
| ºNasal carriage in controls | 1 (reference) | … |
| ºNasal carriage in cases | 5.87 (3.06–11.27) | <.001 |
The Global Approach for Biological Research on Infectious Epidemics in Low Income Countries (GABRIEL) Network, 2010 Through 2014.
aAdjusted on age group, gender, and time period in a multivariate logistic regression.
bNot adjusted on time period (no controls included in quarters 2 and 3).
cNot adjusted on gender.
Overall Description and Geographical Distribution of S. pneumoniae Serotypes Detected in NP Samples From Cases and Controls
Of 605 cases and 412 controls with ≥1 S. pneumoniae strain detected in NP samples, the serotype was not typeable in 90 cases (14.9%) and 90 controls (21.8%) (P = .004) because the serotype had not been included in the PCR assay or because the bacterial load was too low to allow for serotype identification. The proportions of S. pneumoniae serotype detection per 100 patients with NP carriage are presented in Figure 2. The differences in proportions were significant for serotypes 18 (P = .001), 19F (P = .02), and 7C (P = .002). PCV13 serotypes were more frequently detected in cases with NP carriage (n = 406; 67.1%; 95% CI, 63.3–70.8) than in controls with NP carriage (n = 225; 54.6%; 95% CI, 49.8–59.4), P < .001.
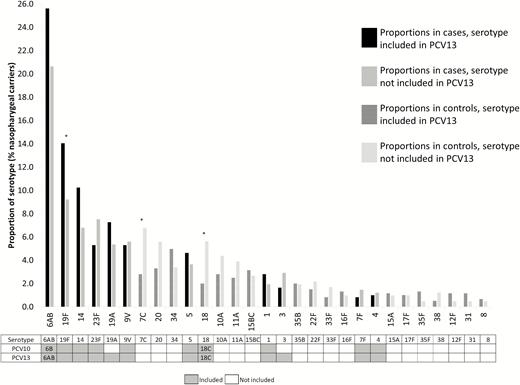
Proportions of Streptococcus pneumoniae serotypes detected, per 100 nasopharyngeal carriers. The Global Approach for Biological Research on Infectious Epidemics in Low Income Countries (GABRIEL) Network, 2010 through 2014. *P < .05. Abbreviations: PCV10, 10-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine. *P < .05.
A detailed geographical distribution of S. pneumoniae serotypes is presented in Supplementary Figure 2 and Supplementary Table 6. The results reveal heterogeneity of the proportions of NP carriage of S. pneumoniae in both cases and controls among sites. However, the dominant serotypes in both groups were, overall, similar and included in PCV13 (serotypes 6AB, 19F, 14, 23F). The results of distribution of the main S. pneumoniae serotypes detected in NP samples from cases and controls are presented in Figure 3. By study center, significant differences in proportions of NP carriage of a specific serotype between cases and controls with NP carriage of S. pneumoniae were observed in Haiti for serotype 6AB (P = .001), in Paraguay for serotype 14 (P < .001), and in Madagascar for serotypes 5 and 7C (P = .048 and P = 0.031, respectively).
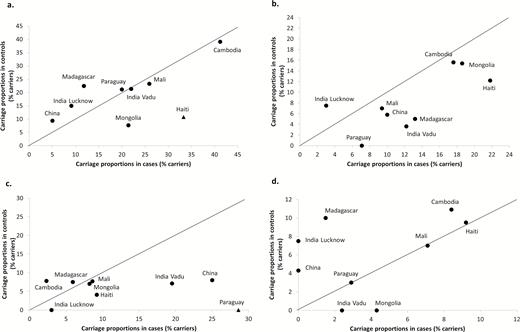
Geographical distribution of the main Streptococcus pneumoniae serotypes (ST) detected in nasopharyngeal carriers. The Global Approach for Biological Research on Infectious Epidemics in Low Income Countries (GABRIEL) Network, 2010 through 2014. (A) ST 6AB. (B) ST 19F. (C) ST 14. (D) ST 23F. Abbreviation: PCV13, 13-valent pneumococcal conjugate vaccine. Black triangle: P < .05 between the NP carriage proportions in cases and controls for the studied study site.
By study center, in cases with NP carriage, the proportions of PCV13 serotypes ranged from 33.3% (95% CI, 18.9–50.5) in India (Lucknow) to 75.9% (95% CI, 66.1–84.0) in Haiti. In controls with NP carriage, the proportions ranged from 40.0% (95% CI, 25.8–55.6) in India (Lucknow) to 78.1% (95% CI, 66.8–87.0) in Cambodia. PCV13 serotypes were more frequently detected in cases with NP carriage than in controls at 3 study sites: Haiti, India (Pune/Vadu), and Paraguay (P < .001, P = .021, and P = .015 respectively; Figure 4).
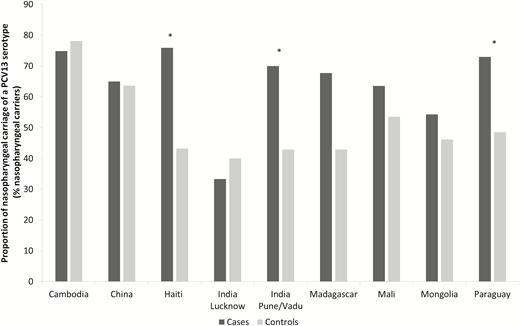
Proportions of 13-valent pneumococcal conjugate vaccine serotype Streptococcus pneumoniae detected in nasopharyngeal samples, by group and study site. The Global Approach for Biological Research on Infectious Epidemics in Low Income Countries (GABRIEL) Network, 2010 through 2014. Abbreviation: PCV13, 13-valent pneumococcal conjugate vaccine. *P < .05.
Distribution of S. pneumoniae Serotypes Detected in Blood From Cases by PCR and Blood Cultures, and Association Between S. pneumoniae Serotypes Detected in NP Samples and in Blood by PCR
Study center–stratified proportions and distribution of S. pneumoniae serotypes detected in blood from cases are shown in Table 2. The proportion of S. pneumoniae detected in blood from cases by PCR was 8.33% (95% CI, 6.65–10.3; n = 74/888). For 40 (40/74, 54.1%) positive blood samples, the serotype was not included in the PCR assay or the bacterial load was too low to allow serotype identification. All of the cases with an S. pneumoniae serotype detected in blood had only 1 serotype detected. There were 26/34 detected serotypes (76.5%) included in PCV13. At the Paraguay and Madagascar study sites, the predominant serotypes detected in NP samples, serotypes 14 and 5, respectively, were also the predominant serotypes detected in blood by PCR.
Study Center–stratified Proportions and Distribution of Streptococcus pneumoniae Serotypes Detected by Polymerase Chain Reaction in Blood From Cases
| . | Positive Polymerase Chain Reaction in Blood, n, proportion per 100 cases (95% Confidence Interval) . | Serotype . |
|---|---|---|
| Study site | ||
| ºCambodia | 5, 2.84 (1.05–6.18) | NT (×5) |
| ºChina | 1, 2.56 (0.13–12.0) | NT |
| ºHaiti | 9, 8.91 (4.43–15.7) | 14 (×2), 6AB, 19F, NT (×5) |
| ºIndia, Lucknow | 6, 6.25 (2.57–12.5) | 1, 7C, 20, NT (×3) |
| ºIndia, Pune/Vadu | 8, 11.3 (5.37–20.3) | 1, 6AB (×2), 8, NT (×4) |
| ºMadagascar | 6, 7.50 (3.10–14.9) | 5 (×5), NT |
| ºMali | 16, 13.6 (8.24–20.7) | 1 (×6), 3, 5, 34, 35F (×3), NT (×4) |
| ºMongolia | 1, 0.93 (0.05–4.48) | 19F |
| ºParaguay | 22, 22.2 (14.9–31.2) | 9V, 14 (×2), 19A, 20, NT (×17) |
| . | Positive Polymerase Chain Reaction in Blood, n, proportion per 100 cases (95% Confidence Interval) . | Serotype . |
|---|---|---|
| Study site | ||
| ºCambodia | 5, 2.84 (1.05–6.18) | NT (×5) |
| ºChina | 1, 2.56 (0.13–12.0) | NT |
| ºHaiti | 9, 8.91 (4.43–15.7) | 14 (×2), 6AB, 19F, NT (×5) |
| ºIndia, Lucknow | 6, 6.25 (2.57–12.5) | 1, 7C, 20, NT (×3) |
| ºIndia, Pune/Vadu | 8, 11.3 (5.37–20.3) | 1, 6AB (×2), 8, NT (×4) |
| ºMadagascar | 6, 7.50 (3.10–14.9) | 5 (×5), NT |
| ºMali | 16, 13.6 (8.24–20.7) | 1 (×6), 3, 5, 34, 35F (×3), NT (×4) |
| ºMongolia | 1, 0.93 (0.05–4.48) | 19F |
| ºParaguay | 22, 22.2 (14.9–31.2) | 9V, 14 (×2), 19A, 20, NT (×17) |
The Global Approach for Biological Research on Infectious Epidemics in Low Income Countries (GABRIEL) Network, 2010 Through 2014.
Abbreviation: NT, not typeable.
Study Center–stratified Proportions and Distribution of Streptococcus pneumoniae Serotypes Detected by Polymerase Chain Reaction in Blood From Cases
| . | Positive Polymerase Chain Reaction in Blood, n, proportion per 100 cases (95% Confidence Interval) . | Serotype . |
|---|---|---|
| Study site | ||
| ºCambodia | 5, 2.84 (1.05–6.18) | NT (×5) |
| ºChina | 1, 2.56 (0.13–12.0) | NT |
| ºHaiti | 9, 8.91 (4.43–15.7) | 14 (×2), 6AB, 19F, NT (×5) |
| ºIndia, Lucknow | 6, 6.25 (2.57–12.5) | 1, 7C, 20, NT (×3) |
| ºIndia, Pune/Vadu | 8, 11.3 (5.37–20.3) | 1, 6AB (×2), 8, NT (×4) |
| ºMadagascar | 6, 7.50 (3.10–14.9) | 5 (×5), NT |
| ºMali | 16, 13.6 (8.24–20.7) | 1 (×6), 3, 5, 34, 35F (×3), NT (×4) |
| ºMongolia | 1, 0.93 (0.05–4.48) | 19F |
| ºParaguay | 22, 22.2 (14.9–31.2) | 9V, 14 (×2), 19A, 20, NT (×17) |
| . | Positive Polymerase Chain Reaction in Blood, n, proportion per 100 cases (95% Confidence Interval) . | Serotype . |
|---|---|---|
| Study site | ||
| ºCambodia | 5, 2.84 (1.05–6.18) | NT (×5) |
| ºChina | 1, 2.56 (0.13–12.0) | NT |
| ºHaiti | 9, 8.91 (4.43–15.7) | 14 (×2), 6AB, 19F, NT (×5) |
| ºIndia, Lucknow | 6, 6.25 (2.57–12.5) | 1, 7C, 20, NT (×3) |
| ºIndia, Pune/Vadu | 8, 11.3 (5.37–20.3) | 1, 6AB (×2), 8, NT (×4) |
| ºMadagascar | 6, 7.50 (3.10–14.9) | 5 (×5), NT |
| ºMali | 16, 13.6 (8.24–20.7) | 1 (×6), 3, 5, 34, 35F (×3), NT (×4) |
| ºMongolia | 1, 0.93 (0.05–4.48) | 19F |
| ºParaguay | 22, 22.2 (14.9–31.2) | 9V, 14 (×2), 19A, 20, NT (×17) |
The Global Approach for Biological Research on Infectious Epidemics in Low Income Countries (GABRIEL) Network, 2010 Through 2014.
Abbreviation: NT, not typeable.
Of the 34 cases in whom an S. pneumoniae serotype was detected in blood by PCR, 27 (79.4%) had the same S. pneumoniae serotype detected in their nasopharynx (Table 3). In 16 cases (47.1%), the serotype detected in the blood was the only serotype detected in the nasopharynx. In 11 cases (32.4%), the serotype detected in blood was also detected in the nasopharynx but associated with 1 or several other serotypes (P = .2).
Association Between Streptococcus pneumoniae Serotypes Detected in Nasopharyngeal Samples and in Blood by Polymerase Chain Reaction in Children Aged <5 Years With Pneumonia
| Serotype . | Positive Blood Sample, n . | Same Serotype Detected in Nose and Blood, n (% of Blood Samples With the Detected Serotype) . | Invasiveness (% of Detection of a Serotype in Blood by Polymerase Chain Reaction Among Nasopharyngeal Carriers) . |
|---|---|---|---|
| 1 | 8 | 6 (75.0) | 6/17 (35.3) |
| 5 | 6 | 6 (100.0) | 6/28 (21.4) |
| 14 | 4 | 4 (100.0) | 4/62 (6.5) |
| 6AB | 3 | 2 (66.7) | 2/155 (1.3) |
| 35F | 3 | 1 (33.3) | 1/8 (12.5) |
| 19F | 2 | 2 (100.0) | 2/85 (2.4) |
| 20 | 2 | 0 (0.0) | 0/20 (0.0) |
| 3 | 1 | 1 (100.0) | 1/10 (10.0) |
| 7C | 1 | 1 (100.0) | 1/17 (5.9) |
| 8 | 1 | 1 (100.0) | 1/4 (25.0) |
| 9V | 1 | 1 (100.0) | 1/32 (3.1) |
| 19A | 1 | 1 (100.0) | 1/44 (2.3) |
| 34 | 1 | 1 (100.0) | 1/30 (3.3) |
| TOTAL | 34 | 27 (79.4) | 27/410 (6.6) |
| Serotype . | Positive Blood Sample, n . | Same Serotype Detected in Nose and Blood, n (% of Blood Samples With the Detected Serotype) . | Invasiveness (% of Detection of a Serotype in Blood by Polymerase Chain Reaction Among Nasopharyngeal Carriers) . |
|---|---|---|---|
| 1 | 8 | 6 (75.0) | 6/17 (35.3) |
| 5 | 6 | 6 (100.0) | 6/28 (21.4) |
| 14 | 4 | 4 (100.0) | 4/62 (6.5) |
| 6AB | 3 | 2 (66.7) | 2/155 (1.3) |
| 35F | 3 | 1 (33.3) | 1/8 (12.5) |
| 19F | 2 | 2 (100.0) | 2/85 (2.4) |
| 20 | 2 | 0 (0.0) | 0/20 (0.0) |
| 3 | 1 | 1 (100.0) | 1/10 (10.0) |
| 7C | 1 | 1 (100.0) | 1/17 (5.9) |
| 8 | 1 | 1 (100.0) | 1/4 (25.0) |
| 9V | 1 | 1 (100.0) | 1/32 (3.1) |
| 19A | 1 | 1 (100.0) | 1/44 (2.3) |
| 34 | 1 | 1 (100.0) | 1/30 (3.3) |
| TOTAL | 34 | 27 (79.4) | 27/410 (6.6) |
The Global Approach for Biological Research on Infectious Epidemics in Low Income Countries (GABRIEL) Network, 2010 Through 2014.
Association Between Streptococcus pneumoniae Serotypes Detected in Nasopharyngeal Samples and in Blood by Polymerase Chain Reaction in Children Aged <5 Years With Pneumonia
| Serotype . | Positive Blood Sample, n . | Same Serotype Detected in Nose and Blood, n (% of Blood Samples With the Detected Serotype) . | Invasiveness (% of Detection of a Serotype in Blood by Polymerase Chain Reaction Among Nasopharyngeal Carriers) . |
|---|---|---|---|
| 1 | 8 | 6 (75.0) | 6/17 (35.3) |
| 5 | 6 | 6 (100.0) | 6/28 (21.4) |
| 14 | 4 | 4 (100.0) | 4/62 (6.5) |
| 6AB | 3 | 2 (66.7) | 2/155 (1.3) |
| 35F | 3 | 1 (33.3) | 1/8 (12.5) |
| 19F | 2 | 2 (100.0) | 2/85 (2.4) |
| 20 | 2 | 0 (0.0) | 0/20 (0.0) |
| 3 | 1 | 1 (100.0) | 1/10 (10.0) |
| 7C | 1 | 1 (100.0) | 1/17 (5.9) |
| 8 | 1 | 1 (100.0) | 1/4 (25.0) |
| 9V | 1 | 1 (100.0) | 1/32 (3.1) |
| 19A | 1 | 1 (100.0) | 1/44 (2.3) |
| 34 | 1 | 1 (100.0) | 1/30 (3.3) |
| TOTAL | 34 | 27 (79.4) | 27/410 (6.6) |
| Serotype . | Positive Blood Sample, n . | Same Serotype Detected in Nose and Blood, n (% of Blood Samples With the Detected Serotype) . | Invasiveness (% of Detection of a Serotype in Blood by Polymerase Chain Reaction Among Nasopharyngeal Carriers) . |
|---|---|---|---|
| 1 | 8 | 6 (75.0) | 6/17 (35.3) |
| 5 | 6 | 6 (100.0) | 6/28 (21.4) |
| 14 | 4 | 4 (100.0) | 4/62 (6.5) |
| 6AB | 3 | 2 (66.7) | 2/155 (1.3) |
| 35F | 3 | 1 (33.3) | 1/8 (12.5) |
| 19F | 2 | 2 (100.0) | 2/85 (2.4) |
| 20 | 2 | 0 (0.0) | 0/20 (0.0) |
| 3 | 1 | 1 (100.0) | 1/10 (10.0) |
| 7C | 1 | 1 (100.0) | 1/17 (5.9) |
| 8 | 1 | 1 (100.0) | 1/4 (25.0) |
| 9V | 1 | 1 (100.0) | 1/32 (3.1) |
| 19A | 1 | 1 (100.0) | 1/44 (2.3) |
| 34 | 1 | 1 (100.0) | 1/30 (3.3) |
| TOTAL | 34 | 27 (79.4) | 27/410 (6.6) |
The Global Approach for Biological Research on Infectious Epidemics in Low Income Countries (GABRIEL) Network, 2010 Through 2014.
The more frequently invasive strains (number of cases with detection of S. pneumoniae serotype both in the nasopharynx and blood divided by the number of cases with the same serotype detected only in the nose) were serotypes 1 (35.3%), 8 (25.0%), 5 (21.4%), 35F (12.5%), and 3 (10.0%). The results of bacteria isolated in blood cultures are listed in Supplementary Table 7.
DISCUSSION
The data presented here essentially represent findings that were made prior to widespread introduction of PCVs. The results reveal heterogeneity for proportions of NP carriage of S. pneumoniae in both cases and controls among countries but they do not show a clear difference for proportions of NP carriage between low-income countries and low- to middle-income countries [18]. The heterogeneity among sites could reflect the spread of S. pneumoniae in the community and the prevalence of pneumonia related to S. pneumoniae. At most of the study sites, S. pneumoniae NP carriage was most frequently detected in cases compared with controls, reflecting the fact that NP carriage is a prerequisite to invasive disease [7, 19]. However, the dominant serotypes in both cases and controls were, overall, similar and included PCV13 serotypes [20, 21].
To our knowledge, no data on the prevalence and serotype distribution of S. pneumoniae in children aged <5 years without pneumonia (ie, in controls) are available for Haiti, Madagascar, Mali, Mongolia, and Paraguay. The results presented here therefore provide valuable data for these countries.
Some interesting differences in serotype distribution were noted. Serotype 14 was retrieved as a major serotype of NP carriage in China, Mongolia, and Paraguay. In Paraguay, this serotype was more frequently detected for NP samples from cases compared with controls. These results support data from Argentina that show that serotype 14 is more frequently detected in IPD in young children [22]. In Haiti, NP carriage of serotype 6AB was more frequently found in cases compared with controls. In China, different studies showed that the most frequent serotypes were 19F, 19A, 14, 6AB, 23F, 15, and 17 [23, 24]. Our results also showed serotypes 19F and 19A as well as serotypes 3, 9V, and 6AB as the most frequently detected in NP samples from cases. Finally, in cases in Madagascar, a higher proportion were NP carriers of serotype 5 compared with the controls. Serotype 5 has already been shown to be a major serotype involved in IPD in Africa [25].
There have been reports that most of these serotypes cause IPD but are also common in carriage (6AB, 19F, 14). Because the capsular serotype is a primary determinant in disease potential, some serotypes appear to have a higher disease potential (1, 5, 7F, 9V) [21, 26, 27]. A low prevalence of carriage of a serotype with high disease potential would not entail a significant IPD burden in the community, while a high prevalence of carriage of a serotype with low disease potential could lead to a significant IPD burden [23].
Despite the similarity of dominant serotypes detected in NP samples from the cases at all project sites, the prevalence of S. pneumoniae detection in blood by PCR differed substantially by study site. These differences could be explained by the heterogeneity of the circulating serotypes, their prevalence, and their disease potential as well as environmental factors (rates of attendance of child care) or genetic factors. A potential overestimation of the detection of S. pneumoniae in blood using PCR could be explained by antibiotic use and organism clearance or by the presence of nonviable organisms merely detected by translocation from the lungs or nasopharynx. The major serotypes found in blood by PCR (1, 5, 14, 35F, 6AB, 19F) and the invasiveness of these serotypes are in accordance with data in the literature [26, 28]. Some studies have shown that serotypes 1, 5, and 14 are “epidemic” serotypes [29–31]. They are included in PCV13. Serotypes 8 and 35F, which are not included in PCV13, are scarce, and their invasive potential is not well understood. Serotype 6AB was found elsewhere to have a low invasiveness [32].
Few studies have assessed S. pneumoniae serotypes for both NP carriage and blood by PCR in children with pneumonia. In our results, for approximately 80% of cases in which an S. pneumoniae serotype was detected in blood by PCR, the same serotype was detected in NP samples. These results strongly suggest that the isolate that carried and caused disease in an individual were of the same serotype [27, 33, 34].
Patients with pneumonia and S. pneumoniae detected in blood by PCR were more frequently NP carriers of PCV serotypes. Since S. pneumoniae–related pneumonia rates decline substantially after introduction of PCV in national immunization programs, this suggests that a PCV vaccination policy would be effective in the study population [35, 36].
Our study has limitations. First, some confounding factors were not taken into account in the regression models, for example, climatic conditions and tools for respiratory virus detection [37]. Antibiotic intake was not available at all study sites. A selection bias cannot be excluded during the inclusion process, particularly if patients were recruited from specific populations (ie, socioeconomic factors, local outbreak, seasonality). This might explain some of the heterogeneity of proportions of NP carriage in pneumonia cases and controls among study sites. A detection bias could exist since the sensitivity of an NP swab is likely to be higher for pneumonia cases owing to larger mucous volumes [38].
Unlike blood cultures, the detection of S. pneumoniae in blood by PCR does not mean that S. pneumoniae is the etiology of the pneumonia. Blood cultures remain the gold standard for confirming diagnosis, but the yield of S. pneumoniae–positive cultures is low and decreased by antibiotic intake before culture [3, 39]. However, blood PCR could be useful by confirming the serotype of presumed S. pneumonia–related pneumonia.
The main strengths of this study are its prospective multicenter design and the standardized procedures that made it possible to adjust analysis of the main confounding factors. It provided valuable data on baseline NP carriage of S. pneumoniae in different low-income and low- to middle-income countries where few data are available, especially in a pre-PCV situation. Moreover, our results support and complete the results of the Pneumonia Etiology Research for Child Health study since the study sites were different with overlap for only 1 country (Mali). Some results from that study are consistent with our results, for example, high NP carriage in the community, an association between NP carriage of serotype 14 and the case status, and that fact that NP carriage was lower among cases with prior antibiotic use [40] (results not published). Further studies are needed to evaluate the effects of implementation of a PCV vaccination policy in the countries in question and particularly the epidemiological change of detected serotypes. For this purpose, a new study is currently being designed and will be performed beginning at the end of 2018.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors acknowledge the Fondation Mérieux for its support and Dr Thomas Bénet, Pr. Florence Ader, Pr. René Écochard, Pr. Étienne Javouhey, and Pr. Gérard Lina for their precious advices. The authors also thank Peter Tucker for editing the manuscript.
Disclaimer. The study funders had no role in data analysis, the decision to publish, or manuscript preparation.
Financial support. This study was supported by the Global Approach for Biological Research on Infectious Epidemics in Low Income Countries (GABRIEL) Network of Fondation Mérieux.
Potential conflicts of interest. A. B. has received grants from Fondation Mérieux. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




