-
PDF
- Split View
-
Views
-
Cite
Cite
Carlos F A Carvalho, Nick Andrews, Gavin Dabrera, Sonia Ribeiro, Julia Stowe, Mary Ramsay, Gayatri Amirthalingam, National Outbreak of Pertussis in England, 2011–2012: A Case-control Study Comparing 3-Component and 5-Component Acellular Vaccines With Whole-cell Pertussis Vaccines, Clinical Infectious Diseases, Volume 70, Issue 2, 15 January 2020, Pages 200–207, https://doi.org/10.1093/cid/ciz199
Close - Share Icon Share
Abstract
In England, acellular pertussis (aP) vaccines replaced whole-cell pertussis (wP) vaccine in the primary immunization course in October 2004. Despite sustained high vaccine coverage, 10 454 cases were confirmed in England in 2011–2012, including 1648 (16%) in those aged 10–19 years. These individuals had been primed with either 3-component (aP3) or 5-component (aP5) aP vaccines or wP vaccine due to temporary supply shortages. We aimed to compare protection provided by different pertussis vaccines.
We conducted a case-control study. Individuals born between 1997 and 2005, fully vaccinated in England, were included. Cases were laboratory confirmed between January 2011 and December 2012. Controls were identified from population vaccination registers, representing 20% of the population. We compared the odds of receiving different types of vaccines in cases and controls, adjusting for year of birth and time since last vaccine received. Odds ratios (ORs) were calculated with 95% confidence intervals (CIs).
We analyzed 403 cases and 581 971 controls with 4 pertussis vaccines recorded. Compared to those who received 3 doses of wP for the primary course, cases had higher odds of receiving 3 doses of aP3 (OR, 3.86 [95% CI, 2.56–5.82]) but no significant association with receipt of 3 doses of aP5 (OR, 0.89 [95% CI, .29–2.73]).
Previous studies have suggested that aP3 and aP5 vaccines provide shorter duration of protection than wP vaccine. Our findings suggest that a primary course with aP3 is associated with increased risk of confirmed pertussis compared with wP. Although follow-up was shorter for aP5 cohorts, their risk did not seem to differ from wP.
(See the Editorial Commentary by Harriman and Healy on pages 208–9.)
Whooping cough, a respiratory disease caused by Bordetella pertussis, remains a disease of global public health importance, with an estimated 16 million cases reported by the World Health Organization (WHO) worldwide in 2008 and 195 000 deaths among young children [1]. Despite a significant reduction in global burden of disease following the introduction of routine whole-cell pertussis (wP) immunization in the 1950s, the disease remains endemic worldwide, with sporadic outbreaks and epidemic peaks observed every 2–5 years [2].
Young unimmunized infants are at highest risk of severe complications and death from pertussis. Protection conferred either through vaccination or natural infection is not lifelong, and older vaccinated individuals can present with milder, often atypical symptoms, leading to delays in diagnosis and risk of transmission to unvaccinated children [3].
In the United Kingdom (UK), the wP vaccine was introduced in the primary infant vaccination schedule in 1957. In the 1970s, following concerns about the safety of wP vaccines, less-reactogenic acellular pertussis (aP) vaccines were developed [4]. Acellular vaccines were first introduced into the routine UK immunization schedule in October 2001 as part of the preschool booster (PSB) dose offered to children aged 3–5 years. In 2004, the UK switched to aP vaccines in the primary infant schedule, offering 3 doses of a 5-component acellular pertussis vaccine (aP5) at 2, 3, and 4 months of age [5]. By 1996–1997, 96% of 2-year-olds in England had completed their primary course (3 doses); in 2011–2012, 87% of 5-year-olds had received a PSB [6].
Following a long period of good pertussis control, from 2011 an increase in laboratory-confirmed cases was observed across England with 10 454 cases between January 2011 and December 2012. These numbers were over and above what would be expected during the regular cyclical increase, with adolescents and adults >19 years old representing 74% of total cases [5, 7]. This had been noted in other countries, leading to some countries such as the United States introducing an adolescent booster [8].
In its 2015 position paper, after reviewing reports of increased cases in a number of countries, WHO concluded that the switch to aP vaccines contributed to a genuine resurgence of pertussis. This led to the recommendation that “national programmes currently administering wP vaccination should continue to use wP vaccines for primary vaccination series,” given the higher cost, shorter duration of protection, and limited protection against infection of aP vaccines [9].
Previous clinical trials have suggested potential differences in efficacy between some aP vaccines [10–12], but the currently available acellular vaccines, and specifically those used in the UK, were not compared. On the basis of the available evidence, the UK Joint Committee on Vaccination and Immunisation recommends that only aP vaccines with ≥3 pertussis components should be used in the national program as vaccines with only 1 or 2 components are less effective [13, 14].
In the UK, vaccines recommended as part of the national program are procured centrally. The types of pertussis vaccines used have changed over time (Table 1).
Types of Pertussis-containing Vaccines Recommended in England Until June 2012, by Birth Cohort
| Birth Cohort . | Age as of 30 June 2012 . | Primary Course . | Preschool Booster (Age 3 y 4 mo to 5 y) . |
|---|---|---|---|
| July 1957–June 1997 | 15–54 y | DTwP (multiple manufacturers; used only for primary course) | … |
| July 1997–June 1999 | 13–14 y | DTwP | DTaP3 |
| July 1999–June 2001 | 11–12 y | DTaP3 or DTwP | DTaP3 or dTap5-low |
| July 2001–June 2004 | 8–10 y | DTwP | dTap5-low or DTaP3 |
| July 2004–June 2012 | 0–7 y | DTaP5 |
| Birth Cohort . | Age as of 30 June 2012 . | Primary Course . | Preschool Booster (Age 3 y 4 mo to 5 y) . |
|---|---|---|---|
| July 1957–June 1997 | 15–54 y | DTwP (multiple manufacturers; used only for primary course) | … |
| July 1997–June 1999 | 13–14 y | DTwP | DTaP3 |
| July 1999–June 2001 | 11–12 y | DTaP3 or DTwP | DTaP3 or dTap5-low |
| July 2001–June 2004 | 8–10 y | DTwP | dTap5-low or DTaP3 |
| July 2004–June 2012 | 0–7 y | DTaP5 |
Abbreviations: d, low-dose diphtheria; D, diphtheria; dTap5-low, low-dose 5-component acellular pertussis (2.5 µg of pertussis toxoid, 5 µg of filamentous hemagglutinin, 3 µg of pertactin, 5 µg of co-purified fimbriae types 2 and 3) used only for preschool booster; manufactured by Sanofi Pasteur/Merck Sharp & Dohme (Repevax); DTaP3, 3-component acellular pertussis (25 µg of pertussis toxoid, 25 µg of filamentous hemagglutinin, 8 µg of pertactin), manufactured by GlaxoSmithKline (Infanrix); DTaP5, 5-component acellular pertussis (20 µg of pertussis toxoid, 20 µg of filamentous hemagglutinin, 3 µg of pertactin, 5 µg of co-purified fimbriae types 2 and 3; manufactured by Sanofi Pasteur/Merck Sharp & Dohme (Pediacel); DTwP, diphtheria–tetanus–whole-cell pertussis;.
Types of Pertussis-containing Vaccines Recommended in England Until June 2012, by Birth Cohort
| Birth Cohort . | Age as of 30 June 2012 . | Primary Course . | Preschool Booster (Age 3 y 4 mo to 5 y) . |
|---|---|---|---|
| July 1957–June 1997 | 15–54 y | DTwP (multiple manufacturers; used only for primary course) | … |
| July 1997–June 1999 | 13–14 y | DTwP | DTaP3 |
| July 1999–June 2001 | 11–12 y | DTaP3 or DTwP | DTaP3 or dTap5-low |
| July 2001–June 2004 | 8–10 y | DTwP | dTap5-low or DTaP3 |
| July 2004–June 2012 | 0–7 y | DTaP5 |
| Birth Cohort . | Age as of 30 June 2012 . | Primary Course . | Preschool Booster (Age 3 y 4 mo to 5 y) . |
|---|---|---|---|
| July 1957–June 1997 | 15–54 y | DTwP (multiple manufacturers; used only for primary course) | … |
| July 1997–June 1999 | 13–14 y | DTwP | DTaP3 |
| July 1999–June 2001 | 11–12 y | DTaP3 or DTwP | DTaP3 or dTap5-low |
| July 2001–June 2004 | 8–10 y | DTwP | dTap5-low or DTaP3 |
| July 2004–June 2012 | 0–7 y | DTaP5 |
Abbreviations: d, low-dose diphtheria; D, diphtheria; dTap5-low, low-dose 5-component acellular pertussis (2.5 µg of pertussis toxoid, 5 µg of filamentous hemagglutinin, 3 µg of pertactin, 5 µg of co-purified fimbriae types 2 and 3) used only for preschool booster; manufactured by Sanofi Pasteur/Merck Sharp & Dohme (Repevax); DTaP3, 3-component acellular pertussis (25 µg of pertussis toxoid, 25 µg of filamentous hemagglutinin, 8 µg of pertactin), manufactured by GlaxoSmithKline (Infanrix); DTaP5, 5-component acellular pertussis (20 µg of pertussis toxoid, 20 µg of filamentous hemagglutinin, 3 µg of pertactin, 5 µg of co-purified fimbriae types 2 and 3; manufactured by Sanofi Pasteur/Merck Sharp & Dohme (Pediacel); DTwP, diphtheria–tetanus–whole-cell pertussis;.
From 2004 to 2012, the UK childhood immunization schedule included 3 doses of Diphtheria, Tetanus, 5-component acellular Pertussis (DTaP5)-Inactivated Poliovirus (IPV)-Haemophilus influenzae type b (Hib) for priming (Pediacel) and 1 dose of either Diphtheria, Tetanus, 3-component acellular Pertussis (DTaP3)–IPV (Infanrix-IPV) or low-dose diphtheria, Tetanus, low-dose 5-component acellular Pertussis (dTap5)–IPV (Repevax) for PSB. For those born before 2004, types and schedules of recommended vaccines depended on the birth cohort (Table 1).
The main objectives of this study were to compare the effectiveness of the aP vaccines currently licensed for the primary childhood immunization program in England (3-component acellular pertussis vaccine [aP3] and aP5) with the wP vaccines previously used, and the effectiveness of the different acellular PSB vaccines. This was done within fully vaccinated children and teenagers, during the national outbreak in 2011 and 2012.
METHODS
Study Design
We conducted a retrospective case-control study, comparing fully vaccinated pertussis cases diagnosed in England from 1 January 2011 to 31 December 2012 with fully vaccinated controls concerning the type of pertussis vaccines received for their primary course (PC) and PSB. We included cases and controls aged between 5 and 15 years at the time of the outbreak to estimate the effectiveness of pertussis vaccination among individuals who were old enough to have received PC and PSB.
Cases were defined as individuals registered in any Primary Care Trust (PCT) in England, born from 1 January 1997 to 31 December 2005, reported to Public Health England (formerly Health Protection Agency) as a laboratory-confirmed case of pertussis according to the national England case definition, who had received 4 documented doses of pertussis vaccine at least 60 days before onset of symptoms (or 90 days before date of specimen collection if onset date was not available). Laboratory-confirmed cases were defined as any person in whom a clinician suspects pertussis infection with either (1) culture result positive for Bordetella pertussis; or (2) serology result of pertussis toxin (PT) immunoglobulin G (IgG) >70 IU/mL (as a diagnostic threshold for recent infection), and no pertussis-containing vaccine administered within 1 year prior to specimen collection [15, 16]. Cases that tested positive by polymerase chain reaction (PCR) were not included in this study as this was only available for infants <1 year of age during the study period in England.
Controls were extracted from local Child Health Information Systems (CHISs), which are population-based registers that record information provided by general practitioners on vaccines administered for all children in the local population. Data from these systems are extracted to measure routine vaccine coverage of the childhood program in England [17]. Data held on those CHISs using the McKesson CarePlus system (McKesson, San Francisco, California) were extracted, representing approximately 20% of the population born from 1997 to 2005. These CHISs were geographically dispersed to include 14 of the 152 PCTs across the country that achieved an average level of coverage of 93% in 2005, close to the national average of 91% in the same year [18]. To prevent cases being included in the control group, individuals were matched on National Health Service number and excluded.
Exposure Assessment
Local PCTs and general practitioners were contacted and asked to complete a form containing dates, batch numbers, and commercial names of the vaccines administered to the cases. Dates of vaccination and respective batch numbers were extracted from the CHIS for controls. No sampling was performed, as vaccine history is routinely collected and stored on the CHIS. Vaccines were identified from batch numbers, using look-up tables provided by the 2 main manufacturers distributing pertussis vaccines in England and by the Department of Health, which is responsible for the procurement and distribution of all vaccines in the childhood routine schedule. Cases and controls without 4 doses of vaccine documented (including at least date of administration) were excluded.
Primary pertussis vaccination course was categorized as “all wP,” “all aP5,” “all aP3,” the combinations “wP/aP5 mix” and “wP/aP3 mix,” or “missing”. The latter category would include any PC with at least 1 missing batch number.
The PSB was recorded as “aP3,” “aP5,” or “aP5-low” (ie, low-dose 5-component vaccine).
Statistical Analysis
Cases and controls were described by year of birth, gender, type of primary course, and PSB received. We calculated odds ratios (ORs) and 95% confidence intervals (CIs) using logistic regression for the association between laboratory-confirmed pertussis and vaccination history. To assess the association between confirmed pertussis and type of pertussis vaccine used for priming, a 3-dose course of wP vaccine was used as the reference.
For the 1999–2002 birth cohorts, schedules with aP3 were compared with the reference. For the 2003–2005 birth cohorts, schedules with aP5 were compared with the reference. The effect of the PSB was analyzed stratifying by single-type primary courses.
Data analysis was performed using Stata software version 12. The multivariable analysis and multiple imputation of missing values are described in full in the Supplementary Materials.
Given the different PT content of the aP3 and aP5 vaccines, antibody titers in those cases diagnosed by serology from 1 January 2011 to 31 December 2012 were compared by type of vaccines received using geometric means and 95% CIs, to identify any potential confounding arising from cases receiving one specific vaccine having a higher probability of being included as a case.
Ethical Considerations
As this study was undertaken as part of a national outbreak response, ethical approval was not required and data collation was covered by existing information governance approvals.
RESULTS
From 1994 to 2012, a total of 17 351 laboratory-confirmed cases of pertussis were followed up by the Health Protection Agency through its national enhanced surveillance. Of those, 3051 were born from January 1997 to December 2005, with 949 having onset of symptoms (or date of specimen collected) in 2011 or 2012. Dates of administration of 4 doses of pertussis vaccine were obtained for 403 (42%), but valid batch numbers were recorded for only 222 patients (Figure 1).
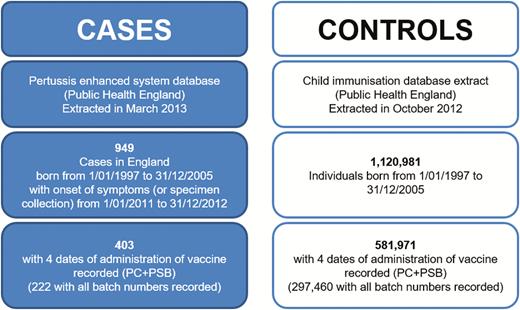
Study flowchart. Abbreviations: PC, primary course; PSB, preschool booster.
The CHIS database included 1 120 981 individuals born from January 1997 to December 2005. Four doses of pertussis vaccine were recorded for 581 971 (52%) controls, including valid batch numbers for PC and PSB in 297 460 controls (Figure 1).
As shown in Table 2 and Figure 2A and 2B, the wP vaccine was used until 2004 for PC, when it was replaced by aP5 in the recommended vaccination schedule. The aP3 vaccine was used concurrently with wP vaccine in the period from 1999 to 2002, but not after that, for PC.
Types of Vaccines Given to the Study Population (Cases and Controls), by Year of Birth
| . | Year of Birth . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of Vaccine . | 1997 . | 1998 . | 1999 . | 2000 . | 2001 . | 2002 . | 2003 . | 2004 . | 2005 . | Total . |
| Primary course | 19 733 | 55 321 | 60 584 | 62 138 | 60 811 | 61 187 | 68 696 | 79 205 | 114 699 | 582 374 |
| all wP | 9637 | 29 977 | 26 721 | 13 084 | 13 161 | 16 837 | 35 965 | 9150 | 0 | 154 532 |
| wP/aP5 mix | 4 | 2 | 6 | 6 | 34 | 111 | 589 | 6590 | 8 | 7350 |
| All aP5 | 1 | 4 | 9 | 8 | 21 | 43 | 126 | 29 148 | 75 227 | 104 587 |
| wP/aP3 mix | 32 | 139 | 6005 | 12 863 | 10 334 | 14 013 | 608 | 149 | 0 | 44 143 |
| All aP3 | 1 | 2 | 601 | 8481 | 10 508 | 4162 | 44 | 27 | 18 | 23 844 |
| Missing/other | 10 058 | 25 197 | 27 242 | 27 696 | 26 753 | 26 021 | 31 364 | 34 141 | 39 446 | 247 918 |
| Preschool booster | 19 733 | 55 321 | 60 584 | 62 138 | 60 811 | 61 187 | 68 696 | 79 205 | 114 699 | 582 374 |
| aP3 | 14 223 | 41 199 | 42 376 | 30 004 | 15 171 | 8751 | 22 289 | 31 310 | 55 188 | 260 511 |
| aP5 | 5 | 25 | 53 | 294 | 699 | 832 | 3933 | 19 064 | 9405 | 34 310 |
| aP5-low | 223 | 460 | 1915 | 16 089 | 29 872 | 36 349 | 24 178 | 8260 | 25 860 | 143 206 |
| Batch missing | 5282 | 13 637 | 16 240 | 15 751 | 15 069 | 15 255 | 18 296 | 20 571 | 24 246 | 144 347 |
| . | Year of Birth . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of Vaccine . | 1997 . | 1998 . | 1999 . | 2000 . | 2001 . | 2002 . | 2003 . | 2004 . | 2005 . | Total . |
| Primary course | 19 733 | 55 321 | 60 584 | 62 138 | 60 811 | 61 187 | 68 696 | 79 205 | 114 699 | 582 374 |
| all wP | 9637 | 29 977 | 26 721 | 13 084 | 13 161 | 16 837 | 35 965 | 9150 | 0 | 154 532 |
| wP/aP5 mix | 4 | 2 | 6 | 6 | 34 | 111 | 589 | 6590 | 8 | 7350 |
| All aP5 | 1 | 4 | 9 | 8 | 21 | 43 | 126 | 29 148 | 75 227 | 104 587 |
| wP/aP3 mix | 32 | 139 | 6005 | 12 863 | 10 334 | 14 013 | 608 | 149 | 0 | 44 143 |
| All aP3 | 1 | 2 | 601 | 8481 | 10 508 | 4162 | 44 | 27 | 18 | 23 844 |
| Missing/other | 10 058 | 25 197 | 27 242 | 27 696 | 26 753 | 26 021 | 31 364 | 34 141 | 39 446 | 247 918 |
| Preschool booster | 19 733 | 55 321 | 60 584 | 62 138 | 60 811 | 61 187 | 68 696 | 79 205 | 114 699 | 582 374 |
| aP3 | 14 223 | 41 199 | 42 376 | 30 004 | 15 171 | 8751 | 22 289 | 31 310 | 55 188 | 260 511 |
| aP5 | 5 | 25 | 53 | 294 | 699 | 832 | 3933 | 19 064 | 9405 | 34 310 |
| aP5-low | 223 | 460 | 1915 | 16 089 | 29 872 | 36 349 | 24 178 | 8260 | 25 860 | 143 206 |
| Batch missing | 5282 | 13 637 | 16 240 | 15 751 | 15 069 | 15 255 | 18 296 | 20 571 | 24 246 | 144 347 |
Abbreviations: aP3, 3-component acellular pertussis vaccine (Infanrix); aP5, 5-component acellular pertussis vaccine (Pediacel); aP5-low, 5-component, low-dose acellular pertussis vaccine (Repevax); wP, whole-cell pertussis vaccine.
Types of Vaccines Given to the Study Population (Cases and Controls), by Year of Birth
| . | Year of Birth . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of Vaccine . | 1997 . | 1998 . | 1999 . | 2000 . | 2001 . | 2002 . | 2003 . | 2004 . | 2005 . | Total . |
| Primary course | 19 733 | 55 321 | 60 584 | 62 138 | 60 811 | 61 187 | 68 696 | 79 205 | 114 699 | 582 374 |
| all wP | 9637 | 29 977 | 26 721 | 13 084 | 13 161 | 16 837 | 35 965 | 9150 | 0 | 154 532 |
| wP/aP5 mix | 4 | 2 | 6 | 6 | 34 | 111 | 589 | 6590 | 8 | 7350 |
| All aP5 | 1 | 4 | 9 | 8 | 21 | 43 | 126 | 29 148 | 75 227 | 104 587 |
| wP/aP3 mix | 32 | 139 | 6005 | 12 863 | 10 334 | 14 013 | 608 | 149 | 0 | 44 143 |
| All aP3 | 1 | 2 | 601 | 8481 | 10 508 | 4162 | 44 | 27 | 18 | 23 844 |
| Missing/other | 10 058 | 25 197 | 27 242 | 27 696 | 26 753 | 26 021 | 31 364 | 34 141 | 39 446 | 247 918 |
| Preschool booster | 19 733 | 55 321 | 60 584 | 62 138 | 60 811 | 61 187 | 68 696 | 79 205 | 114 699 | 582 374 |
| aP3 | 14 223 | 41 199 | 42 376 | 30 004 | 15 171 | 8751 | 22 289 | 31 310 | 55 188 | 260 511 |
| aP5 | 5 | 25 | 53 | 294 | 699 | 832 | 3933 | 19 064 | 9405 | 34 310 |
| aP5-low | 223 | 460 | 1915 | 16 089 | 29 872 | 36 349 | 24 178 | 8260 | 25 860 | 143 206 |
| Batch missing | 5282 | 13 637 | 16 240 | 15 751 | 15 069 | 15 255 | 18 296 | 20 571 | 24 246 | 144 347 |
| . | Year of Birth . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of Vaccine . | 1997 . | 1998 . | 1999 . | 2000 . | 2001 . | 2002 . | 2003 . | 2004 . | 2005 . | Total . |
| Primary course | 19 733 | 55 321 | 60 584 | 62 138 | 60 811 | 61 187 | 68 696 | 79 205 | 114 699 | 582 374 |
| all wP | 9637 | 29 977 | 26 721 | 13 084 | 13 161 | 16 837 | 35 965 | 9150 | 0 | 154 532 |
| wP/aP5 mix | 4 | 2 | 6 | 6 | 34 | 111 | 589 | 6590 | 8 | 7350 |
| All aP5 | 1 | 4 | 9 | 8 | 21 | 43 | 126 | 29 148 | 75 227 | 104 587 |
| wP/aP3 mix | 32 | 139 | 6005 | 12 863 | 10 334 | 14 013 | 608 | 149 | 0 | 44 143 |
| All aP3 | 1 | 2 | 601 | 8481 | 10 508 | 4162 | 44 | 27 | 18 | 23 844 |
| Missing/other | 10 058 | 25 197 | 27 242 | 27 696 | 26 753 | 26 021 | 31 364 | 34 141 | 39 446 | 247 918 |
| Preschool booster | 19 733 | 55 321 | 60 584 | 62 138 | 60 811 | 61 187 | 68 696 | 79 205 | 114 699 | 582 374 |
| aP3 | 14 223 | 41 199 | 42 376 | 30 004 | 15 171 | 8751 | 22 289 | 31 310 | 55 188 | 260 511 |
| aP5 | 5 | 25 | 53 | 294 | 699 | 832 | 3933 | 19 064 | 9405 | 34 310 |
| aP5-low | 223 | 460 | 1915 | 16 089 | 29 872 | 36 349 | 24 178 | 8260 | 25 860 | 143 206 |
| Batch missing | 5282 | 13 637 | 16 240 | 15 751 | 15 069 | 15 255 | 18 296 | 20 571 | 24 246 | 144 347 |
Abbreviations: aP3, 3-component acellular pertussis vaccine (Infanrix); aP5, 5-component acellular pertussis vaccine (Pediacel); aP5-low, 5-component, low-dose acellular pertussis vaccine (Repevax); wP, whole-cell pertussis vaccine.
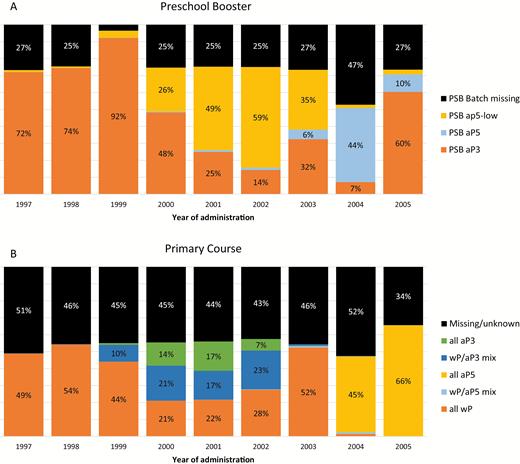
Types of vaccines administered for the preschool booster (A) and primary course (B). Abbreviations: aP3, 3-component acellular pertussis vaccine (Infanrix); aP5, 5-component acellular pertussis vaccine (Pediacel); aP5-low, 5-component, low-dose acellular pertussis vaccine (Repevax); PSB, preschool booster; wP, whole-cell pertussis vaccine.
For PSB, aP3 was the main vaccine used for those born between 1997 and 2000 (administered in 2000–2005). The aP5-low vaccine became the most frequently used vaccine for the cohorts born after 2000, but aP5 and aP3 were used as alternatives, especially in those born after 2003. Analysis of dates of vaccination indicates that use of aP5 in children born before 2004 reflects delayed vaccination in these children.
In the study population, cases tended to be older and consequently to have received their PSB earlier than controls (Table 3). The vaccines administered to the study population varied according to the year of birth, reflecting the historical changes to vaccines used in the national program.
Description of Cases and Controls With at Least 3 Doses of Vaccine Recorded (Primary Course)
| Characteristics . | Cases (n = 403) . | Controls (n = 581 971) . |
|---|---|---|
| Year of birth | ||
| 1997 | 31 (7.7) | 19 702 (3.4) |
| 1998 | 76 (18.9) | 55 245 (9.5) |
| 1999 | 79 (19.6) | 60 505 (10.4) |
| 2000 | 79 (19.6) | 62 059 (10.7) |
| 2001 | 54 (13.4) | 60 757 (10.4) |
| 2002 | 38 (9.4) | 61 149 (10.5) |
| 2003 | 17 (4.2) | 68 679 (11.8) |
| 2004 | 18 (4.5) | 79 187 (13.6) |
| 2005 | 11 (2.7) | 114 688 (19.7) |
| Gender | ||
| Male | 194 (48.1) | 297 041 (51.0) |
| Female | 209 (51.9) | 284 930 (49.0) |
| Year of PSB | ||
| 2000 | 2 (0.5) | 540 (0.1) |
| 2001 | 17 (4.2) | 7387 (1.3) |
| 2002 | 69 (17.1) | 58 045 (10.0) |
| 2003 | 66 (16.4) | 57 781 (9.9) |
| 2004 | 94 (23.3) | 62 762 (10.8) |
| 2005 | 68 (16.9) | 62 484 (10.7) |
| 2006 | 41 (10.2) | 60 284 (10.4) |
| 2007 | 15 (3.7) | 74 158 (12.7) |
| 2008 | 22 (5.5) | 109 848 (18.9) |
| 2009–2010 | 9 (2.2) | 88 682 (15.2) |
| PC type | ||
| All wP | 119 (29.5) | 154 413 (26.5) |
| wP/aP5 mix | 4 (1.0) | 7346 (1.3) |
| All aP5 | 17 (4.2) | 104 570 (18.0) |
| wP/aP3 mix | 62 (15.4) | 44 081 (7.6) |
| All aP3 | 47 (11.7) | 23 797 (4.1) |
| Missing | 154 (38.2) | 247 764 (42.6) |
| PSB type | ||
| aP3 | 189 (46.9) | 260 322 (44.7) |
| aP5 | 8 (2.0) | 34 302 (5.9) |
| aP5-low | 98 (24.3) | 143 108 (24.6) |
| Missing | 108 (26.8) | 144 239 (24.8) |
| Characteristics . | Cases (n = 403) . | Controls (n = 581 971) . |
|---|---|---|
| Year of birth | ||
| 1997 | 31 (7.7) | 19 702 (3.4) |
| 1998 | 76 (18.9) | 55 245 (9.5) |
| 1999 | 79 (19.6) | 60 505 (10.4) |
| 2000 | 79 (19.6) | 62 059 (10.7) |
| 2001 | 54 (13.4) | 60 757 (10.4) |
| 2002 | 38 (9.4) | 61 149 (10.5) |
| 2003 | 17 (4.2) | 68 679 (11.8) |
| 2004 | 18 (4.5) | 79 187 (13.6) |
| 2005 | 11 (2.7) | 114 688 (19.7) |
| Gender | ||
| Male | 194 (48.1) | 297 041 (51.0) |
| Female | 209 (51.9) | 284 930 (49.0) |
| Year of PSB | ||
| 2000 | 2 (0.5) | 540 (0.1) |
| 2001 | 17 (4.2) | 7387 (1.3) |
| 2002 | 69 (17.1) | 58 045 (10.0) |
| 2003 | 66 (16.4) | 57 781 (9.9) |
| 2004 | 94 (23.3) | 62 762 (10.8) |
| 2005 | 68 (16.9) | 62 484 (10.7) |
| 2006 | 41 (10.2) | 60 284 (10.4) |
| 2007 | 15 (3.7) | 74 158 (12.7) |
| 2008 | 22 (5.5) | 109 848 (18.9) |
| 2009–2010 | 9 (2.2) | 88 682 (15.2) |
| PC type | ||
| All wP | 119 (29.5) | 154 413 (26.5) |
| wP/aP5 mix | 4 (1.0) | 7346 (1.3) |
| All aP5 | 17 (4.2) | 104 570 (18.0) |
| wP/aP3 mix | 62 (15.4) | 44 081 (7.6) |
| All aP3 | 47 (11.7) | 23 797 (4.1) |
| Missing | 154 (38.2) | 247 764 (42.6) |
| PSB type | ||
| aP3 | 189 (46.9) | 260 322 (44.7) |
| aP5 | 8 (2.0) | 34 302 (5.9) |
| aP5-low | 98 (24.3) | 143 108 (24.6) |
| Missing | 108 (26.8) | 144 239 (24.8) |
Data are no. (%).
Abbreviations: aP3, 3-component acellular pertussis vaccine (Infanrix); aP5, 5-component acellular pertussis vaccine (Pediacel); aP5-low, 5-component, low-dose acellular pertussis vaccine (Repevax); PC, primary course; PSB, preschool booster; wP, whole-cell pertussis vaccine.
Description of Cases and Controls With at Least 3 Doses of Vaccine Recorded (Primary Course)
| Characteristics . | Cases (n = 403) . | Controls (n = 581 971) . |
|---|---|---|
| Year of birth | ||
| 1997 | 31 (7.7) | 19 702 (3.4) |
| 1998 | 76 (18.9) | 55 245 (9.5) |
| 1999 | 79 (19.6) | 60 505 (10.4) |
| 2000 | 79 (19.6) | 62 059 (10.7) |
| 2001 | 54 (13.4) | 60 757 (10.4) |
| 2002 | 38 (9.4) | 61 149 (10.5) |
| 2003 | 17 (4.2) | 68 679 (11.8) |
| 2004 | 18 (4.5) | 79 187 (13.6) |
| 2005 | 11 (2.7) | 114 688 (19.7) |
| Gender | ||
| Male | 194 (48.1) | 297 041 (51.0) |
| Female | 209 (51.9) | 284 930 (49.0) |
| Year of PSB | ||
| 2000 | 2 (0.5) | 540 (0.1) |
| 2001 | 17 (4.2) | 7387 (1.3) |
| 2002 | 69 (17.1) | 58 045 (10.0) |
| 2003 | 66 (16.4) | 57 781 (9.9) |
| 2004 | 94 (23.3) | 62 762 (10.8) |
| 2005 | 68 (16.9) | 62 484 (10.7) |
| 2006 | 41 (10.2) | 60 284 (10.4) |
| 2007 | 15 (3.7) | 74 158 (12.7) |
| 2008 | 22 (5.5) | 109 848 (18.9) |
| 2009–2010 | 9 (2.2) | 88 682 (15.2) |
| PC type | ||
| All wP | 119 (29.5) | 154 413 (26.5) |
| wP/aP5 mix | 4 (1.0) | 7346 (1.3) |
| All aP5 | 17 (4.2) | 104 570 (18.0) |
| wP/aP3 mix | 62 (15.4) | 44 081 (7.6) |
| All aP3 | 47 (11.7) | 23 797 (4.1) |
| Missing | 154 (38.2) | 247 764 (42.6) |
| PSB type | ||
| aP3 | 189 (46.9) | 260 322 (44.7) |
| aP5 | 8 (2.0) | 34 302 (5.9) |
| aP5-low | 98 (24.3) | 143 108 (24.6) |
| Missing | 108 (26.8) | 144 239 (24.8) |
| Characteristics . | Cases (n = 403) . | Controls (n = 581 971) . |
|---|---|---|
| Year of birth | ||
| 1997 | 31 (7.7) | 19 702 (3.4) |
| 1998 | 76 (18.9) | 55 245 (9.5) |
| 1999 | 79 (19.6) | 60 505 (10.4) |
| 2000 | 79 (19.6) | 62 059 (10.7) |
| 2001 | 54 (13.4) | 60 757 (10.4) |
| 2002 | 38 (9.4) | 61 149 (10.5) |
| 2003 | 17 (4.2) | 68 679 (11.8) |
| 2004 | 18 (4.5) | 79 187 (13.6) |
| 2005 | 11 (2.7) | 114 688 (19.7) |
| Gender | ||
| Male | 194 (48.1) | 297 041 (51.0) |
| Female | 209 (51.9) | 284 930 (49.0) |
| Year of PSB | ||
| 2000 | 2 (0.5) | 540 (0.1) |
| 2001 | 17 (4.2) | 7387 (1.3) |
| 2002 | 69 (17.1) | 58 045 (10.0) |
| 2003 | 66 (16.4) | 57 781 (9.9) |
| 2004 | 94 (23.3) | 62 762 (10.8) |
| 2005 | 68 (16.9) | 62 484 (10.7) |
| 2006 | 41 (10.2) | 60 284 (10.4) |
| 2007 | 15 (3.7) | 74 158 (12.7) |
| 2008 | 22 (5.5) | 109 848 (18.9) |
| 2009–2010 | 9 (2.2) | 88 682 (15.2) |
| PC type | ||
| All wP | 119 (29.5) | 154 413 (26.5) |
| wP/aP5 mix | 4 (1.0) | 7346 (1.3) |
| All aP5 | 17 (4.2) | 104 570 (18.0) |
| wP/aP3 mix | 62 (15.4) | 44 081 (7.6) |
| All aP3 | 47 (11.7) | 23 797 (4.1) |
| Missing | 154 (38.2) | 247 764 (42.6) |
| PSB type | ||
| aP3 | 189 (46.9) | 260 322 (44.7) |
| aP5 | 8 (2.0) | 34 302 (5.9) |
| aP5-low | 98 (24.3) | 143 108 (24.6) |
| Missing | 108 (26.8) | 144 239 (24.8) |
Data are no. (%).
Abbreviations: aP3, 3-component acellular pertussis vaccine (Infanrix); aP5, 5-component acellular pertussis vaccine (Pediacel); aP5-low, 5-component, low-dose acellular pertussis vaccine (Repevax); PC, primary course; PSB, preschool booster; wP, whole-cell pertussis vaccine.
Table 4 shows the analysis of the association between pertussis and type of PC and PSB vaccines, stratified according to birth cohort and type of PC.
Association Between Pertussis and Type of Primary Course and Preschool Booster Vaccines, Stratified According to Birth Cohort and Type of Primary Course Received
| Vaccination . | Analysis Strata . | Vaccination Type . | Cases . | Controls . | Odds Ratioa (95% CI) . | Multiple Imputation Odds Ratioa (95% CI) . | Multiple Imputation P Value . |
|---|---|---|---|---|---|---|---|
| Primary course | Year of birth 1999–2002 | 3 wP | 47 | 69 756 | Ref | ||
| wP/aP3 mix | 62 | 43 153 | 2.23 (1.51–3.31) | 2.47 (1.71–3.56) | < .001 | ||
| 3 aP3 | 47 | 23 705 | 3.18 (2.05–4.92) | 3.86 (2.56–5.82) | < .001 | ||
| Any missing | 94 | 107 618 | 1.35 (.93–1.96) | … | |||
| Year of birth 2003–2005 | 3 wP | 17 | 45 098 | Ref | … | ||
| wP/aP5 mix | 4 | 7183 | 1.35 (.38–4.86) | 1.82 (.52–6.37) | .35 | ||
| 3 aP5 | 7 | 104 484 | 0.64 (.22–1.88) | 0.89 (.29–2.73) | .85 | ||
| Any missing | 8 | 104 943 | 0.22 (.08–.60) | … | |||
| Preschool booster | Primary course with 3 doses of wP | aP3 | 86 | 93 857 | Ref | … | |
| aP5 | 2 | 4673 | 1.11 (.22–5.62) | 0.91 (.18–4.58) | .91 | ||
| aP5-low | 16 | 39 918 | 1.09 (.50–2.40) | 0.95 (.47–1.93) | .89 | ||
| Missing | 15 | 15 965 | 1.22 (.69–2.15) | … | |||
| Primary course with 3 doses of aP5 | aP3 | 8 | 51 897 | Ref | … | ||
| aP5 | 3 | 16 725 | 0.96 (.23–3.93) | 1.07 (.30–3.78) | .92 | ||
| aP5-low | 2 | 22 815 | 0.58 (.12–2.75) | 0.52 (.11–2.44) | .41 | ||
| Missing | 4 | 13 133 | 1.92 (.58–6.38) | … | |||
| Primary course with 3 doses of aP3 | aP3 | 14 | 8233 | Ref | … | ||
| aP5 | 0 | 212 | … | … | |||
| aP5-low | 29 | 12 943 | 1.94 (.94–3.98) | 1.94 (1.04–3.61) | .04 | ||
| Missing | 4 | 2409 | 1.20 (.39–3.71) | … |
| Vaccination . | Analysis Strata . | Vaccination Type . | Cases . | Controls . | Odds Ratioa (95% CI) . | Multiple Imputation Odds Ratioa (95% CI) . | Multiple Imputation P Value . |
|---|---|---|---|---|---|---|---|
| Primary course | Year of birth 1999–2002 | 3 wP | 47 | 69 756 | Ref | ||
| wP/aP3 mix | 62 | 43 153 | 2.23 (1.51–3.31) | 2.47 (1.71–3.56) | < .001 | ||
| 3 aP3 | 47 | 23 705 | 3.18 (2.05–4.92) | 3.86 (2.56–5.82) | < .001 | ||
| Any missing | 94 | 107 618 | 1.35 (.93–1.96) | … | |||
| Year of birth 2003–2005 | 3 wP | 17 | 45 098 | Ref | … | ||
| wP/aP5 mix | 4 | 7183 | 1.35 (.38–4.86) | 1.82 (.52–6.37) | .35 | ||
| 3 aP5 | 7 | 104 484 | 0.64 (.22–1.88) | 0.89 (.29–2.73) | .85 | ||
| Any missing | 8 | 104 943 | 0.22 (.08–.60) | … | |||
| Preschool booster | Primary course with 3 doses of wP | aP3 | 86 | 93 857 | Ref | … | |
| aP5 | 2 | 4673 | 1.11 (.22–5.62) | 0.91 (.18–4.58) | .91 | ||
| aP5-low | 16 | 39 918 | 1.09 (.50–2.40) | 0.95 (.47–1.93) | .89 | ||
| Missing | 15 | 15 965 | 1.22 (.69–2.15) | … | |||
| Primary course with 3 doses of aP5 | aP3 | 8 | 51 897 | Ref | … | ||
| aP5 | 3 | 16 725 | 0.96 (.23–3.93) | 1.07 (.30–3.78) | .92 | ||
| aP5-low | 2 | 22 815 | 0.58 (.12–2.75) | 0.52 (.11–2.44) | .41 | ||
| Missing | 4 | 13 133 | 1.92 (.58–6.38) | … | |||
| Primary course with 3 doses of aP3 | aP3 | 14 | 8233 | Ref | … | ||
| aP5 | 0 | 212 | … | … | |||
| aP5-low | 29 | 12 943 | 1.94 (.94–3.98) | 1.94 (1.04–3.61) | .04 | ||
| Missing | 4 | 2409 | 1.20 (.39–3.71) | … |
Abbreviations: aP3, 3-component acellular pertussis vaccine (Infanrix); aP5, 5-component acellular pertussis vaccine (Pediacel); aP5-low, 5-component, low-dose acellular pertussis vaccine (Repevax); CI, confidence interval; Ref, reference; wP, whole-cell pertussis vaccine.
aOdds ratio adjusted for year of birth and type and year of administration of preschool booster. Multiple imputation of missing values was performed taking into consideration the date at which the vaccines were given.
Association Between Pertussis and Type of Primary Course and Preschool Booster Vaccines, Stratified According to Birth Cohort and Type of Primary Course Received
| Vaccination . | Analysis Strata . | Vaccination Type . | Cases . | Controls . | Odds Ratioa (95% CI) . | Multiple Imputation Odds Ratioa (95% CI) . | Multiple Imputation P Value . |
|---|---|---|---|---|---|---|---|
| Primary course | Year of birth 1999–2002 | 3 wP | 47 | 69 756 | Ref | ||
| wP/aP3 mix | 62 | 43 153 | 2.23 (1.51–3.31) | 2.47 (1.71–3.56) | < .001 | ||
| 3 aP3 | 47 | 23 705 | 3.18 (2.05–4.92) | 3.86 (2.56–5.82) | < .001 | ||
| Any missing | 94 | 107 618 | 1.35 (.93–1.96) | … | |||
| Year of birth 2003–2005 | 3 wP | 17 | 45 098 | Ref | … | ||
| wP/aP5 mix | 4 | 7183 | 1.35 (.38–4.86) | 1.82 (.52–6.37) | .35 | ||
| 3 aP5 | 7 | 104 484 | 0.64 (.22–1.88) | 0.89 (.29–2.73) | .85 | ||
| Any missing | 8 | 104 943 | 0.22 (.08–.60) | … | |||
| Preschool booster | Primary course with 3 doses of wP | aP3 | 86 | 93 857 | Ref | … | |
| aP5 | 2 | 4673 | 1.11 (.22–5.62) | 0.91 (.18–4.58) | .91 | ||
| aP5-low | 16 | 39 918 | 1.09 (.50–2.40) | 0.95 (.47–1.93) | .89 | ||
| Missing | 15 | 15 965 | 1.22 (.69–2.15) | … | |||
| Primary course with 3 doses of aP5 | aP3 | 8 | 51 897 | Ref | … | ||
| aP5 | 3 | 16 725 | 0.96 (.23–3.93) | 1.07 (.30–3.78) | .92 | ||
| aP5-low | 2 | 22 815 | 0.58 (.12–2.75) | 0.52 (.11–2.44) | .41 | ||
| Missing | 4 | 13 133 | 1.92 (.58–6.38) | … | |||
| Primary course with 3 doses of aP3 | aP3 | 14 | 8233 | Ref | … | ||
| aP5 | 0 | 212 | … | … | |||
| aP5-low | 29 | 12 943 | 1.94 (.94–3.98) | 1.94 (1.04–3.61) | .04 | ||
| Missing | 4 | 2409 | 1.20 (.39–3.71) | … |
| Vaccination . | Analysis Strata . | Vaccination Type . | Cases . | Controls . | Odds Ratioa (95% CI) . | Multiple Imputation Odds Ratioa (95% CI) . | Multiple Imputation P Value . |
|---|---|---|---|---|---|---|---|
| Primary course | Year of birth 1999–2002 | 3 wP | 47 | 69 756 | Ref | ||
| wP/aP3 mix | 62 | 43 153 | 2.23 (1.51–3.31) | 2.47 (1.71–3.56) | < .001 | ||
| 3 aP3 | 47 | 23 705 | 3.18 (2.05–4.92) | 3.86 (2.56–5.82) | < .001 | ||
| Any missing | 94 | 107 618 | 1.35 (.93–1.96) | … | |||
| Year of birth 2003–2005 | 3 wP | 17 | 45 098 | Ref | … | ||
| wP/aP5 mix | 4 | 7183 | 1.35 (.38–4.86) | 1.82 (.52–6.37) | .35 | ||
| 3 aP5 | 7 | 104 484 | 0.64 (.22–1.88) | 0.89 (.29–2.73) | .85 | ||
| Any missing | 8 | 104 943 | 0.22 (.08–.60) | … | |||
| Preschool booster | Primary course with 3 doses of wP | aP3 | 86 | 93 857 | Ref | … | |
| aP5 | 2 | 4673 | 1.11 (.22–5.62) | 0.91 (.18–4.58) | .91 | ||
| aP5-low | 16 | 39 918 | 1.09 (.50–2.40) | 0.95 (.47–1.93) | .89 | ||
| Missing | 15 | 15 965 | 1.22 (.69–2.15) | … | |||
| Primary course with 3 doses of aP5 | aP3 | 8 | 51 897 | Ref | … | ||
| aP5 | 3 | 16 725 | 0.96 (.23–3.93) | 1.07 (.30–3.78) | .92 | ||
| aP5-low | 2 | 22 815 | 0.58 (.12–2.75) | 0.52 (.11–2.44) | .41 | ||
| Missing | 4 | 13 133 | 1.92 (.58–6.38) | … | |||
| Primary course with 3 doses of aP3 | aP3 | 14 | 8233 | Ref | … | ||
| aP5 | 0 | 212 | … | … | |||
| aP5-low | 29 | 12 943 | 1.94 (.94–3.98) | 1.94 (1.04–3.61) | .04 | ||
| Missing | 4 | 2409 | 1.20 (.39–3.71) | … |
Abbreviations: aP3, 3-component acellular pertussis vaccine (Infanrix); aP5, 5-component acellular pertussis vaccine (Pediacel); aP5-low, 5-component, low-dose acellular pertussis vaccine (Repevax); CI, confidence interval; Ref, reference; wP, whole-cell pertussis vaccine.
aOdds ratio adjusted for year of birth and type and year of administration of preschool booster. Multiple imputation of missing values was performed taking into consideration the date at which the vaccines were given.
For PC vaccination, aP3 was less protective than wP (OR, 3.86 [95% CI, 2.56–5.82] for 3 doses of aP3; OR, 2.47 [95% CI, 1.71–3.56] for a wP/aP3 mix when compared to 3 doses of wP), whereas aP5 appeared to be at least as protective as the whole-cell vaccine (OR, 0.89 [95% CI, .29–2.73] for 3 doses of aP5; OR, 1.82 [95% CI, .52–6.37] for a wP/aP5 mix when compared to 3 doses of wP). These results were consistent with the results prior to multiple imputation of missing batch numbers, indicating that those with a primary course of wP/aP3 or all-aP3 were more likely to become a laboratory-confirmed case of pertussis compared with those receiving a complete PC of wP.
This association was observed regardless of the PSB received by those born during 1999–2002 (results not shown); when compared to those receiving 3 doses of wP for PC, those receiving PC with aP3 would have an adjusted OR of 2.9 (95% CI, 1.35–5.30) if the booster was aP3 and 5.2 (95% CI, 2.8–9.7) if the booster was aP5-low.
PSB with aP3, aP5, or aP5-low seemed to have similar protective effects when the PC consisted of 3 doses of whole-cell vaccine (Table 4). In the multiple imputation results, there was a marginally increased odds of pertussis in the aP5-low group compared to aP3 within those who had 3 primary doses of aP, but this was not seen in the aP5-low group for other primary schedules.
Laboratory confirmation of pertussis was made by serological testing in 383 of the 403 cases included in this study (95%). There were no statistically significant differences in geometric means of titers of PT IgG of cases, regardless of the vaccines that they had received (P = .60, Kruskal-Wallis test). Detailed information can be found in the Supplementary Materials.
DISCUSSION
Our findings from a case-control study during a national outbreak of pertussis in 2011–2012 in England suggest that primary vaccination courses consisting of 3 doses of aP3 were more likely to be associated with disease than primary courses consisting of 3 doses of wP.
There was no significant difference in protection among individuals who received 3 doses of aP5 and those who received 3 doses of wP, but the fact that aP5 cohorts were younger in age and therefore closer to vaccination limits the interpretation of this finding. However it is consistent with other studies, where it was shown that fimbriae (FIM) antigens, present in aP5 but not in aP3, could enhance the response to pertussis toxoid, leading to an increased protection against the disease [19].
Previous findings from studies in the United States [20, 21] and Australia [22] concluded that primary vaccination with acellular vaccines is less effective than with whole-cell vaccines, although these did not distinguish between different aP vaccines and may have masked potential differences. It is possible that those studies’ results had been influenced by the relative proportions of aP3 and aP5 vaccines, as both were available during the study periods [23–25].
A strength of our study was that the aP3 and aP5 vaccines were studied separately rather than as a homogenous group. The phased replacement of wP by aP vaccines over a time period acted as a natural experiment, providing an opportunity to compare protection by different pertussis vaccines within a large national outbreak. The central procurement of vaccines for the national program in England has made it possible to identify vaccines received based on the birth cohorts within the population, even when batch number was not recorded.
An additional finding from our study is that the type of vaccine used for the PSB (whether aP3, aP5, and aP5-low) appears to have little impact on the overall protection against pertussis, with the type of vaccine used in the primary course appearing to be more important [21].
The finding that a PSB with aP5-low could be less effective than PSB with aP3 when PC consisted of 3 doses of aP3 (OR, 1.94 [95% CI, 1.04–3.61]) raises the hypothesis that boosting with the same formulation used for PC could be preferable (boosting aP5 with aP3 does not boost the FIM; for those primed with aP3, an aP5 boost is unlikely to give good FIM response and does not boost the PT and pertactin as strongly as would aP3). This finding could be spurious, though, as numbers are small; the impact of pertussis boosting after priming with a more limited range of vaccine antigens deserves further investigation.
This study has some potential limitations. Direct comparison between the 2 acellular vaccines available in England (aP5 and aP3) was not possible, as they were recommended and administered during different time periods. Each of these periods, however, overlapped with use of whole-cell vaccine, and so wP was used as the reference for the comparisons. In addition, including both year of birth and the year of last vaccine dose in our regression models and stratifying the analysis by the relevant birth cohorts will have helped to reduce the impact of this limitation.
Although the CHIS data used for population controls come from only 14 PCTs, the geographic spread was considered representative of the general English population, and the coverage for these was very similar to national coverage.
In England, diagnosis of pertussis in the age groups included in this study in England relies predominantly on a single high titer of serum PT IgG. Given that the PT antigen content of the vaccines is different, it could be argued that the probability of being included as a case varied by vaccine received. However, our results suggest that antibody responses to disease do not vary significantly depending on the type of vaccine received. During the study period, PCR diagnosis was only available for infants <1 year of age during hospital visits for suspected pertussis.
With the data collected for this study, including only children or teenagers aged ≤15 years, it is still not possible to generalize the results for longer-term protection. Given the change in the recommended schedule in 2004, most children receiving aP5 would be <8 years old during the outbreak in 2011–2012. The shorter follow-up period for aP5 cohorts is a limitation of our study, and the long-term effectiveness of the aP5 vaccine should therefore be confirmed in future studies.
Approximately half of the cases and controls did not have valid batch numbers recorded for all the 4 doses of vaccine they received. This could reflect population movement during childhood so that not all doses were completely recorded. However, analysis of the data after multiple imputation of unknown vaccination courses yielded consistent results, adding strength to the findings.
Our study results confirm the hypothesis raised in reviews published in the late 1990s, when it was suggested that 5-component aP vaccines would provide similar protection against disease as wP vaccines [26, 27].
These findings provide important information for policy makers currently considering decisions around the choice between wP and aP vaccines in their routine vaccination programs [9]. Many South American, African, and Southeast Asian countries still use whole-cell vaccines, although there is considerable debate about switching to acellular vaccines in these countries. The observation of large community outbreaks of pertussis reported in the last years in countries with high uptake of aP vaccines [28] has prompted some of the concerns relating to aP use. At the center of this debate is a trade-off between waning immunity with aP vaccines and the higher reactogenicity of wP vaccines. The findings of this study provide important evidence that priming with a 5-component acellular pertussis vaccine might have a similar protective effect to the wP vaccine previously used in the accelerated UK schedule; similar studies using alternative primary infant schedules as in other countries would provide useful additional information. Furthermore, as the type of pertussis vaccine used for PSB appears to have little impact on the overall protection, there remains greater flexibility over the choice of pertussis vaccine for booster doses.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. C. F. A. C. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. C. F. A. C., G. A., N. A., G. D., and M. R. were all involved in the study design. C. F. A. C., G. A., N. A., S. R., and J. S. contributed to the implementation of data collection. C. F. A. C. and N. A. undertook data analysis. C. F. A. C. and G. D. drafted the manuscript, and all other authors contributed to the revision of this prior to submission.
Acknowledgments. The authors are grateful to Dr Richard Pebody (European Programme for Intervention Epidemiology Training [EPIET] supervisor) and Dr Helen Maguire (EPIET front-line coordinator) for their scientific support to C. F. A. C. during this project; Kim Taylor and Adolphe Bukasa for their assistance with data collection and entry; Ross Harris for his support on the multiple imputation methods; Liz Miller for her feedback and support; and all the general practitioners who assisted with this investigation.
Financial support. This work was supported by the European Centre for Disease Prevention and Control, through EPIET, for which C. F. A. C. was a fellow based at Public Health England. The study was undertaken by authors at Public Health England as part of the routine functions of surveillance and control of communicable diseases.
Potential conflicts of interest. The authors report that the Immunisation Department has provided vaccine manufactures with postmarketing surveillance reports (not pertussis-containing vaccines to date), which the companies are required to submit to the United Kingdom Licensing authority in compliance with their risk management strategy. A cost recovery charge is made for these reports. The authors report no other potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
World Health Organization.
Public Health England.
Health Protection Agency (HPA).
World Health Organization.
National Health Service.
Centers for Disease Control and Prevention.
US Food and Drug Administration, Center for Biologics Evaluation and Research.
Australian Department of Health.
World Health Organization.




