-
PDF
- Split View
-
Views
-
Cite
Cite
Christiana Graf, Marcus M Mücke, Georg Dultz, Kai-Henrik Peiffer, Alica Kubesch, Patrick Ingiliz, Stefan Zeuzem, Eva Herrmann, Johannes Vermehren, Efficacy of Direct-acting Antivirals for Chronic Hepatitis C Virus Infection in People Who Inject Drugs or Receive Opioid Substitution Therapy: A Systematic Review and Meta-analysis, Clinical Infectious Diseases, Volume 70, Issue 11, 1 June 2020, Pages 2355–2365, https://doi.org/10.1093/cid/ciz696
Close - Share Icon Share
Abstract
Treatment uptake for hepatitis C virus (HCV) infection in people who inject drugs (PWID) and patients on opioid substitution therapy (OST) is still low despite treatment guidelines that advocate the use of direct-acting antivirals (DAAs) in all patients. Our aim in this review was to investigate treatment outcomes among PWID and patients on OST in comparison to control cohorts.
A search of Embase, Medline, PubMed, and Web of Science (from October 2010 to March 2018) was conducted to assess sustained virologic response (SVR), discontinuation rates, adherence, and HCV reinfection in PWID and patients on OST.
We identified 11 primary articles and 12 conference abstracts comprising 1702 patients on OST, 538 PWID, and 19 723 patients who served as controls. Among patients on OST, the pooled SVR was 90% (95% confidence interval [CI], 87% to 93%) and pooled treatment discontinuation rate was 7% (95% CI, 4% to 11%). Similarly, the pooled SVR was 88% (95% CI, 80% to 93%) in PWID and the pooled treatment discontinuation rate was 9% (95% CI, 5% to 15%). There was no significant difference regarding pooled rates of SVR, adherence, and discontinuation between patients on OST and controls as well as between PWID and controls. HCV reinfection rates among patients on OST ranged from 0.0 to 12.5 per 100 person-years.
HCV treatment outcomes in PWID and patients on OST are similar to those in patients without a history of injecting drugs, supporting current guideline recommendations to treat HCV in these patient populations.
(See the Editorial Commentary by Norton and Litwin on pages 2366–8.)
Chronic infection with the hepatitis C virus (HCV) is a major global health concern, affecting approximately 71 million persons or 1% of the world’s population [1]. Injection drug use (IDU) accounts for most new infections in Europe and the United States and is responsible for much of the ongoing spread of the disease [2]. The recent approval of direct-acting antivirals (DAAs) that target specific nonstructural proteins of the viral life cycle was a major breakthrough in HCV care. HCV can now be cured in >95% of patients across diverse patient populations [1]. This prompted the World Health Organization (WHO) to issue its first global strategy on viral hepatitis B and C in May 2016, with the goal to reach elimination by 2030 [1]. The WHO elimination strategy includes, among others, a 90% reduction in new HCV infections and a 65% reduction in HCV mortality. However, few national plans for combating viral hepatitis have been implemented to date [1].
The prevalence of HCV is disproportionately high in people who inject drugs (PWID) who are at increased risk of liver-related morbidity and mortality [3]. However, elimination strategies will only be successful if special efforts that target this particular patient population are made [4]. Historically, the uptake of HCV treatment among PWID has been low, mainly due to the poor tolerability and lack of efficacy of interferon-based treatments [5, 6]. With the advent of highly effective and well-tolerated interferon-free DAA regimens, the path has been paved for expanding access to care in populations of PWID with or without opioid substitution therapy (OST). However, even today, some clinicians are reluctant to prescribe DAAs to PWID or people receiving OST despite universal treatment recommendations in national and international clinical practice guidelines [7–9]. This may be based on concerns over poor adherence, reduced tolerability, and the risk of HCV reinfection [10]. However, there is accumulating evidence that suggests that DAAs are equally effective in patients with recent and ongoing IDU. Moreover, PWID receiving OST were not excluded from many clinical trials that evaluated newer DAAs, with subanalyses showing adherence and response rates that were comparable to those in patients without a history of drug use [11–14].
Here, we carried out a systematic review and metaanalysis of HCV treatment outcomes including sustained virologic response (SVR), treatment completion, adherence, and HCV reinfection of interferon-free DAA therapies in patients with active IDU and those on OST.
METHODS
Search Strategy and Selection Criteria
This systematic review and metaanalysis was conducted applying standard methods according to the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses; see Supplementary Materials, pp. 1–2) [15].
We did a systematic search of Medline, Embase, PubMed, and Web of Science for English-language articles and conference abstracts published from 1 October 2010, which is the date of the first publication on sofosbuvir, the first DAA available that was used in interferon-free direct-acting antiviral regimens, to 1 March 2018. The search strategy for PubMed, Embase, and Web of Science databases can be found in the Supplementary Materials, pp. 4–7). The reference lists of selected studies and reviews as well as conference proceedings were also examined for relevant articles and ongoing research. All types of articles were eligible for study inclusion. The study and its protocol were prospectively registered with the PROSPERO register for systematic reviews (CRD42018090027).
All studies of patients with chronic HCV infection treated with interferon-free DAA regimens and who were actively injecting drugs (PWID), defined as active injecting drug use during DAA therapy or as having injected drugs within 6–12 months prior to study entry, or who were receiving OST at the time of study entry were eligible for study inclusion. All patients who received interferon-containing regimens (including those with sofosbuvir) were excluded from this analysis.
As both study populations showed some overlap because participants with active IDU were also receiving OST and the 2 groups could not be clearly differentiated in most trials, we analyzed both cohorts separately and compared them with controls (no PWID or no OST) but not with each other. Controls were defined as patients who received DAA therapy with no prior IDU or former IDU >12 months prior to study inclusion or who were not on OST.
Corresponding authors of conference abstracts were contacted and patients were included if all relevant data could be provided. In case of insufficient data in published articles, corresponding authors were contacted to collect additional data. We excluded studies that involved pediatric patients (aged <18 years) as well as studies or patient subgroups who received interferon-based treatments. In case more than 1 study reported data from the same source, the study that provided the most up-to-date data and the most comprehensive account of the study population was selected.
Study Outcomes
Our primary outcome was an SVR defined as the proportion of patients by intention-to-treat (ITT) analysis who had undetectable HCV RNA at least 12 weeks after the end of DAA treatment. SVR was calculated using the principles of ITT as this is the most widely accepted study endpoint in HCV studies. To acknowledge the specific patient populations that are reported by on-treatment response or per protocol analysis, we also reported discontinuation rates as a secondary outcome. Treatment discontinuations were defined as the proportion of patients by ITT analysis who did not complete a full course of treatment including follow-up until 12 weeks after the end of treatment (including early treatment discontinuation due to breakthrough, side effects, or lost to follow-up); additional secondary outcomes of interest were treatment adherence and HCV reinfection. In line with previously published studies, taking 90% of scheduled pills was defined as a clinically relevant threshold for treatment adherence [16]. Evaluation of medication adherence was defined as the proportion of patients by ITT analysis who took at least 90% of DAA cumulative doses. This definition was consistent across all included studies. HCV reinfection was documented by positive HCV RNA with another HCV genotype or phylogenetic analysis suggesting HCV reinfection following SVR.
Data Extraction and Quality Assessment
Two authors (C. G. and J. V.) independently extracted all data in duplicate, resolving discrepancies through discussion or by a third author (E. H.). Extracted data included study author, publication date, study location, study design, patient age and sex, HCV genotype, presence of cirrhosis, type of DAA treatment, length of follow-up, and study outcome. The risk of bias for the included studies was assessed using the Newcastle-Ottawa scale for nonrandomized studies, including 9 items [17]. Studies with a score of 4 or less, 5–8, and more than 9 were considered as having high, moderate, and low risk of bias, respectively.
Data Synthesis
Metaanalyses were performed with R (R Foundation for Statistical Computing, Vienna, Austria) and the R-package meta by Guido Schwarzer.
Proportions for SVR, adherence, and discontinuation were summarized with a fixed-effects model or DerSimonian and Laird random-effects model by use of the logit transformation [18]. Pooled risk differences were analyzed with a fixed-effects model or DerSimonian and Laird random-effects model by use of the inverse variance approach.
Heterogeneity and subgroup differences were tested with the Q test. A sensitivity analysis was done to assess the effects of including only articles that had been published as primary articles. Moreover, a subgroup analysis was performed to obtain ITT SVRs of all included real-world studies that examined HCV treatment outcomes of PWID and patients on OST in order to determine whether there is a difference concerning this endpoint between registration trials and real-world studies.
HCV reinfection rates were calculated as the number of reinfection events per 100 person-years of follow-up.
RESULTS
Study and Patient Characteristics
The systematic review identified 5163 publications. One additional study from a conference proceeding not published online at the time of our search was included after all relevant data were provided [19]. After screening of titles and abstracts for relevant publications and removal of duplicates, 78 potential articles were eligible for full-text screening, of which 23 met our inclusion criteria and were included in the metaanalysis. Five case-control studies [11, 12, 14, 19, 20], 12 prospective studies [13, 21–31], and 6 retrospective observational cohort studies [32–37] were identified.
A total of 55 studies were excluded for not meeting the inclusion criteria or because information was lacking with regard to study outcomes. Six studies with substantial overlap of the relevant patient population were excluded [38–43].
SVR as the primary study outcome was assessed in all included studies. Moreover, 17 studies reported on discontinuation [11–14, 19, 21–27, 31–35] and 10 on adherence [11–14, 19, 22, 24, 27, 28, 31]. Ten studies also reported on HCV reinfection following SVR [11–14, 19, 22, 24, 31, 32, 35].
Among the 23 studies, 12 were from Europe [12, 20, 21, 24–26, 28, 30–32, 34, 37], 7 from Australia [11, 13, 14, 19, 22, 23, 33], 3 from the United States [27, 29, 35], and 1 from Canada [36]. Thirteen of the included studies were multicenter studies [11–14, 19, 20, 22, 23, 25, 30–33], and 10 were single-center studies [21, 24, 26, 27, 29, 31, 33–36].
The study characteristics of HCV patients who were on OST or actively injecting drugs are summarized in Tables 1 and 2.
Characteristics of Studies That Examined People Who Inject Drugs Treated With Direct-acting Antivirals for Chronic Hepatitis C
| Study and Year (Reference) . | Center . | Country . | Study Design . | HCV Treatment Regime . | Total Number of Patients . | Age (Mean or Median) . | Male . | PWID . | Non- PWID . | Definition of Active Injecting Drug Use . | HCV Genotype (1/2/3/4–6) . | No. of Patients With Cirrhosis . | Treatment Setting . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alimohammadi et al 2016 [36] | Single | Canada | Retrospective cohort study | Not specified | 40 | ... | ... | 15 | 0 | Current injection drug use during DAA treatment | ... | ... | ... |
| Bielen et al 2017 [32] | Multiple | Belgium | Retrospective cohort study | Various | 579 | 52,3 | 359 | 18 | 464 | Injection drug use during DAA treatment | 413/0/78/82 | 341 | Hospital-based |
| Boglione et al 2017 [21] | Single | Italy | Prospective cohort study | Various | 163 | ... | ... | 163 | 0 | Injection drug use in 12 months prior to starting treatment | ... | ... | Hospital-based |
| Foster et al 2017 [12] | Multiple | United Kingdom | Retrospective case- control study | GLE/PIB | 1666 | 45 | 900 | 67 | 1599 | Injection drug use in 12 months prior to starting treatment | 852/245/401/168 | 163 | ... |
| Grebely et al 2018 [22] | Multiple | Australia | Prospective cohort study | SOF/VEL | 103 | 48 | 74 | 103 | 0 | Injection drug use in 6 months prior to starting treatment | 36/5/60/2 | 9 | Community- and hospital-based |
| Morris et al 2017 [23] | Multiple | Australia | Prospective cohort study | Various | 120 | … | 82 | 60 | 60 | Injection drug use in 12 months prior to starting treatment | NA | NA | Community-based |
| Read et al 2017 [33] | Single | Australia | Retrospective cohort study | Various | 72 | 45 | 48 | 53 | 19 | Injection drug use in 6 months prior to starting treatment | 47/1/24/0 | 7 | Community- and hospital-based |
| Schütz et al 2018 [24] | Single | Austria | Prospective cohort study | Various | 40 | 38,9 | 29 | 23 | 17 | Injection drug use in 3 months prior to starting treatment | 40/0/0/0 | 0 | Community- and hospital-based |
| Sulkowski et al 2017 [29] | Single | United States | Prospective cohort study | SOF/LDV | 144 | 55 | 88 | 36 | 108 | Recent injection drug use prior to starting treatment | … | 16 | Hospital-based |
| Study and Year (Reference) . | Center . | Country . | Study Design . | HCV Treatment Regime . | Total Number of Patients . | Age (Mean or Median) . | Male . | PWID . | Non- PWID . | Definition of Active Injecting Drug Use . | HCV Genotype (1/2/3/4–6) . | No. of Patients With Cirrhosis . | Treatment Setting . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alimohammadi et al 2016 [36] | Single | Canada | Retrospective cohort study | Not specified | 40 | ... | ... | 15 | 0 | Current injection drug use during DAA treatment | ... | ... | ... |
| Bielen et al 2017 [32] | Multiple | Belgium | Retrospective cohort study | Various | 579 | 52,3 | 359 | 18 | 464 | Injection drug use during DAA treatment | 413/0/78/82 | 341 | Hospital-based |
| Boglione et al 2017 [21] | Single | Italy | Prospective cohort study | Various | 163 | ... | ... | 163 | 0 | Injection drug use in 12 months prior to starting treatment | ... | ... | Hospital-based |
| Foster et al 2017 [12] | Multiple | United Kingdom | Retrospective case- control study | GLE/PIB | 1666 | 45 | 900 | 67 | 1599 | Injection drug use in 12 months prior to starting treatment | 852/245/401/168 | 163 | ... |
| Grebely et al 2018 [22] | Multiple | Australia | Prospective cohort study | SOF/VEL | 103 | 48 | 74 | 103 | 0 | Injection drug use in 6 months prior to starting treatment | 36/5/60/2 | 9 | Community- and hospital-based |
| Morris et al 2017 [23] | Multiple | Australia | Prospective cohort study | Various | 120 | … | 82 | 60 | 60 | Injection drug use in 12 months prior to starting treatment | NA | NA | Community-based |
| Read et al 2017 [33] | Single | Australia | Retrospective cohort study | Various | 72 | 45 | 48 | 53 | 19 | Injection drug use in 6 months prior to starting treatment | 47/1/24/0 | 7 | Community- and hospital-based |
| Schütz et al 2018 [24] | Single | Austria | Prospective cohort study | Various | 40 | 38,9 | 29 | 23 | 17 | Injection drug use in 3 months prior to starting treatment | 40/0/0/0 | 0 | Community- and hospital-based |
| Sulkowski et al 2017 [29] | Single | United States | Prospective cohort study | SOF/LDV | 144 | 55 | 88 | 36 | 108 | Recent injection drug use prior to starting treatment | … | 16 | Hospital-based |
Abbreviations: DAA, direct acting antiviral; GLE/PIB, glecaprevir/pibrentasvir; HCV, hepatitis C virus; NA, not available; PWID, people who inject drugs; SOF/LDV, sofosbuvir/ledipasvir.
Characteristics of Studies That Examined People Who Inject Drugs Treated With Direct-acting Antivirals for Chronic Hepatitis C
| Study and Year (Reference) . | Center . | Country . | Study Design . | HCV Treatment Regime . | Total Number of Patients . | Age (Mean or Median) . | Male . | PWID . | Non- PWID . | Definition of Active Injecting Drug Use . | HCV Genotype (1/2/3/4–6) . | No. of Patients With Cirrhosis . | Treatment Setting . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alimohammadi et al 2016 [36] | Single | Canada | Retrospective cohort study | Not specified | 40 | ... | ... | 15 | 0 | Current injection drug use during DAA treatment | ... | ... | ... |
| Bielen et al 2017 [32] | Multiple | Belgium | Retrospective cohort study | Various | 579 | 52,3 | 359 | 18 | 464 | Injection drug use during DAA treatment | 413/0/78/82 | 341 | Hospital-based |
| Boglione et al 2017 [21] | Single | Italy | Prospective cohort study | Various | 163 | ... | ... | 163 | 0 | Injection drug use in 12 months prior to starting treatment | ... | ... | Hospital-based |
| Foster et al 2017 [12] | Multiple | United Kingdom | Retrospective case- control study | GLE/PIB | 1666 | 45 | 900 | 67 | 1599 | Injection drug use in 12 months prior to starting treatment | 852/245/401/168 | 163 | ... |
| Grebely et al 2018 [22] | Multiple | Australia | Prospective cohort study | SOF/VEL | 103 | 48 | 74 | 103 | 0 | Injection drug use in 6 months prior to starting treatment | 36/5/60/2 | 9 | Community- and hospital-based |
| Morris et al 2017 [23] | Multiple | Australia | Prospective cohort study | Various | 120 | … | 82 | 60 | 60 | Injection drug use in 12 months prior to starting treatment | NA | NA | Community-based |
| Read et al 2017 [33] | Single | Australia | Retrospective cohort study | Various | 72 | 45 | 48 | 53 | 19 | Injection drug use in 6 months prior to starting treatment | 47/1/24/0 | 7 | Community- and hospital-based |
| Schütz et al 2018 [24] | Single | Austria | Prospective cohort study | Various | 40 | 38,9 | 29 | 23 | 17 | Injection drug use in 3 months prior to starting treatment | 40/0/0/0 | 0 | Community- and hospital-based |
| Sulkowski et al 2017 [29] | Single | United States | Prospective cohort study | SOF/LDV | 144 | 55 | 88 | 36 | 108 | Recent injection drug use prior to starting treatment | … | 16 | Hospital-based |
| Study and Year (Reference) . | Center . | Country . | Study Design . | HCV Treatment Regime . | Total Number of Patients . | Age (Mean or Median) . | Male . | PWID . | Non- PWID . | Definition of Active Injecting Drug Use . | HCV Genotype (1/2/3/4–6) . | No. of Patients With Cirrhosis . | Treatment Setting . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alimohammadi et al 2016 [36] | Single | Canada | Retrospective cohort study | Not specified | 40 | ... | ... | 15 | 0 | Current injection drug use during DAA treatment | ... | ... | ... |
| Bielen et al 2017 [32] | Multiple | Belgium | Retrospective cohort study | Various | 579 | 52,3 | 359 | 18 | 464 | Injection drug use during DAA treatment | 413/0/78/82 | 341 | Hospital-based |
| Boglione et al 2017 [21] | Single | Italy | Prospective cohort study | Various | 163 | ... | ... | 163 | 0 | Injection drug use in 12 months prior to starting treatment | ... | ... | Hospital-based |
| Foster et al 2017 [12] | Multiple | United Kingdom | Retrospective case- control study | GLE/PIB | 1666 | 45 | 900 | 67 | 1599 | Injection drug use in 12 months prior to starting treatment | 852/245/401/168 | 163 | ... |
| Grebely et al 2018 [22] | Multiple | Australia | Prospective cohort study | SOF/VEL | 103 | 48 | 74 | 103 | 0 | Injection drug use in 6 months prior to starting treatment | 36/5/60/2 | 9 | Community- and hospital-based |
| Morris et al 2017 [23] | Multiple | Australia | Prospective cohort study | Various | 120 | … | 82 | 60 | 60 | Injection drug use in 12 months prior to starting treatment | NA | NA | Community-based |
| Read et al 2017 [33] | Single | Australia | Retrospective cohort study | Various | 72 | 45 | 48 | 53 | 19 | Injection drug use in 6 months prior to starting treatment | 47/1/24/0 | 7 | Community- and hospital-based |
| Schütz et al 2018 [24] | Single | Austria | Prospective cohort study | Various | 40 | 38,9 | 29 | 23 | 17 | Injection drug use in 3 months prior to starting treatment | 40/0/0/0 | 0 | Community- and hospital-based |
| Sulkowski et al 2017 [29] | Single | United States | Prospective cohort study | SOF/LDV | 144 | 55 | 88 | 36 | 108 | Recent injection drug use prior to starting treatment | … | 16 | Hospital-based |
Abbreviations: DAA, direct acting antiviral; GLE/PIB, glecaprevir/pibrentasvir; HCV, hepatitis C virus; NA, not available; PWID, people who inject drugs; SOF/LDV, sofosbuvir/ledipasvir.
Characteristics of Studies That Examined Opioid Substitution Therapy Patients Treated With Direct-acting Antivirals for Chronic Hepatitis C
| Study Year (Reference) . | Center . | Country . | Study Design . | HCV Treatment Regime . | Total Number of Patients . | Age (Mean or Median) . | Male . | OST . | Non- OST . | Definition of OST . | HCV Genotype (1/2/3/4–6) . | No. of Patients With Cirrhosis . | Treatment Setting . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bielen et al 2017 [32] | Multiple | Belgium | Retrospective cohort study | Various | 579 | 52 | 359 | 35 | ... | Opiate substitution during DAA treatment | 413/0/78/82 | ... | Hospital-based |
| Boyle et al 2017 [37] | Multiple | United Kingdom | Retrospective cohort study | 2D/3D | 175 | 48 | ... | 56 | 0 | ... | ... | ... | Community-based |
| Christensen et al 2018 [30] | Multiple | Germany | Prospective cohort study | Various | 5413 | ... | ... | 413 | 5000 | Opiate substitution during DAA treatment | ... | ... | Community- and hospital-based |
| Dillon et al 2017 [20] | Multiple | United Kingdom | Retrospective case- control study | Not specified | 849 | ... | ... | 37 | 812 | Opiate substitution at start of treatment | ... | ... | Community- and hospital-based |
| Dore et al 2016 [13] | Multiple | Australia | Prospective cohort study | EBV/GZR | 201 | ... | 153 | 201 | 0 | Opiate substitution 3 months before enrollment | 184/0/0/17 | 40 | Community- and hospital-based |
| Filippovych et al 2017 [25] | Multiple | Ukraine | Prospective cohort study | Not specified | 109 | ... | ... | 98 | ... | ... | ... | ... | Community- and hospital-based |
| Grebely et al. 2017 [11]a | Multiple | Australia | Retrospective case- control study | 2D/3D | 4747 | ... | 2751 | 149 | 4598 | ... | 4747/0/0/0 | 855 | ... |
| Grebely et al. 2018 [22] | Multiple | Australia | Prospective cohort study | SOF/VEL | 103 | 48 | 74 | 58 | 45 | Opiate substitution during DAA treatment | 36/5/60/2 | 9 | Community- and hospital-based |
| Grebely et al. 2018 [14]b | Multiple | Australia | Retrospective case- control study | Various | 4743 | 51 | 2911 | 194 | 4549 | ... | 3021/423/861/419 | 1461 | ... |
| Grebely et al. 2017 [19] | Multiple | Australia | Retrospective case- control study | GLE/PIB | 2256 | ... | 1236 | 157 | 2099 | ... | 889/466/643/258 | 277 | Community- and hospital-based |
| Morris et al 2017 [23] | Multiple | Australia | Prospective cohort study | Various | 120 | ... | 82 | 45 | 75 | ... | ... | ... | Community-based |
| Øvrehus et al 2018 [31] | Single | Denmark | Prospective cohort study | SOF/LDV | 16 | 39 | 11 | 16 | 0 | ... | 9/1/6/0 | ... | Hospital-based |
| Read et al 2017 [33] | Single | Australia | Retrospective cohort study | Various | 72 | 45 | 48 | 18 | 54 | ... | 47/1/24/0 | 7 | ... |
| Scherz et al 2017 [34] | Single | Switzerland | Retrospective cohort study | Not specified | 52 | 49 | 41 | 52 | 0 | Opioid agonist treatment and heroin-assisted treatment | 25/1/18/8 | 31 | ... |
| Schütz et al 2016 [26] | Single | Austria | Prospective cohort study | Various | 15 | 41 | 13 | 15 | 0 | Opioid substitution therapy under direct observation | 11/0/4/0 | 5 | ... |
| Schütz et al 2018 [24] | Single | Austria | Prospective cohort study | SOF/LDV | 40 | 39 | 29 | 40 | 0 | Opiate substitution 3 months before DAA treatment | 40/0/0/0 | 0 | Community- and hospital-based |
| Sylvestre et al 2017 [27] | Single | United States | Prospective cohort study | 2D/3D | 35 | … | 22 | 35 | 0 | ... | 35/0/0/0 | 5 | Community-based |
| Talal et al 2017 [35] | Single | United States | Retrospective cohort study | Not specified | 45 | ... | ... | 45 | 0 | ... | ... | ... | Community- and hospital-based |
| Teuber et al 2017 [28] | Multiple | Germany | Prospective cohort study | 2D/3D | 252 | 51 | 155 | 27 | 225 | … | 226/0/0/26 | 19 | Community- and hospital-based |
| Study Year (Reference) . | Center . | Country . | Study Design . | HCV Treatment Regime . | Total Number of Patients . | Age (Mean or Median) . | Male . | OST . | Non- OST . | Definition of OST . | HCV Genotype (1/2/3/4–6) . | No. of Patients With Cirrhosis . | Treatment Setting . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bielen et al 2017 [32] | Multiple | Belgium | Retrospective cohort study | Various | 579 | 52 | 359 | 35 | ... | Opiate substitution during DAA treatment | 413/0/78/82 | ... | Hospital-based |
| Boyle et al 2017 [37] | Multiple | United Kingdom | Retrospective cohort study | 2D/3D | 175 | 48 | ... | 56 | 0 | ... | ... | ... | Community-based |
| Christensen et al 2018 [30] | Multiple | Germany | Prospective cohort study | Various | 5413 | ... | ... | 413 | 5000 | Opiate substitution during DAA treatment | ... | ... | Community- and hospital-based |
| Dillon et al 2017 [20] | Multiple | United Kingdom | Retrospective case- control study | Not specified | 849 | ... | ... | 37 | 812 | Opiate substitution at start of treatment | ... | ... | Community- and hospital-based |
| Dore et al 2016 [13] | Multiple | Australia | Prospective cohort study | EBV/GZR | 201 | ... | 153 | 201 | 0 | Opiate substitution 3 months before enrollment | 184/0/0/17 | 40 | Community- and hospital-based |
| Filippovych et al 2017 [25] | Multiple | Ukraine | Prospective cohort study | Not specified | 109 | ... | ... | 98 | ... | ... | ... | ... | Community- and hospital-based |
| Grebely et al. 2017 [11]a | Multiple | Australia | Retrospective case- control study | 2D/3D | 4747 | ... | 2751 | 149 | 4598 | ... | 4747/0/0/0 | 855 | ... |
| Grebely et al. 2018 [22] | Multiple | Australia | Prospective cohort study | SOF/VEL | 103 | 48 | 74 | 58 | 45 | Opiate substitution during DAA treatment | 36/5/60/2 | 9 | Community- and hospital-based |
| Grebely et al. 2018 [14]b | Multiple | Australia | Retrospective case- control study | Various | 4743 | 51 | 2911 | 194 | 4549 | ... | 3021/423/861/419 | 1461 | ... |
| Grebely et al. 2017 [19] | Multiple | Australia | Retrospective case- control study | GLE/PIB | 2256 | ... | 1236 | 157 | 2099 | ... | 889/466/643/258 | 277 | Community- and hospital-based |
| Morris et al 2017 [23] | Multiple | Australia | Prospective cohort study | Various | 120 | ... | 82 | 45 | 75 | ... | ... | ... | Community-based |
| Øvrehus et al 2018 [31] | Single | Denmark | Prospective cohort study | SOF/LDV | 16 | 39 | 11 | 16 | 0 | ... | 9/1/6/0 | ... | Hospital-based |
| Read et al 2017 [33] | Single | Australia | Retrospective cohort study | Various | 72 | 45 | 48 | 18 | 54 | ... | 47/1/24/0 | 7 | ... |
| Scherz et al 2017 [34] | Single | Switzerland | Retrospective cohort study | Not specified | 52 | 49 | 41 | 52 | 0 | Opioid agonist treatment and heroin-assisted treatment | 25/1/18/8 | 31 | ... |
| Schütz et al 2016 [26] | Single | Austria | Prospective cohort study | Various | 15 | 41 | 13 | 15 | 0 | Opioid substitution therapy under direct observation | 11/0/4/0 | 5 | ... |
| Schütz et al 2018 [24] | Single | Austria | Prospective cohort study | SOF/LDV | 40 | 39 | 29 | 40 | 0 | Opiate substitution 3 months before DAA treatment | 40/0/0/0 | 0 | Community- and hospital-based |
| Sylvestre et al 2017 [27] | Single | United States | Prospective cohort study | 2D/3D | 35 | … | 22 | 35 | 0 | ... | 35/0/0/0 | 5 | Community-based |
| Talal et al 2017 [35] | Single | United States | Retrospective cohort study | Not specified | 45 | ... | ... | 45 | 0 | ... | ... | ... | Community- and hospital-based |
| Teuber et al 2017 [28] | Multiple | Germany | Prospective cohort study | 2D/3D | 252 | 51 | 155 | 27 | 225 | … | 226/0/0/26 | 19 | Community- and hospital-based |
Abbreviations: DAA, direct acting antiviral; EBV/GZR, elbasvir/grazoprevir; GLE/PIB, glecaprevir/pibrentasvir; HCV, hepatitis C virus; OST, opioid substitution therapy; SOF/LDV, sofosbuvir/ledipasvir; 2D/3D, ombitasvir, ritonavir/paritaprevir (2D) and dasabuvir (3D).
Characteristics of Studies That Examined Opioid Substitution Therapy Patients Treated With Direct-acting Antivirals for Chronic Hepatitis C
| Study Year (Reference) . | Center . | Country . | Study Design . | HCV Treatment Regime . | Total Number of Patients . | Age (Mean or Median) . | Male . | OST . | Non- OST . | Definition of OST . | HCV Genotype (1/2/3/4–6) . | No. of Patients With Cirrhosis . | Treatment Setting . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bielen et al 2017 [32] | Multiple | Belgium | Retrospective cohort study | Various | 579 | 52 | 359 | 35 | ... | Opiate substitution during DAA treatment | 413/0/78/82 | ... | Hospital-based |
| Boyle et al 2017 [37] | Multiple | United Kingdom | Retrospective cohort study | 2D/3D | 175 | 48 | ... | 56 | 0 | ... | ... | ... | Community-based |
| Christensen et al 2018 [30] | Multiple | Germany | Prospective cohort study | Various | 5413 | ... | ... | 413 | 5000 | Opiate substitution during DAA treatment | ... | ... | Community- and hospital-based |
| Dillon et al 2017 [20] | Multiple | United Kingdom | Retrospective case- control study | Not specified | 849 | ... | ... | 37 | 812 | Opiate substitution at start of treatment | ... | ... | Community- and hospital-based |
| Dore et al 2016 [13] | Multiple | Australia | Prospective cohort study | EBV/GZR | 201 | ... | 153 | 201 | 0 | Opiate substitution 3 months before enrollment | 184/0/0/17 | 40 | Community- and hospital-based |
| Filippovych et al 2017 [25] | Multiple | Ukraine | Prospective cohort study | Not specified | 109 | ... | ... | 98 | ... | ... | ... | ... | Community- and hospital-based |
| Grebely et al. 2017 [11]a | Multiple | Australia | Retrospective case- control study | 2D/3D | 4747 | ... | 2751 | 149 | 4598 | ... | 4747/0/0/0 | 855 | ... |
| Grebely et al. 2018 [22] | Multiple | Australia | Prospective cohort study | SOF/VEL | 103 | 48 | 74 | 58 | 45 | Opiate substitution during DAA treatment | 36/5/60/2 | 9 | Community- and hospital-based |
| Grebely et al. 2018 [14]b | Multiple | Australia | Retrospective case- control study | Various | 4743 | 51 | 2911 | 194 | 4549 | ... | 3021/423/861/419 | 1461 | ... |
| Grebely et al. 2017 [19] | Multiple | Australia | Retrospective case- control study | GLE/PIB | 2256 | ... | 1236 | 157 | 2099 | ... | 889/466/643/258 | 277 | Community- and hospital-based |
| Morris et al 2017 [23] | Multiple | Australia | Prospective cohort study | Various | 120 | ... | 82 | 45 | 75 | ... | ... | ... | Community-based |
| Øvrehus et al 2018 [31] | Single | Denmark | Prospective cohort study | SOF/LDV | 16 | 39 | 11 | 16 | 0 | ... | 9/1/6/0 | ... | Hospital-based |
| Read et al 2017 [33] | Single | Australia | Retrospective cohort study | Various | 72 | 45 | 48 | 18 | 54 | ... | 47/1/24/0 | 7 | ... |
| Scherz et al 2017 [34] | Single | Switzerland | Retrospective cohort study | Not specified | 52 | 49 | 41 | 52 | 0 | Opioid agonist treatment and heroin-assisted treatment | 25/1/18/8 | 31 | ... |
| Schütz et al 2016 [26] | Single | Austria | Prospective cohort study | Various | 15 | 41 | 13 | 15 | 0 | Opioid substitution therapy under direct observation | 11/0/4/0 | 5 | ... |
| Schütz et al 2018 [24] | Single | Austria | Prospective cohort study | SOF/LDV | 40 | 39 | 29 | 40 | 0 | Opiate substitution 3 months before DAA treatment | 40/0/0/0 | 0 | Community- and hospital-based |
| Sylvestre et al 2017 [27] | Single | United States | Prospective cohort study | 2D/3D | 35 | … | 22 | 35 | 0 | ... | 35/0/0/0 | 5 | Community-based |
| Talal et al 2017 [35] | Single | United States | Retrospective cohort study | Not specified | 45 | ... | ... | 45 | 0 | ... | ... | ... | Community- and hospital-based |
| Teuber et al 2017 [28] | Multiple | Germany | Prospective cohort study | 2D/3D | 252 | 51 | 155 | 27 | 225 | … | 226/0/0/26 | 19 | Community- and hospital-based |
| Study Year (Reference) . | Center . | Country . | Study Design . | HCV Treatment Regime . | Total Number of Patients . | Age (Mean or Median) . | Male . | OST . | Non- OST . | Definition of OST . | HCV Genotype (1/2/3/4–6) . | No. of Patients With Cirrhosis . | Treatment Setting . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bielen et al 2017 [32] | Multiple | Belgium | Retrospective cohort study | Various | 579 | 52 | 359 | 35 | ... | Opiate substitution during DAA treatment | 413/0/78/82 | ... | Hospital-based |
| Boyle et al 2017 [37] | Multiple | United Kingdom | Retrospective cohort study | 2D/3D | 175 | 48 | ... | 56 | 0 | ... | ... | ... | Community-based |
| Christensen et al 2018 [30] | Multiple | Germany | Prospective cohort study | Various | 5413 | ... | ... | 413 | 5000 | Opiate substitution during DAA treatment | ... | ... | Community- and hospital-based |
| Dillon et al 2017 [20] | Multiple | United Kingdom | Retrospective case- control study | Not specified | 849 | ... | ... | 37 | 812 | Opiate substitution at start of treatment | ... | ... | Community- and hospital-based |
| Dore et al 2016 [13] | Multiple | Australia | Prospective cohort study | EBV/GZR | 201 | ... | 153 | 201 | 0 | Opiate substitution 3 months before enrollment | 184/0/0/17 | 40 | Community- and hospital-based |
| Filippovych et al 2017 [25] | Multiple | Ukraine | Prospective cohort study | Not specified | 109 | ... | ... | 98 | ... | ... | ... | ... | Community- and hospital-based |
| Grebely et al. 2017 [11]a | Multiple | Australia | Retrospective case- control study | 2D/3D | 4747 | ... | 2751 | 149 | 4598 | ... | 4747/0/0/0 | 855 | ... |
| Grebely et al. 2018 [22] | Multiple | Australia | Prospective cohort study | SOF/VEL | 103 | 48 | 74 | 58 | 45 | Opiate substitution during DAA treatment | 36/5/60/2 | 9 | Community- and hospital-based |
| Grebely et al. 2018 [14]b | Multiple | Australia | Retrospective case- control study | Various | 4743 | 51 | 2911 | 194 | 4549 | ... | 3021/423/861/419 | 1461 | ... |
| Grebely et al. 2017 [19] | Multiple | Australia | Retrospective case- control study | GLE/PIB | 2256 | ... | 1236 | 157 | 2099 | ... | 889/466/643/258 | 277 | Community- and hospital-based |
| Morris et al 2017 [23] | Multiple | Australia | Prospective cohort study | Various | 120 | ... | 82 | 45 | 75 | ... | ... | ... | Community-based |
| Øvrehus et al 2018 [31] | Single | Denmark | Prospective cohort study | SOF/LDV | 16 | 39 | 11 | 16 | 0 | ... | 9/1/6/0 | ... | Hospital-based |
| Read et al 2017 [33] | Single | Australia | Retrospective cohort study | Various | 72 | 45 | 48 | 18 | 54 | ... | 47/1/24/0 | 7 | ... |
| Scherz et al 2017 [34] | Single | Switzerland | Retrospective cohort study | Not specified | 52 | 49 | 41 | 52 | 0 | Opioid agonist treatment and heroin-assisted treatment | 25/1/18/8 | 31 | ... |
| Schütz et al 2016 [26] | Single | Austria | Prospective cohort study | Various | 15 | 41 | 13 | 15 | 0 | Opioid substitution therapy under direct observation | 11/0/4/0 | 5 | ... |
| Schütz et al 2018 [24] | Single | Austria | Prospective cohort study | SOF/LDV | 40 | 39 | 29 | 40 | 0 | Opiate substitution 3 months before DAA treatment | 40/0/0/0 | 0 | Community- and hospital-based |
| Sylvestre et al 2017 [27] | Single | United States | Prospective cohort study | 2D/3D | 35 | … | 22 | 35 | 0 | ... | 35/0/0/0 | 5 | Community-based |
| Talal et al 2017 [35] | Single | United States | Retrospective cohort study | Not specified | 45 | ... | ... | 45 | 0 | ... | ... | ... | Community- and hospital-based |
| Teuber et al 2017 [28] | Multiple | Germany | Prospective cohort study | 2D/3D | 252 | 51 | 155 | 27 | 225 | … | 226/0/0/26 | 19 | Community- and hospital-based |
Abbreviations: DAA, direct acting antiviral; EBV/GZR, elbasvir/grazoprevir; GLE/PIB, glecaprevir/pibrentasvir; HCV, hepatitis C virus; OST, opioid substitution therapy; SOF/LDV, sofosbuvir/ledipasvir; 2D/3D, ombitasvir, ritonavir/paritaprevir (2D) and dasabuvir (3D).
Across the 23 included studies, there were 1702 individuals receiving OST and 538 were active PWID. Active injecting drug use was defined as self-reported injecting drug use within the last 12 months by 3 studies [12, 21, 23], within the last 6 months by 2 studies [22, 33], and within the last 3 months by 1 study [24]. The remaining 3 studies described their participants as active or current injection drug users during DAA therapy [29, 32, 36].
The results of the risk of bias assessment of the included studies are reported in the Supplementary Materials (pp. 11, 12). Seventeen studies had a moderate risk of bias (score 5–8), and 6 studies had a low risk of bias (score >9). Concerning the mode of treatment delivery, 4 studies provided hospital-based DAA treatment [21, 29, 31, 32], 3 studies provided community-based treatment [23, 27, 37], and 10 studies reported a mix of hospital- and community-based treatment [13, 19, 20, 22, 24, 25, 28, 30, 33, 35]. In the remaining studies, the mode of treatment delivery was not clearly stated [11, 12, 14, 26, 34, 36].
Antiviral regimens for treatment of HCV infection included ledipasvir/sofosbuvir in 9 studies [14, 21, 23–26, 30, 31, 33], sofosbuvir/simeprevir in 1 study [32], sofosbuvir/daclatasvir in 4 studies [23, 26, 32, 33], glecaprevir/pibrentasvir in 2 studies [12, 19], sofosbuvir/velpatasvir in 2 studies [14, 22], sofosbuvir/velpatasvir/voxilaprevir in 1 study [14], elbasvir/grazoprevir in 1 study [13], and ombitasvir plus ritonavir-boosted paritaprevir with or without dasabuvir in 9 studies [11, 21, 23, 27, 28, 30, 32, 33, 37]. The remaining studies reported various (>3) DAA regimens [21, 30] or did not specify the DAA regimen that was used [20, 25, 34–37].
SVR Analysis
Based on a pooled ITT analysis of 19 studies, the overall SVR rate was 90% (95% confidence interval [CI], 87% to 93%) among DAA-treated patients on OST compared to 93% (95% CI, 89% to 95%; P = .19) in patients not receiving OST. When only studies with 2 groups of participants (OST vs non-OST) were compared, the difference between SVR rates (rate difference) was significant, with a lower response for patients on OST (–0.03; 95% CI, –0.05 to –0.01; P = .0002; Figure 1A). No significant heterogeneity was observed across the included studies (I2 = 0%; P = .43).
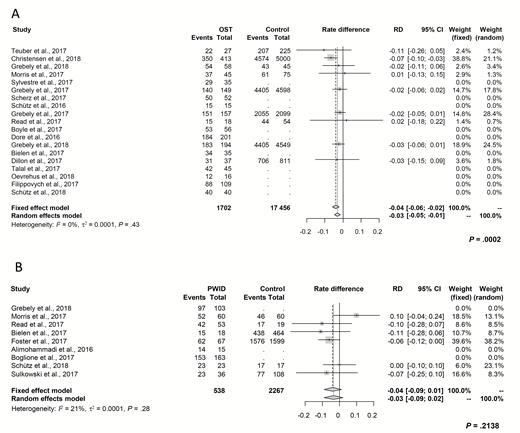
Forest plot demonstrating rate differences of intention-to-treat sustained virologic response and associated 95% CIs following direct-acting antiviral therapy. A, Between patients on OST and controls. B, Between PWID and controls. Abbreviations: CI, confidence interval; OST, opioid substitution therapy; PWID, people who inject drugs; RD, rate difference.
Based on a pooled ITT analysis of 9 studies, the overall SVR rate was 88% (95% CI, 80% to 93%) among PWID compared to 92% in patients with no active drug use (95% CI, 75% to 98%; P = .62). Among studies with 2 groups of participants (PWID vs no PWID), the difference in SVR rates was not significantly lower for PWID compared to controls (–0.03; 95% CI, –0.09 to 0.02; P = .21). No significant study heterogeneity was observed (I2 = 21%; P = .28; Figure 1B).
A sensitivity analysis with restriction to studies that had only been published as full articles showed a difference in SVR rates of –0.04 (95% CI, –0.07 to –0.02; P = .0015) for patients on OST and –0.01 for PWID (95% CI, –0.10 to 0.07; P = .76) compared to the related controls (Figure 2).
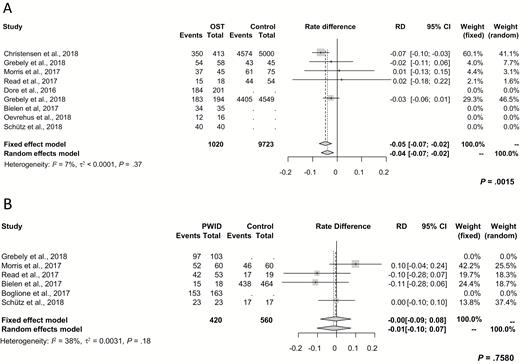
Subgroup analysis for rate differences of intention-to-treat sustained virologic response derived from studies that were published as full articles. A, Between patients on OST and those not receiving OST. B, Between PWID and non-PWID. Abbreviations: CI, confidence interval; OST, opioid substitution therapy; PWID, people who inject drugs; RD, rate difference.
In order to determine whether ITT SVR rates were different in real-world studies compared to those of registration trials among patients on OST, we examined SVR rates derived from real-world studies. The resulting rate difference was –0.06 (95% CI, –0.09 to –0.03; P = .0002), indicating that the study type had no impact on SVR (Figure 3).
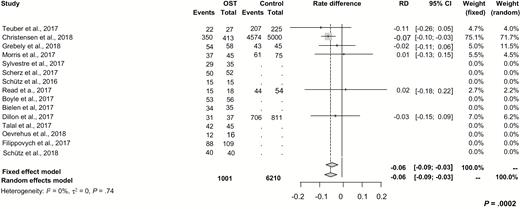
Subgroup analysis for differences of intention-to-treat sustained virologic response rates derived from real-world studies between patients on OST and related controls. Abbreviations: CI, confidence interval; OST, opioid substitution therapy; RD, rate difference.
Treatment Discontinuation
Thirteen studies reported on treatment discontinuation among patients on OST. The pooled estimate was comparable between patients on OST (0.07; 95% CI, 0.04 to 0.11) and controls (0.04; 95% CI, 0.01 to 0.10; P = .33). Moreover, the discontinuation rate was not significantly different between patients on OST and controls (0.02; 95% CI, 0.00 to 0.03; P = .016; Figure 4A).
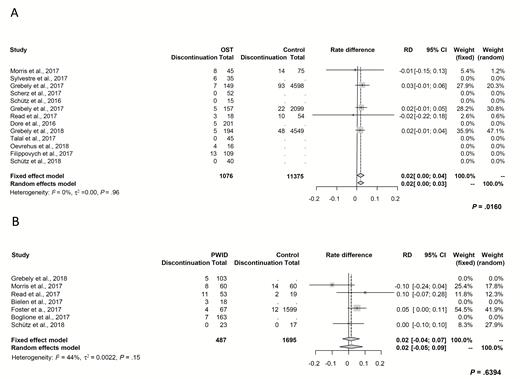
Forest plot demonstrating rate differences of treatment discontinuation and associated 95% CIs. A, Between patients on OST and controls. B, Between PWID and controls. Abbreviations: CI, confidence interval; OST, opioid substitution therapy; PWID, people who inject drugs; RD, rate difference.
Among PWID, the pooled treatment discontinuation estimate was 0.09 (95% CI, 0.05 to 0.15) compared to 0.05 (95% CI, 0.01 to 0.37) in controls (patients not actively injecting drugs). A significant difference between both cohorts could not be detected (Figure 4B; P = .64). There was no significant heterogeneity in this effect size with an estimated I2 of 0% (P = .96; Figure 4A) across studies reporting on patients receiving OST and I2 of 44% (P = .15; Figure 4B) for studies examining PWID compared to controls.
HCV Reinfection
HCV reinfection rates were reported from 7 studies comprising 837 patients on OST and 2 studies with 188 PWID. HCV reinfection risks among patients on OST ranged from 0.0 [11, 14, 19, 32] to 4.6 [13], 10 [24], and 12.5 [31] per 100 person-years. Among PWID the risk of HCV reinfection ranged from 0.0 [32] to 2.6 [22] per 100 person-years.
DISCUSSION
In this systematic review and metaanalysis, we found that all-oral DAA treatment for patients with chronic HCV infection who receive OST or who are actively injecting drugs (PWID) resulted in SVR rates that were comparable to those in controls. This constitutes a major improvement over previous studies of interferon-based treatment in these patient populations [44]. Until recently, treatment of chronic HCV for PWID was not offered by many clinicians despite the fact that it was shown to be cost-effective [45]. Traditionally, major concerns with PWID and even patients on OST included poor tolerability of interferon-based treatments, lack of adherence, and the risk for HCV reinfection. The recent introduction of highly effective interferon-free all-oral HCV therapies has helped to overcome barriers associated with poor treatment uptake in PWID and patients on OST [4]. Recent studies have shown that a scale-up of HCV treatment in patients at high risk of HCV transmission leads to a substantial reduction in HCV prevalence [46]. However, this has to be accompanied by other interventions, including uptake of OST and needle-exchange programs. Interestingly, the risk of HCV reinfection in PWID is low, which further supports unrestricted access to DAAs in drug users [19, 45].
In our study, pooled SVR rates were not significantly different among patients on OST compared to those not receiving OST (90% vs 93%). However, when only studies involving both OST and non-OST controls were taken into account, the difference in SVR rates indicated significantly lower response for patients on OST (rate difference [RD] = –0.03; P = .0002). This finding was mainly driven by studies that also contained DAA combinations that are no longer in use due to suboptimal antiviral activity [30]. Moreover, study heterogeneity among patients on OST was high, indicating publication bias, possibly due to differences in inclusion criteria, risk behavior definitions, involvement of support services, and provision of assistance during antiviral treatment.
Interestingly, SVR rates were not significantly different between PWID and controls (RD = –0.03; P = .21). However, the overall number of PWID that was studied was low.
We also investigated the impact of the trial design (real-word studies vs registrational trials) on treatment outcomes. Our results show that real-world treatment is not associated with lower SVR rates, indicating that results from highly selected patient populations from pharmaceutical trials can be replicated in a real-world setting.
As expected, treatment adherence was high in patients on OST, which is in line with previous studies (Figure 5) [44, 47]. While medication adherence is an important measure of effectiveness of HCV elimination interventions, only 2 of 9 studies evaluated this endpoint in PWID. Overall, we observed relatively low treatment discontinuation rates, which supports the excellent tolerability of all DAAs. Moreover, the majority of patients who did not achieve SVR were not found to be virologic failures but mainly patients who were lost to follow-up. Forty-three percent of treatment discontinuations were observed between the end of treatment and 12 weeks thereafter (SVR12). These patients may in fact have been cured. Several factors, including use of ribavirin as well as interventions such as directly observed therapy, group treatment, and nurse or peer support, may have influenced treatment discontinuation rates. Nevertheless, the effect of these aspects on treatment outcome was not assessed in this metaanalysis since no such subanalyses were performed.
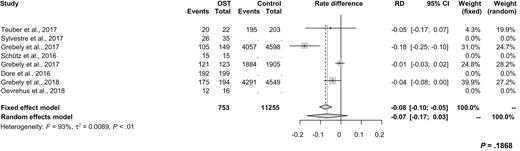
Difference of adherence rates and associated 95% CIs between patients on OST and related control cohorts. Abbreviations: CI, confidence interval; OST, opioid substitution therapy; RD, rate difference.
HCV reinfection is a major concern with current and former drug users, and this constitutes an important barrier to treatment uptake in this patient population. Recent studies suggest that concerted efforts are required to combine a scale-up of HCV treatment with harm-reduction measures to reduce the prevalence of HCV infection in PWID to minimal levels. This will ultimately also lead to a lower risk of HCV reinfection [46]. In our study, the risk of HCV reinfection among patients on OST varied greatly between different studies, ranging from 0.0 per 100 person-years reported in 4 studies [11, 14, 19, 32] to 4.6 per 100 person-years in 1 study [13], 10, and even 12.5 per 100 person-years in 2 studies [24, 31]. This may be mainly explained by heterogeneous study populations, including differences in risk behavior, ongoing drug use after DAA treatment, as well as differences in and lacking information about the availability of syringe-exchange and harm-reduction programs. Moreover, the median follow-up time was 24 weeks (range, 12–24 weeks). Therefore, data on reinfection rates should be interpreted with caution; further prospective studies are needed to assess the long-term effectiveness of HCV treatment in PWID and patients on OST.
In conclusion, our systematic review and metaanalysis showed SVR rates, treatment adherence, and treatment discontinuation for DAA-treated PWID and patients on OST that were comparable to those of controls. These results indicate that DAA treatment for HCV is highly effective and safe in PWID and patients on OST, thereby supporting current guideline recommendations to prioritize access to HCV treatment in this patient population [8, 9]. In keeping with the WHO goal of global HCV eradication by 2030, we believe that current drug use or OST should not be a barrier to HCV treatment. However, long-term follow-up studies are needed to investigate the durability of SVR and the impact on overall morbidity and mortality in PWID and patients on OST.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. The authors have contributed to the manuscript by planning the study (C. G., J. V.), reviewing the literature (C. G., J. V.), collecting the data (all authors), performing the metaanalyses (E. H., C. G., J. V.), and assessing and interpreting the data (all authors). C. G. and J. V. wrote the manuscript, and all authors read, revised, and approved the final version of the manuscript.
Potential conflicts of interest. C. G. reports travel support from Gilead and speaking fees from AbbVie outside the submitted work. M. M. M. reports travel support from AbbVie, Gilead, and Intercept; speaking fees from AbbVie; and a research grant from Gilead outside the submitted work. G. D. reports travel support and speaking fees from AbbVie outside the submitted work. K.-H. P. reports speaking fees from Gilead and travel support from Gilead, Abbott, and Bristol-Myers Squibb. P. I. has received lecture or consultancy fees from AbbVie, BMS, Gilead, Janssen, and MSD. S. Z. reports speaking and/or consulting fees from AbbVie, Bristol-Myers Squibb, Falk, Gilead, Janssen, and Merck/MSD outside the submitted work. J. V. reports speaking and/or consulting fees from Abbott, AbbVie, Bristol-Myers Squibb, Gilead, Medtronic, Merck/MSD, and Roche outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
E. H. and J. V. contributed equally to this work.




