-
PDF
- Split View
-
Views
-
Cite
Cite
Jean T Coulibaly, Noemi Hiroshige, Yves K N’Gbesso, Jan Hattendorf, Jennifer Keiser, Efficacy and Safety of Ascending Dosages of Tribendimidine Against Hookworm Infections in Children: A Randomized Controlled Trial, Clinical Infectious Diseases, Volume 69, Issue 5, 1 September 2019, Pages 845–852, https://doi.org/10.1093/cid/ciy999
Close - Share Icon Share
Abstract
The global strategy to control soil-transmitted helminthiasis is mainly focused on preventive chemotherapy with albendazole and mebendazole. We assessed the efficacy and safety of ascending tribendimidine doses against hookworm infections in African school-aged children, key information for the development of tribendimidine.
We performed a single blind, randomized, controlled trial in Côte d’Ivoire between June and August 2017. Eligible participants were randomly assigned to placebo, 100, 200, or 400 mg tribendimidine. Cure rates (CRs, primary outcome) and egg reduction rates (ERRs) were determined 14–21 days after treatment. Clinical symptoms were assessed before treatment and adverse events monitored 3 and 24 hours posttreatment.
CRs calculated for 130 children dose-dependently increased. The observed CRs were 20.6% (7/34), 21.2% (7/33), 38.7% (12/31), and 53.1% (17/32) for placebo, 100, 200, and 400 mg of tribendimidine, respectively. The Emax model predicted a placebo corrected net effect of 34.3 percentage points (95% confidence interval [CI], 13.3–54.4) for the 400-mg tribendimidine dose. The ERRs (geometric mean) were 30.6% (95% CI, −24.7 to 64.1), 65.4% (95% CI, 24.5–85.9), 82.1% (95% CI, 58.4–92.5) and 92.2% (95% CI, 81.0–97.1) for placebo, 100, 200, and 400 mg tribendimidine, respectively. The Emax model predicted an ERR of 95% at 500 mg. Only mild adverse events and no abnormal biochemical parameters were observed.
A 400-mg dose of tribendimidine yielded the highest efficacy and was well tolerated. Because children were mostly lightly infected, further investigations with tribendimidine against moderate/heavy hookworm infection are needed.
The trial is registered at www.isrctn.com number ISRCTN81391471.
Over 1.5 billion people are infected with at least one of the main soil-transmitted helminth (STH) species, Ascaris lumbricoides, Trichuris trichiura, and hookworms (Ancylostoma duodenale and Necator americanus) [1]. The current global strategy to control soil-transmitted helminthiases is mainly focused on preventive chemotherapy, that is, the regular large-scale administration of the 2 benzimidazoles albendazole and mebendazole to at-risk populations, especially school-aged children [2, 3]. After relying on this strategy for decades, there is considerable concern that growing drug pressure and using only one class of anthelmintics might result in the development of resistant parasite strains and hence increasing the risk of reduced drug efficacy. Evidence of extensive resistance to anthelmintic treatments in veterinary practice raises fears for a similar scenario in humans, which would have a major impact on global health [4]. Indeed, a recent systematic review and meta-analysis suggested that the efficacy of albendazole and mebendazole might have decreased over time, particularly for Trichuris trichiura [5]. Access to a new drug with a distinct mechanism of action could mitigate that risk. Unfortunately, there are no new drugs to treat infections with soil-transmitted helminths in drug discovery. Moreover, only a handful of alternative drugs used for other indications (ie, moxidectin for onchocerciasis) or undergoing clinical development (tribendimidine) might complement the small number of anthelmintic drugs in the near future [6].
Tribendimidine is an anthelmintic drug developed and approved in China for treatment of STH infection. Tribendimidine has been shown to have a broad spectrum of activity against several nematode, trematode, and cestode species and most importantly has a different mechanism of action to that of the benzimidazole drugs, albendazole and mebendazole, acting on bephenium-sensitive, B-acetylcholine receptor subtype [7]. The approval was based on the evidence of safety and efficacy gathered in a series of clinical trials in China, including children [8]. However, to date, dose-finding studies were conducted in adults only. In more detail, cure rates (CRs) following ascending tribendimidine doses of 200, 300, or 400 mg were 52.8% (19/36), 77.8% (28/36), and 83.3% (30/36), respectively [9]. In children doses of 200 mg were arbitrarily selected for the treatment of STH infections. Moreover, the majority of studies have been implemented in Asia [8] with the exception of a recent randomized controlled trial evaluating tribendimidine monotherapy and tribendimidine-ivermectin and tribendimidine-oxantel pamoate combination chemotherapy in Côte d’Ivoire and Tanzania [5]. As part of the clinical development program for tribendimidine, further studies are therefore required to identify the correct dose in the target population for preventive chemotherapy, that is, children. We assessed, to our knowledge, for the first time the efficacy and safety of tribendimidine in African children aged 6 to 12 years infected with hookworm using ascending tribendimidine doses of 100, 200, and 400 mg. This study will be useful by providing evidence for an efficacious and safe tribendimidine dose for children and in the long term by adding a new drug into the depleted drug development pipeline for helminthiasis control in African children.
METHODS
Study Design, Participants, and Ethical Consideration
We conducted this single blind, randomized, placebo controlled trial between 8 June and 27 August 2017 in Rubino and its surrounding villages in southern Côte d’Ivoire involving children between 6 and 12 years of age.
The study was approved by the ethics committee of the Ministère de la Santé et de l’Hygiène Publique in Côte d’Ivoire (reference number 053//MSHP/CNER-kp) and the ethical authorities of Northwestern and Central Switzerland (EKNZ, reference number 2017-00139). Communities were informed about the objectives, the procedures, the benefits and potential risks of the study in the central part of each village and being encouraged asking questions in an open discussion forum. Then, written informed consent was obtained from all parents and guardians of the children aged between 6 and 12 years in each community. Children were assenting for their participation to the study by writing their name on an assent sheet in addition to their fingerprint. The participation was voluntary, and parents/guardians and children were aware that any participant could withdraw at any time without obligation. At the end of the study, all participating children were treated regardless of their infection status with single oral dose of 400 mg of albendazole according to the national guidelines.
Randomization and Treatment
Children were randomly assigned (1:1:1:1) to one of the 4 treatment arms (placebo, 100, 200, or 400 mg of tribendimidine) using a computer-generated stratified block randomization code. The random allocation sequence with varying random blocks sizes of 4 and 8 was generated by a statistician not involved in the data collection. The codes were held in a locked cabinet at the Swiss Tropical and Public Health Institute, and a copy of this code was kept in a sealed envelope by one of the co-investigators to be opened in emergency situations determined by the principal investigator upon consultation with the co-investigators.
Only the investigator dealing with drug administration was aware of the treatment assignment. The physician assessing the adverse events and laboratory technicians reading urine filtration slide for eggs count were masked to treatment allocation. In sum, 50 and 200 mg tablets of tribendimidine were obtained from Shandong Xinhua Pharmaceutical Co., Ltd. (Shandong, China). Participants were asked to undergo treatment with an empty stomach. All drugs were administered in the presence of the investigators, ingestion confirmed and time recorded. After swallowing the tablets, the children received a small snack (biscuits) and lunch (rice, fish, vegetable oil) approximately 4 hours after treatment. Participants were asked not to take any drugs other than those prescribed by the study medical team. After ingestion of the medication, participants were observed for 3 hours. Vomiting within 1 hour posttreatment required retreatment. Participants were not given more than one repeated dose. No re-administration of tribendimidine was needed for participants vomiting after 1 hour posttreatment.
Eligibility Criteria
Children between 6 and 12 years old, hookworm positive, and provided a written informed consent from their parents/guardians were eligible for enrollment. Children were excluded from trial participation if any major systemic illnesses, diabetes, chronic heart, liver, or renal problem as assessed by a medical doctor, upon baseline clinical and biochemical assessment was diagnosed or they had received a recent anthelmintic treatment (within past 4 weeks).
Outcome
The primary outcome was the CR (conversion from being egg positive pretreatment to egg negative posttreatment) against hookworm infection assessed by quadruplicate Kato-Katz thick smears at baseline and 14–21 days after treatment. Secondary endpoints were egg reduction rates (ERR) against hookworm, CRs, and ERRs for concomitant infection with T. trichiura and A. lumbricoides, the tolerability of tribendimidine and pharmacokinetic parameters [10].
Procedures
The Kato-Katz technique was used to quantitatively assess STH infections (hookworm, T. trichiura and A. lumbricoides) before and between 14 and 21 days after treatment. Children were asked to provide 2 consecutive stool samples over a period of 5 days. Each stool sample was assessed with duplicate Kato-Katz thick smears (41.7 mg each) [11]. After a clearance of the slides for 30 minutes, the slides were analyzed under a microscope by 2 experienced technicians, and a subsequent independent quality control of sample results (approximately 10%) was conducted as described elsewhere [12, 13]. A finger prick blood sample was taken for the diagnosis of Plasmodium falciparum using a rapid malaria diagnostic test (ICT Malaria P. falciparum (HRPII), Cape Town, South Africa) according to the manufactures’ instructions.
The medical history of hookworm-infected children was assessed using a standardized questionnaire in addition to clinical examination by the study physician at baseline and 3 and 24 hours posttreatment. A sample of venous blood was taken from each child to assess clinical chemistry parameters (eg, urea, creatinine, liver enzymes, and albumin) at baseline and 72 hours posttreatment.
Sample Size Calculation and Statistical Analysis
The main purpose of this study was to elucidate the nature of the dose–response relationship of tribendimidine against hookworm. We run a series of simulations to determine the required sample size. We assumed true cure rates of 2.5%, 20%, 40%, and 60% (for 0, 100, 200, and 400 mg) and a loss to follow up of 5%. We estimated that enrolling 40 participants per arm will be sufficient to predict the dose response curve with a precision (defined as median distance between prediction and confidence band) of about 10 percentage points.
Data were double entered into an Excel spreadsheet, transferred into EpiInfo version 3.5.2 (Centers for Disease Control and Prevention, Atlanta, GA, USA) and cross-checked.
We used the full analysis set (available case population) according to the intention to treat principle for the primary analysis, defined as all randomized subjects who provide any follow-up data. Only subjects who were negative at baseline and erroneously randomized were excluded from the analysis. We also performed a per protocol analysis in which 3 children absent on treatment day were excluded from analysis (one each in placebo, 100 mg, and 200 mg arms).
Eggs per gram of stool (EPGs) were assessed by calculating the mean of fecal egg counts from quadruplicate Kato-Katz thick smears and multiplying this number by a factor of 24. The ERR based on arithmetic or geometric mean of EPG was assessed as follows:
with 5000 replicates was used to calculate 95% confidence intervals (CIs) for ERRs.
Emax models were used to predict the dose-response curves in terms of CRs and ERRs. The placebo corrected net effect of a certain dose was estimated as the predicted CR minus the predicted CR associated with the placebo (E0). Confidence limits were constructed via bootstrap resampling. The analysis was conducted using the DoseFinding package (version 0.9–14) of the statistical software environment R (v3.4.0).
RESULTS
Study Flow and Baseline Characteristics
Figure 1 show the study flow. Overall, 2044 children were invited to participate in the eligibility survey. Two hundred ninety-five children were absent during the survey. Of the 1749 children screened, 148 were infected with hookworm (prevalence of 8.6%). These 148 children were randomly assigned to the 4 treatment arms, placebo (n = 38), 100 mg (n = 36), 200 mg (n = 37), and 400 mg (n = 37). Eighteen children were lost to follow-up.
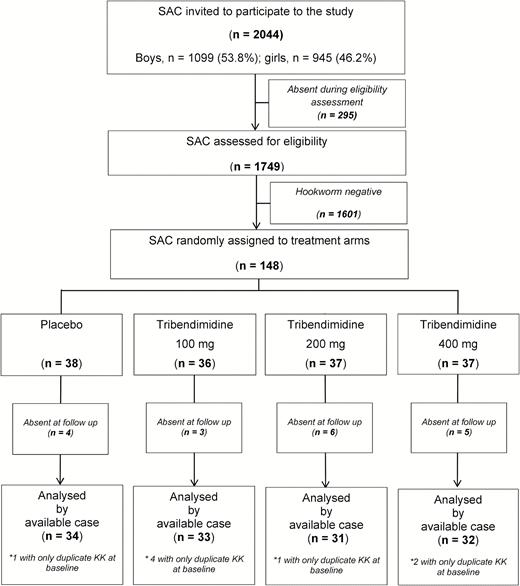
Treatment arms were well balanced regarding, age, weight, height, geometric mean of EPG of stool, infection intensity categories, and the coinfection profile (Table 1). At baseline EPGs ranged from 6 to 3612 with the majority of children harbored a light infections. More boys than girls participated in the study. Overall, prevalences of coinfections among hookworm-infected individuals with A. lumbricoides, T. trichiura, and P. falciparum were 2.7 (4/148), 10.1 (15/148), and 54.7 (81/148), respectively.
| Characteristics . | Treatment Arm . | ||||
|---|---|---|---|---|---|
| Placebo . | 100 mg . | 200 mg . | 400 mg . | Total . | |
| N . | 38 . | 36 . | 37 . | 37 . | 148 . |
| Female, n (%) | 15 (39.5) | 14 (38.9) | 10 (27.0) | 12 (32.4) | 51 (34.2) |
| Age, years; median (IQR) | 8.5 (7–11) | 9 (7–10.5) | 9 (7–10) | 8 (7–11) | 9 (7–11) |
| Weight, kg; median (IQR) | 26.5 (22–30) | 26 (22–29) | 25 (20–29) | 23 (20–28) | 25.5 (21–29) |
| Height, cm; median (IQR) | 129.5 (122–137) | 127.5 (119–139) | 129 (120–136) | 123 (114–132) | 127 (119–137) |
| Temperature, °C, median (IQR) | 36.7 (36.5–36.9) | 36.7 (36.2–37.0) | 36.7 (36.3–37.0) | 36.7 (36.4–37.0) | 36.7 (36.4–37.0) |
| Pool, beats/minute, median (IQR) | 98.5 (88–104) | 92 (82–107) | 97 (84–104) | 100 (84–104) | 98 (84–104) |
| EPG median (IQR) | 84 (24–366) | 150 (48–294) | 90 (36–150) | 108 (54–210) | 99 (39–240) |
| GM EPG | 85.2 | 129.3 | 92.1 | 110.9 | 102.7 |
| Infection intensity, n (%) | |||||
| Low | 37 (97.4) | 35 (97.2) | 35 (94.6) | 37 (100) | 144 (97.3) |
| Moderate | 1 (2.6) | 1 (2.8) | 2 (5.4) | 0 (0.0) | 4 (2.7) |
| Heavy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Coinfections, n (%) | |||||
| Ascaris lumbricoïdes | 2 (5.3) | 1 (2.8) | 1 (2.7) | 0 (0.0) | 4 (2.7) |
| Trichuris trichiura | 6 (15.8) | 1 (2.8) | 3 (8.1) | 5 (13.5) | 15 (10.1) |
| Plasmodium falciparum | 21 (55.3) | 17 (47.2) | 22 (59.5) | 21 (56.8) | 81 (54.7) |
| Characteristics . | Treatment Arm . | ||||
|---|---|---|---|---|---|
| Placebo . | 100 mg . | 200 mg . | 400 mg . | Total . | |
| N . | 38 . | 36 . | 37 . | 37 . | 148 . |
| Female, n (%) | 15 (39.5) | 14 (38.9) | 10 (27.0) | 12 (32.4) | 51 (34.2) |
| Age, years; median (IQR) | 8.5 (7–11) | 9 (7–10.5) | 9 (7–10) | 8 (7–11) | 9 (7–11) |
| Weight, kg; median (IQR) | 26.5 (22–30) | 26 (22–29) | 25 (20–29) | 23 (20–28) | 25.5 (21–29) |
| Height, cm; median (IQR) | 129.5 (122–137) | 127.5 (119–139) | 129 (120–136) | 123 (114–132) | 127 (119–137) |
| Temperature, °C, median (IQR) | 36.7 (36.5–36.9) | 36.7 (36.2–37.0) | 36.7 (36.3–37.0) | 36.7 (36.4–37.0) | 36.7 (36.4–37.0) |
| Pool, beats/minute, median (IQR) | 98.5 (88–104) | 92 (82–107) | 97 (84–104) | 100 (84–104) | 98 (84–104) |
| EPG median (IQR) | 84 (24–366) | 150 (48–294) | 90 (36–150) | 108 (54–210) | 99 (39–240) |
| GM EPG | 85.2 | 129.3 | 92.1 | 110.9 | 102.7 |
| Infection intensity, n (%) | |||||
| Low | 37 (97.4) | 35 (97.2) | 35 (94.6) | 37 (100) | 144 (97.3) |
| Moderate | 1 (2.6) | 1 (2.8) | 2 (5.4) | 0 (0.0) | 4 (2.7) |
| Heavy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Coinfections, n (%) | |||||
| Ascaris lumbricoïdes | 2 (5.3) | 1 (2.8) | 1 (2.7) | 0 (0.0) | 4 (2.7) |
| Trichuris trichiura | 6 (15.8) | 1 (2.8) | 3 (8.1) | 5 (13.5) | 15 (10.1) |
| Plasmodium falciparum | 21 (55.3) | 17 (47.2) | 22 (59.5) | 21 (56.8) | 81 (54.7) |
Abbreviations: CI, confidence interval; EPG, eggs per gram of stool; GM, geometric mean; IQR, interquartile range.
| Characteristics . | Treatment Arm . | ||||
|---|---|---|---|---|---|
| Placebo . | 100 mg . | 200 mg . | 400 mg . | Total . | |
| N . | 38 . | 36 . | 37 . | 37 . | 148 . |
| Female, n (%) | 15 (39.5) | 14 (38.9) | 10 (27.0) | 12 (32.4) | 51 (34.2) |
| Age, years; median (IQR) | 8.5 (7–11) | 9 (7–10.5) | 9 (7–10) | 8 (7–11) | 9 (7–11) |
| Weight, kg; median (IQR) | 26.5 (22–30) | 26 (22–29) | 25 (20–29) | 23 (20–28) | 25.5 (21–29) |
| Height, cm; median (IQR) | 129.5 (122–137) | 127.5 (119–139) | 129 (120–136) | 123 (114–132) | 127 (119–137) |
| Temperature, °C, median (IQR) | 36.7 (36.5–36.9) | 36.7 (36.2–37.0) | 36.7 (36.3–37.0) | 36.7 (36.4–37.0) | 36.7 (36.4–37.0) |
| Pool, beats/minute, median (IQR) | 98.5 (88–104) | 92 (82–107) | 97 (84–104) | 100 (84–104) | 98 (84–104) |
| EPG median (IQR) | 84 (24–366) | 150 (48–294) | 90 (36–150) | 108 (54–210) | 99 (39–240) |
| GM EPG | 85.2 | 129.3 | 92.1 | 110.9 | 102.7 |
| Infection intensity, n (%) | |||||
| Low | 37 (97.4) | 35 (97.2) | 35 (94.6) | 37 (100) | 144 (97.3) |
| Moderate | 1 (2.6) | 1 (2.8) | 2 (5.4) | 0 (0.0) | 4 (2.7) |
| Heavy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Coinfections, n (%) | |||||
| Ascaris lumbricoïdes | 2 (5.3) | 1 (2.8) | 1 (2.7) | 0 (0.0) | 4 (2.7) |
| Trichuris trichiura | 6 (15.8) | 1 (2.8) | 3 (8.1) | 5 (13.5) | 15 (10.1) |
| Plasmodium falciparum | 21 (55.3) | 17 (47.2) | 22 (59.5) | 21 (56.8) | 81 (54.7) |
| Characteristics . | Treatment Arm . | ||||
|---|---|---|---|---|---|
| Placebo . | 100 mg . | 200 mg . | 400 mg . | Total . | |
| N . | 38 . | 36 . | 37 . | 37 . | 148 . |
| Female, n (%) | 15 (39.5) | 14 (38.9) | 10 (27.0) | 12 (32.4) | 51 (34.2) |
| Age, years; median (IQR) | 8.5 (7–11) | 9 (7–10.5) | 9 (7–10) | 8 (7–11) | 9 (7–11) |
| Weight, kg; median (IQR) | 26.5 (22–30) | 26 (22–29) | 25 (20–29) | 23 (20–28) | 25.5 (21–29) |
| Height, cm; median (IQR) | 129.5 (122–137) | 127.5 (119–139) | 129 (120–136) | 123 (114–132) | 127 (119–137) |
| Temperature, °C, median (IQR) | 36.7 (36.5–36.9) | 36.7 (36.2–37.0) | 36.7 (36.3–37.0) | 36.7 (36.4–37.0) | 36.7 (36.4–37.0) |
| Pool, beats/minute, median (IQR) | 98.5 (88–104) | 92 (82–107) | 97 (84–104) | 100 (84–104) | 98 (84–104) |
| EPG median (IQR) | 84 (24–366) | 150 (48–294) | 90 (36–150) | 108 (54–210) | 99 (39–240) |
| GM EPG | 85.2 | 129.3 | 92.1 | 110.9 | 102.7 |
| Infection intensity, n (%) | |||||
| Low | 37 (97.4) | 35 (97.2) | 35 (94.6) | 37 (100) | 144 (97.3) |
| Moderate | 1 (2.6) | 1 (2.8) | 2 (5.4) | 0 (0.0) | 4 (2.7) |
| Heavy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Coinfections, n (%) | |||||
| Ascaris lumbricoïdes | 2 (5.3) | 1 (2.8) | 1 (2.7) | 0 (0.0) | 4 (2.7) |
| Trichuris trichiura | 6 (15.8) | 1 (2.8) | 3 (8.1) | 5 (13.5) | 15 (10.1) |
| Plasmodium falciparum | 21 (55.3) | 17 (47.2) | 22 (59.5) | 21 (56.8) | 81 (54.7) |
Abbreviations: CI, confidence interval; EPG, eggs per gram of stool; GM, geometric mean; IQR, interquartile range.
Efficacy Against Hookworm
Table 2 summarizes the CRs and ERRs of ascending tribendimidine doses against hookworm infections. CRs and ERRs were increasing with highest values observed at 400 mg of tribendimidine. In more detail, 21.2% (7/33), 38.7% (12/31), and 53.1% (17/32) of children were cured following 100 mg, 200 mg, and 400 mg tribendimidine. In the placebo group, no eggs were found in 20.6% (7/34) of the children. The Emax model predicted a placebo corrected net effect of 34.3 percentage points (95% CI, 13.3−54.4) for the 400-mg tribendimidine dose. The ERRs based on geometric mean were 30.6% (95% CI, −24.7 to 64.1) following placebo; 65.4% (95% CI, 24.5–85.9) following 100 mg; 82.1% (95% CI, 58.4–92.5) following 200 mg, and 92.2% (95% CI, 81.0–97.1) following 400 mg. An ERR of 95% can be expected at a dosage of about 500 mg. The dose response curves are presented in Figures 2 and 3.
Cure Rate and Egg Reduction Rates in Hookworm-Infected Children Aged Between 6 and 12 Years Treated With Ascending Doses of Tribendimidine Based on Available Case Analysis
| Treatment Outcome . | Treatment Arm . | |||
|---|---|---|---|---|
| Placebo . | 100 mg . | 200 mg . | 400 mg . | |
| Hookworm | ||||
| Infected children before treatment (N) | 34 | 33 | 31 | 32 |
| Cured children after treatment, n (%) | 7 (20.6) | 7 (21.2) | 12 (38.7) | 17 (53.1) |
| 95% CI | 8.7–37.9 | 9.0–38.9 | 21.8–57.8 | 34.7–70.9 |
| Cured children according to sex | ||||
| Male, n (%) | 4 (57.1) | 6 (85.7) | 7 (58.3) | 12 (70.6) |
| 95% CI | 18.4–90.1 | 42.1–99.6 | 27.7–84.8 | 44.0–89.7 |
| Female, n (%) | 3 (42.8) | 1 (14.3) | 5 (41.7) | 5 (29.4) |
| 95% CI | 9.9–81.6 | 0.4–57.9 | 15.2–72.3 | 10.3–55.9 |
| Cured children according to infection intensitya, % (n/N) | ||||
| Low | 100 (7/7) | 85.7 (6/7) | 100 (12/12) | 100 (17/17) |
| Moderate | 0 (0/1) | 100 (1/1) | 0 (0/2) | NA |
| Heavy | NA | NA | NA | NA |
| Geometric mean EPG | ||||
| Before treatment | 86.6 | 129.7 | 97.0 | 118.3 |
| After treatment | 60.1 | 44.9 | 17.4 | 9.2 |
| Egg reduction rate (%) | 30.6 | 65.4 | 82.1 | 92.2 |
| 95% CI | -24.7–64.1 | 24.5–85.9 | 58.4–92.5 | 81.0–97.1 |
| Arithmetic mean EPG | ||||
| Before treatment | 334.1 | 354.7 | 313.3 | 213.6 |
| After treatment | 273.9 | 258.4 | 130.3 | 218.1 |
| Egg reduction rate (%) | 18.0 | 27.2 | 58.4 | -2.1b |
| 95% CI | -32.0–46.2 | -41.7–65.9 | 3.0–79.4 | -1.7–71.2 |
| T. trichiura | ||||
| Cured children after treatment, n/N (%) | 0/5 (0.0) | 0/0 (0.0) | 2/2 (100) | 2/3 (66.7) |
| A. lumbricoides | ||||
| Cured children after treatment, n/N (%) | 1/2 (50.0) | 0/1 (0.0) | 0/0 (0.0) | 0/0 (0.0) |
| Treatment Outcome . | Treatment Arm . | |||
|---|---|---|---|---|
| Placebo . | 100 mg . | 200 mg . | 400 mg . | |
| Hookworm | ||||
| Infected children before treatment (N) | 34 | 33 | 31 | 32 |
| Cured children after treatment, n (%) | 7 (20.6) | 7 (21.2) | 12 (38.7) | 17 (53.1) |
| 95% CI | 8.7–37.9 | 9.0–38.9 | 21.8–57.8 | 34.7–70.9 |
| Cured children according to sex | ||||
| Male, n (%) | 4 (57.1) | 6 (85.7) | 7 (58.3) | 12 (70.6) |
| 95% CI | 18.4–90.1 | 42.1–99.6 | 27.7–84.8 | 44.0–89.7 |
| Female, n (%) | 3 (42.8) | 1 (14.3) | 5 (41.7) | 5 (29.4) |
| 95% CI | 9.9–81.6 | 0.4–57.9 | 15.2–72.3 | 10.3–55.9 |
| Cured children according to infection intensitya, % (n/N) | ||||
| Low | 100 (7/7) | 85.7 (6/7) | 100 (12/12) | 100 (17/17) |
| Moderate | 0 (0/1) | 100 (1/1) | 0 (0/2) | NA |
| Heavy | NA | NA | NA | NA |
| Geometric mean EPG | ||||
| Before treatment | 86.6 | 129.7 | 97.0 | 118.3 |
| After treatment | 60.1 | 44.9 | 17.4 | 9.2 |
| Egg reduction rate (%) | 30.6 | 65.4 | 82.1 | 92.2 |
| 95% CI | -24.7–64.1 | 24.5–85.9 | 58.4–92.5 | 81.0–97.1 |
| Arithmetic mean EPG | ||||
| Before treatment | 334.1 | 354.7 | 313.3 | 213.6 |
| After treatment | 273.9 | 258.4 | 130.3 | 218.1 |
| Egg reduction rate (%) | 18.0 | 27.2 | 58.4 | -2.1b |
| 95% CI | -32.0–46.2 | -41.7–65.9 | 3.0–79.4 | -1.7–71.2 |
| T. trichiura | ||||
| Cured children after treatment, n/N (%) | 0/5 (0.0) | 0/0 (0.0) | 2/2 (100) | 2/3 (66.7) |
| A. lumbricoides | ||||
| Cured children after treatment, n/N (%) | 1/2 (50.0) | 0/1 (0.0) | 0/0 (0.0) | 0/0 (0.0) |
Abbreviations: CI, confidence interval; EPG, eggs per gram of stool; NA, not applicable.
aLow (1–1999 EPG), moderate (2000–3999 EPG); heavy (≥4000 EPG).
bValue includes data from an outlier who had a much higher follow-up EPG (4003) compared to baseline (169). When excluding this participant, the AM EPG is 216 at baseline and 97 at follow-up resulting in an ERR of 55.1%.
Cure Rate and Egg Reduction Rates in Hookworm-Infected Children Aged Between 6 and 12 Years Treated With Ascending Doses of Tribendimidine Based on Available Case Analysis
| Treatment Outcome . | Treatment Arm . | |||
|---|---|---|---|---|
| Placebo . | 100 mg . | 200 mg . | 400 mg . | |
| Hookworm | ||||
| Infected children before treatment (N) | 34 | 33 | 31 | 32 |
| Cured children after treatment, n (%) | 7 (20.6) | 7 (21.2) | 12 (38.7) | 17 (53.1) |
| 95% CI | 8.7–37.9 | 9.0–38.9 | 21.8–57.8 | 34.7–70.9 |
| Cured children according to sex | ||||
| Male, n (%) | 4 (57.1) | 6 (85.7) | 7 (58.3) | 12 (70.6) |
| 95% CI | 18.4–90.1 | 42.1–99.6 | 27.7–84.8 | 44.0–89.7 |
| Female, n (%) | 3 (42.8) | 1 (14.3) | 5 (41.7) | 5 (29.4) |
| 95% CI | 9.9–81.6 | 0.4–57.9 | 15.2–72.3 | 10.3–55.9 |
| Cured children according to infection intensitya, % (n/N) | ||||
| Low | 100 (7/7) | 85.7 (6/7) | 100 (12/12) | 100 (17/17) |
| Moderate | 0 (0/1) | 100 (1/1) | 0 (0/2) | NA |
| Heavy | NA | NA | NA | NA |
| Geometric mean EPG | ||||
| Before treatment | 86.6 | 129.7 | 97.0 | 118.3 |
| After treatment | 60.1 | 44.9 | 17.4 | 9.2 |
| Egg reduction rate (%) | 30.6 | 65.4 | 82.1 | 92.2 |
| 95% CI | -24.7–64.1 | 24.5–85.9 | 58.4–92.5 | 81.0–97.1 |
| Arithmetic mean EPG | ||||
| Before treatment | 334.1 | 354.7 | 313.3 | 213.6 |
| After treatment | 273.9 | 258.4 | 130.3 | 218.1 |
| Egg reduction rate (%) | 18.0 | 27.2 | 58.4 | -2.1b |
| 95% CI | -32.0–46.2 | -41.7–65.9 | 3.0–79.4 | -1.7–71.2 |
| T. trichiura | ||||
| Cured children after treatment, n/N (%) | 0/5 (0.0) | 0/0 (0.0) | 2/2 (100) | 2/3 (66.7) |
| A. lumbricoides | ||||
| Cured children after treatment, n/N (%) | 1/2 (50.0) | 0/1 (0.0) | 0/0 (0.0) | 0/0 (0.0) |
| Treatment Outcome . | Treatment Arm . | |||
|---|---|---|---|---|
| Placebo . | 100 mg . | 200 mg . | 400 mg . | |
| Hookworm | ||||
| Infected children before treatment (N) | 34 | 33 | 31 | 32 |
| Cured children after treatment, n (%) | 7 (20.6) | 7 (21.2) | 12 (38.7) | 17 (53.1) |
| 95% CI | 8.7–37.9 | 9.0–38.9 | 21.8–57.8 | 34.7–70.9 |
| Cured children according to sex | ||||
| Male, n (%) | 4 (57.1) | 6 (85.7) | 7 (58.3) | 12 (70.6) |
| 95% CI | 18.4–90.1 | 42.1–99.6 | 27.7–84.8 | 44.0–89.7 |
| Female, n (%) | 3 (42.8) | 1 (14.3) | 5 (41.7) | 5 (29.4) |
| 95% CI | 9.9–81.6 | 0.4–57.9 | 15.2–72.3 | 10.3–55.9 |
| Cured children according to infection intensitya, % (n/N) | ||||
| Low | 100 (7/7) | 85.7 (6/7) | 100 (12/12) | 100 (17/17) |
| Moderate | 0 (0/1) | 100 (1/1) | 0 (0/2) | NA |
| Heavy | NA | NA | NA | NA |
| Geometric mean EPG | ||||
| Before treatment | 86.6 | 129.7 | 97.0 | 118.3 |
| After treatment | 60.1 | 44.9 | 17.4 | 9.2 |
| Egg reduction rate (%) | 30.6 | 65.4 | 82.1 | 92.2 |
| 95% CI | -24.7–64.1 | 24.5–85.9 | 58.4–92.5 | 81.0–97.1 |
| Arithmetic mean EPG | ||||
| Before treatment | 334.1 | 354.7 | 313.3 | 213.6 |
| After treatment | 273.9 | 258.4 | 130.3 | 218.1 |
| Egg reduction rate (%) | 18.0 | 27.2 | 58.4 | -2.1b |
| 95% CI | -32.0–46.2 | -41.7–65.9 | 3.0–79.4 | -1.7–71.2 |
| T. trichiura | ||||
| Cured children after treatment, n/N (%) | 0/5 (0.0) | 0/0 (0.0) | 2/2 (100) | 2/3 (66.7) |
| A. lumbricoides | ||||
| Cured children after treatment, n/N (%) | 1/2 (50.0) | 0/1 (0.0) | 0/0 (0.0) | 0/0 (0.0) |
Abbreviations: CI, confidence interval; EPG, eggs per gram of stool; NA, not applicable.
aLow (1–1999 EPG), moderate (2000–3999 EPG); heavy (≥4000 EPG).
bValue includes data from an outlier who had a much higher follow-up EPG (4003) compared to baseline (169). When excluding this participant, the AM EPG is 216 at baseline and 97 at follow-up resulting in an ERR of 55.1%.

Cure rates of tribendimidine against hookworm in children. Circles and numbers therein show observed cure rates with 95% CIs (vertical lines). Dashed lines represent the estimated dose-response curve and corresponding 95% CIs predicted by the Emax models. Gray symbols: full analysis set (available case population), black symbols: per protocol population. Abbreviation: CI, confidence interval.
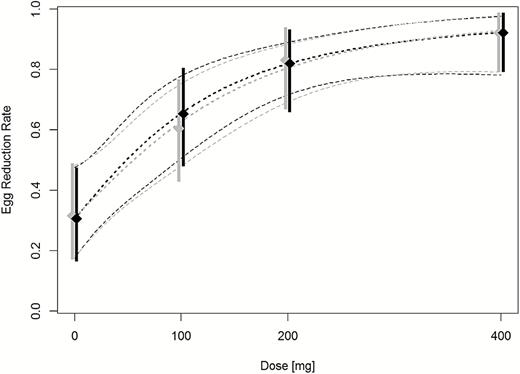
Egg reduction rates of tribendimidine against hookworm in children. Diamonds represent observed ERRs with 95% CIs (vertical lines). Dashed lines represent the estimated dose-response curve and corresponding 95% CIs predicted by the Emax models. Gray symbols: full analysis set (available case population analyzed according to ITT principles), black symbols: per protocol population. Abbreviations: CI, confidence interval; ERR, egg reduction rates; ITT, intention to treat.
The per protocol analysis did not change the results of note (Table S1). Data on efficacy against the small numbers of coinfections are presented in Table 2.
Safety
Table 3 shows adverse events occurrence in participants before treatment and 3 hours and 24 hours posttreatment. Overall, 33% (42/127), 33% (42/127), and 8% (10/127) children were reporting adverse events before treatment and 3 and 24 hours posttreatment, respectively. Most adverse events were mild. Only 2 moderate adverse events, headache in both cases, were observed following 100 mg and 400 mg tribendimidine treatment at 3 and 24 hours posttreatment, respectively. No heavy/severe adverse events occurred. Adverse events were similar among treatment arms, with headache, stomach ache, thrill, itching, and diarrhea most frequently reported. No increase in the number of adverse events was seen with increasing tribendimidine doses. Adverse events were not more abundant in children who had received active ingredient over children who had received placebo.
Frequency of Pretreatment Symptoms and Adverse Events as Experienced by Children According to Treatment Arms in the Per Protocol Population
| Symptoms . | Placebo . | 100 mg . | 200 mg . | 400 mg . | Overall . |
|---|---|---|---|---|---|
| 33 . | 32 . | 30 . | 32 . | (n = 127a) . | |
| Before treatment | |||||
| Moderate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mild | 11 (33.3) | 15 (46.9) | 7 (23.3) | 9 (28.1) | 42 (30.1) |
| None | 22 (66.7) | 17 (53.1) | 23 (76.7) | 23 (71.9) | 85 (66.9) |
| Headache | 6 (18.2) | 5 (15.6) | 1 (3.3) | 4 (12.5) | 16 (12.6) |
| Stomach ache | 5 (15.2) | 4 (12.5) | 3 (10.0) | 3 (9.4) | 15 (11.8) |
| Itching | 3 (9.1) | 4 (12.5) | 3 (10.0) | 2 (6.3) | 12 (9.4) |
| Thrill | 0 (0.0) | 5 (15.6) | 2 | 4 (12.5) | 11 (8.7) |
| Nausea | 0 (0.0) | 0 (0.0) | 1 (3.3) | 0 (0.0) | 1 (0.8) |
| Vomiting | 0 (0.0) | 1 (3.1) | 1 (3.3) | 0 (0.0) | 2 (1.6) |
| Diarrhea | 2 (6.1) | 1 (3.1) | 3 (10.0) | 0 (0.0) | 6 (4.7) |
| 3 h posttreatment | |||||
| Moderate | 0 (0.0) | 1 (3.1) | 0 (0.0) | 0 (0.0) | 1 (0.8) |
| Mild | 9 (27.3) | 13 (40.6) | 9 (30.0) | 10 (31.3) | 41(32.3) |
| None | 24 (72.7) | 18 (56.3) | 21 (70.0) | 22 (68.8) | 85 (66.9) |
| Headache | 2 (6.1) | 4 (12.5) | 4 (13.3) | 2 (6.3) | 12 (9.4) |
| Stomach ache | 4 (12.1) | 5 (15.6) | 1 (3.3) | 6 (18.8) | 16 (12.6) |
| Itching | 1 (3.0) | 2 (6.3) | 2 (6.7) | 1 (3.1) | 6 (4.7) |
| Thrill | 3 (9.1) | 6 (18.8) | 6 (20.0) | 3 (9.4) | 18 (14.2) |
| Nausea | 0 (0.0) | 0 (0.0) | 1 (3.3) | 1 (3.1) | 2 (1.6) |
| Vomiting | 1 (3.0) | 0 (0.0) | 1 (3.3) | 2 (6.3) | 4 (3.1) |
| Diarrhea | 2 (6.1) | 2 (6.3) | 2 (6.7) | 4 (12.5) | 10 (7.9) |
| 24 h posttreatment | |||||
| Moderate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.1) | 1 (0.8) |
| Mild | 1 (3.0) | 3 (9.4) | 3 (10.0) | 2 (6.3) | 9 (7.1) |
| None | 32 (97.0) | 29 (90.6) | 27 (90.0) | 29 (90.6) | 117 (92.1) |
| Headache | 1 (3.0) | 3 (9.4) | 1 (3.3) | 1 (3.1) | 6 (4.7) |
| Stomach ache | 1 (3.0) | 2 (6.3) | 1 (3.3) | 0 (0.0) | 4 (3.1) |
| Itching | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.1) | 1 (0.8) |
| Thrill | 1 (3.0) | 1 (3.1) | 0 (0.0) | 1 (3.1) | 3 (2.4) |
| Nausea | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vomiting | 0 (0.0) | 0 (0.0) | 1 (3.3) | 1 (3.1) | 2 (1.6) |
| Diarrhea | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Symptoms . | Placebo . | 100 mg . | 200 mg . | 400 mg . | Overall . |
|---|---|---|---|---|---|
| 33 . | 32 . | 30 . | 32 . | (n = 127a) . | |
| Before treatment | |||||
| Moderate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mild | 11 (33.3) | 15 (46.9) | 7 (23.3) | 9 (28.1) | 42 (30.1) |
| None | 22 (66.7) | 17 (53.1) | 23 (76.7) | 23 (71.9) | 85 (66.9) |
| Headache | 6 (18.2) | 5 (15.6) | 1 (3.3) | 4 (12.5) | 16 (12.6) |
| Stomach ache | 5 (15.2) | 4 (12.5) | 3 (10.0) | 3 (9.4) | 15 (11.8) |
| Itching | 3 (9.1) | 4 (12.5) | 3 (10.0) | 2 (6.3) | 12 (9.4) |
| Thrill | 0 (0.0) | 5 (15.6) | 2 | 4 (12.5) | 11 (8.7) |
| Nausea | 0 (0.0) | 0 (0.0) | 1 (3.3) | 0 (0.0) | 1 (0.8) |
| Vomiting | 0 (0.0) | 1 (3.1) | 1 (3.3) | 0 (0.0) | 2 (1.6) |
| Diarrhea | 2 (6.1) | 1 (3.1) | 3 (10.0) | 0 (0.0) | 6 (4.7) |
| 3 h posttreatment | |||||
| Moderate | 0 (0.0) | 1 (3.1) | 0 (0.0) | 0 (0.0) | 1 (0.8) |
| Mild | 9 (27.3) | 13 (40.6) | 9 (30.0) | 10 (31.3) | 41(32.3) |
| None | 24 (72.7) | 18 (56.3) | 21 (70.0) | 22 (68.8) | 85 (66.9) |
| Headache | 2 (6.1) | 4 (12.5) | 4 (13.3) | 2 (6.3) | 12 (9.4) |
| Stomach ache | 4 (12.1) | 5 (15.6) | 1 (3.3) | 6 (18.8) | 16 (12.6) |
| Itching | 1 (3.0) | 2 (6.3) | 2 (6.7) | 1 (3.1) | 6 (4.7) |
| Thrill | 3 (9.1) | 6 (18.8) | 6 (20.0) | 3 (9.4) | 18 (14.2) |
| Nausea | 0 (0.0) | 0 (0.0) | 1 (3.3) | 1 (3.1) | 2 (1.6) |
| Vomiting | 1 (3.0) | 0 (0.0) | 1 (3.3) | 2 (6.3) | 4 (3.1) |
| Diarrhea | 2 (6.1) | 2 (6.3) | 2 (6.7) | 4 (12.5) | 10 (7.9) |
| 24 h posttreatment | |||||
| Moderate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.1) | 1 (0.8) |
| Mild | 1 (3.0) | 3 (9.4) | 3 (10.0) | 2 (6.3) | 9 (7.1) |
| None | 32 (97.0) | 29 (90.6) | 27 (90.0) | 29 (90.6) | 117 (92.1) |
| Headache | 1 (3.0) | 3 (9.4) | 1 (3.3) | 1 (3.1) | 6 (4.7) |
| Stomach ache | 1 (3.0) | 2 (6.3) | 1 (3.3) | 0 (0.0) | 4 (3.1) |
| Itching | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.1) | 1 (0.8) |
| Thrill | 1 (3.0) | 1 (3.1) | 0 (0.0) | 1 (3.1) | 3 (2.4) |
| Nausea | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vomiting | 0 (0.0) | 0 (0.0) | 1 (3.3) | 1 (3.1) | 2 (1.6) |
| Diarrhea | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
aThree children absent for adverse event examination are excluded.
Frequency of Pretreatment Symptoms and Adverse Events as Experienced by Children According to Treatment Arms in the Per Protocol Population
| Symptoms . | Placebo . | 100 mg . | 200 mg . | 400 mg . | Overall . |
|---|---|---|---|---|---|
| 33 . | 32 . | 30 . | 32 . | (n = 127a) . | |
| Before treatment | |||||
| Moderate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mild | 11 (33.3) | 15 (46.9) | 7 (23.3) | 9 (28.1) | 42 (30.1) |
| None | 22 (66.7) | 17 (53.1) | 23 (76.7) | 23 (71.9) | 85 (66.9) |
| Headache | 6 (18.2) | 5 (15.6) | 1 (3.3) | 4 (12.5) | 16 (12.6) |
| Stomach ache | 5 (15.2) | 4 (12.5) | 3 (10.0) | 3 (9.4) | 15 (11.8) |
| Itching | 3 (9.1) | 4 (12.5) | 3 (10.0) | 2 (6.3) | 12 (9.4) |
| Thrill | 0 (0.0) | 5 (15.6) | 2 | 4 (12.5) | 11 (8.7) |
| Nausea | 0 (0.0) | 0 (0.0) | 1 (3.3) | 0 (0.0) | 1 (0.8) |
| Vomiting | 0 (0.0) | 1 (3.1) | 1 (3.3) | 0 (0.0) | 2 (1.6) |
| Diarrhea | 2 (6.1) | 1 (3.1) | 3 (10.0) | 0 (0.0) | 6 (4.7) |
| 3 h posttreatment | |||||
| Moderate | 0 (0.0) | 1 (3.1) | 0 (0.0) | 0 (0.0) | 1 (0.8) |
| Mild | 9 (27.3) | 13 (40.6) | 9 (30.0) | 10 (31.3) | 41(32.3) |
| None | 24 (72.7) | 18 (56.3) | 21 (70.0) | 22 (68.8) | 85 (66.9) |
| Headache | 2 (6.1) | 4 (12.5) | 4 (13.3) | 2 (6.3) | 12 (9.4) |
| Stomach ache | 4 (12.1) | 5 (15.6) | 1 (3.3) | 6 (18.8) | 16 (12.6) |
| Itching | 1 (3.0) | 2 (6.3) | 2 (6.7) | 1 (3.1) | 6 (4.7) |
| Thrill | 3 (9.1) | 6 (18.8) | 6 (20.0) | 3 (9.4) | 18 (14.2) |
| Nausea | 0 (0.0) | 0 (0.0) | 1 (3.3) | 1 (3.1) | 2 (1.6) |
| Vomiting | 1 (3.0) | 0 (0.0) | 1 (3.3) | 2 (6.3) | 4 (3.1) |
| Diarrhea | 2 (6.1) | 2 (6.3) | 2 (6.7) | 4 (12.5) | 10 (7.9) |
| 24 h posttreatment | |||||
| Moderate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.1) | 1 (0.8) |
| Mild | 1 (3.0) | 3 (9.4) | 3 (10.0) | 2 (6.3) | 9 (7.1) |
| None | 32 (97.0) | 29 (90.6) | 27 (90.0) | 29 (90.6) | 117 (92.1) |
| Headache | 1 (3.0) | 3 (9.4) | 1 (3.3) | 1 (3.1) | 6 (4.7) |
| Stomach ache | 1 (3.0) | 2 (6.3) | 1 (3.3) | 0 (0.0) | 4 (3.1) |
| Itching | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.1) | 1 (0.8) |
| Thrill | 1 (3.0) | 1 (3.1) | 0 (0.0) | 1 (3.1) | 3 (2.4) |
| Nausea | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vomiting | 0 (0.0) | 0 (0.0) | 1 (3.3) | 1 (3.1) | 2 (1.6) |
| Diarrhea | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Symptoms . | Placebo . | 100 mg . | 200 mg . | 400 mg . | Overall . |
|---|---|---|---|---|---|
| 33 . | 32 . | 30 . | 32 . | (n = 127a) . | |
| Before treatment | |||||
| Moderate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mild | 11 (33.3) | 15 (46.9) | 7 (23.3) | 9 (28.1) | 42 (30.1) |
| None | 22 (66.7) | 17 (53.1) | 23 (76.7) | 23 (71.9) | 85 (66.9) |
| Headache | 6 (18.2) | 5 (15.6) | 1 (3.3) | 4 (12.5) | 16 (12.6) |
| Stomach ache | 5 (15.2) | 4 (12.5) | 3 (10.0) | 3 (9.4) | 15 (11.8) |
| Itching | 3 (9.1) | 4 (12.5) | 3 (10.0) | 2 (6.3) | 12 (9.4) |
| Thrill | 0 (0.0) | 5 (15.6) | 2 | 4 (12.5) | 11 (8.7) |
| Nausea | 0 (0.0) | 0 (0.0) | 1 (3.3) | 0 (0.0) | 1 (0.8) |
| Vomiting | 0 (0.0) | 1 (3.1) | 1 (3.3) | 0 (0.0) | 2 (1.6) |
| Diarrhea | 2 (6.1) | 1 (3.1) | 3 (10.0) | 0 (0.0) | 6 (4.7) |
| 3 h posttreatment | |||||
| Moderate | 0 (0.0) | 1 (3.1) | 0 (0.0) | 0 (0.0) | 1 (0.8) |
| Mild | 9 (27.3) | 13 (40.6) | 9 (30.0) | 10 (31.3) | 41(32.3) |
| None | 24 (72.7) | 18 (56.3) | 21 (70.0) | 22 (68.8) | 85 (66.9) |
| Headache | 2 (6.1) | 4 (12.5) | 4 (13.3) | 2 (6.3) | 12 (9.4) |
| Stomach ache | 4 (12.1) | 5 (15.6) | 1 (3.3) | 6 (18.8) | 16 (12.6) |
| Itching | 1 (3.0) | 2 (6.3) | 2 (6.7) | 1 (3.1) | 6 (4.7) |
| Thrill | 3 (9.1) | 6 (18.8) | 6 (20.0) | 3 (9.4) | 18 (14.2) |
| Nausea | 0 (0.0) | 0 (0.0) | 1 (3.3) | 1 (3.1) | 2 (1.6) |
| Vomiting | 1 (3.0) | 0 (0.0) | 1 (3.3) | 2 (6.3) | 4 (3.1) |
| Diarrhea | 2 (6.1) | 2 (6.3) | 2 (6.7) | 4 (12.5) | 10 (7.9) |
| 24 h posttreatment | |||||
| Moderate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.1) | 1 (0.8) |
| Mild | 1 (3.0) | 3 (9.4) | 3 (10.0) | 2 (6.3) | 9 (7.1) |
| None | 32 (97.0) | 29 (90.6) | 27 (90.0) | 29 (90.6) | 117 (92.1) |
| Headache | 1 (3.0) | 3 (9.4) | 1 (3.3) | 1 (3.1) | 6 (4.7) |
| Stomach ache | 1 (3.0) | 2 (6.3) | 1 (3.3) | 0 (0.0) | 4 (3.1) |
| Itching | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.1) | 1 (0.8) |
| Thrill | 1 (3.0) | 1 (3.1) | 0 (0.0) | 1 (3.1) | 3 (2.4) |
| Nausea | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vomiting | 0 (0.0) | 0 (0.0) | 1 (3.3) | 1 (3.1) | 2 (1.6) |
| Diarrhea | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
aThree children absent for adverse event examination are excluded.
Safety data based on biochemical indicators from blood samples before treatment and 72 hours posttreatment are presented in Table S2. We did not observe any abnormal values before or after treatment.
DISCUSSION
Tribendimidine, a broad spectrum anthelmintic, discovered and developed in China, is the only anthelmintic drug approved in the past 3 decades [8, 9]. Though a wealth of data is available on the efficacy and safety of this drug [8, 9, 13] pivotal data are still missing to support its global use in preventive chemotherapy programs. We conducted for the first time to our knowledge a randomized, controlled, single blind trial assessing efficacy and safety of ascending doses of tribendimidine in school-aged children infected with hookworm.
Our study shows that higher tribendimidine doses reveal a better performance against hookworm in children. Increasing efficacy was observed with ascending tribendimidine doses with CRs of 21.2% for the lowest dose (100 mg) and 53.1% for the highest dose (400 mg). ERRs that were based on GM ranged between 65.4% (100 mg) and 92.2% (400 mg).
The efficacy of tribendimidine was lower in our study when compared to previous studies. The CR obtained with 200 mg (38.7%) against hookworm was lower compared to the CRs (67.4–78.4%) found in earlier studies in Asia using this dose in the same age group [8, 14]. However, the efficacy of the 400-mg dose (53.1% CR) was very similar to the one observed in adolescents in a nearby setting (CR 53.6%) [13] but lower than the ones observed in adults in previous studies in China (CRs 72.2–93.1%) [8]. The comparison of drug efficacy between trials, when using imperfect diagnostic tools such as Kato-Katz, should be done with caution. It has been well documented that the Kato-Katz method is less sensitive especially in populations with low-infection intensity [15]. Therefore, multiple Kato-Katz thick smears are required to overcome this gap. In the present study a relatively high diagnostic standard, namely, quadruplicate Kato-Katz thick smears was applied. However, differences between African and Asian populations in drug disposition could also explain this varying efficacy [10]. Moreover, the lower CRs observed in our study could be related to the hookworm species prevalent in the study setting. Note that the efficacy of tribendimidine is hookworm species dependent [8]. Tang and colleagues reported 94.9% and 78.6% of CRs, respectively, for Necator americanus and Ancylostoma duodenale [14]. Although we did not rigorously assess the efficacy of tribendimidine on the hookworm species in our study setting, preliminary data from baseline samples based on hatching eggs and morphological examination of third-stage larvae (L3) and molecular techniques documents that N. americanus is exclusively present in the study area (data not shown). It is therefore unlikely that the lower efficacy can be explained by this.
Because few coinfections with T. trichiura and A. lumbricoides occurred, no conclusion can be drawn on the dose-response on tribendimidine on these parasites in children. Moreover, we could not compare the efficacy of the drug against moderate and heavy hookworm infections because almost all trial participants had light hookworm infections. Further investigations in settings where hookworm moderate or heavy infection occurs and T. trichiura and A. lumbricoides infections are prevalent are necessary to allow a rigorous assessment of the optimal dose in the young age group.
Tribendimidine was well tolerated, and no acute kidney or liver injury was observed at all doses tested. Our finding is in line with a previous study investigating tribendimidine in hookworm-infected adolescent in Côte d’Ivoire and Pemba, Tanzania, and studies in children and adults in China [8, 13, 14, 16]. A dose-finding study in children (treated with 50–400 mg tribendimidine) and adults (treated with 25–600 mg) infected with Opisthorchis viverrini in Laos demonstrated that the drug was even better tolerated in children when compared to adults [17]. As in this study, adverse events did not increase with ascending dosages. Opisthorchis viverrini-infected children treated with tribendimidine showed more symptoms prior to treatment, even at the highest dose of 400 mg administered [17]. Our data add to the wealth of data demonstrating that tribendimidine is a safe drug, a prerequisite for its use in global preventive chemotherapy programs.
In summary, a 400-mg tribendimidine dose showed the highest efficacy in children and was well tolerated. Moreover, using the same dose in adults and school-aged children would simplify treatment programs. We advise that further investigation should be launched in moderate to heavy hookworm infection settings in order to assess the efficacy and safety of tribendimidine in these epidemiological contexts.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Authors’ contributions. J. K., J. T. C., and J. H. designed the study. J. T. C., N. H., Y. G., and J. K. conducted the study. J.T.C., N.H., J.H., and J.K. analyzed and interpreted the data. J. T. C. and J. K. wrote the first draft of the report. N. H. and J. H. revised the manuscript. All authors read and approved the final version of the report prior to submission.
Ackowledgments. We are grateful to the participants of the study and their parents for their availability. We also deeply thank ommunity Health workers (CHWs) for their strong support without which this work could have not been done.
Funding. This work was supported by a grant of the European Research Council (ERC) (grant number ERC-2013-CoG 614739-A_HERO) held by J. K.. The funders had no role in study design, data collection, analysis and decision to publish the work.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
World Health Organization.
World Health Organisation.




