-
PDF
- Split View
-
Views
-
Cite
Cite
Jan A Roth, Fabrice Juchler, Marc Dangel, Friedrich S Eckstein, Manuel Battegay, Andreas F Widmer, Frequent Door Openings During Cardiac Surgery Are Associated With Increased Risk for Surgical Site Infection: A Prospective Observational Study, Clinical Infectious Diseases, Volume 69, Issue 2, 15 July 2019, Pages 290–294, https://doi.org/10.1093/cid/ciy879
Close - Share Icon Share
Abstract
Preliminary studies that analyzed surrogate markers have suggested that operating room (OR) door openings may be a risk factor for surgical site infection (SSI). We therefore aimed to estimate the effect of OR door openings on SSI risk in patients undergoing cardiac surgery.
This prospective, observational study involved consecutive patients undergoing cardiac surgery in 2 prespecified ORs equipped with automatic door-counting devices from June 2016 to October 2017. Occurrence of an SSI within 30 days after cardiac surgery was our primary outcome measure. Respective outcome data were obtained from a national SSI surveillance cohort. We analyzed the relationship between mean OR door opening frequencies and SSI risk by use of uni- and multivariable Cox regression models.
A total of 301 594 OR door openings were recorded during the study period, with 87 676 eligible door openings being logged between incision and skin closure. There were 688 patients included in the study, of whom 24 (3.5%) developed an SSI within 30 days after surgery. In uni- and multivariable analysis, an increased mean door opening frequency during cardiac surgery was associated with higher risk for consecutive SSI (adjusted hazard ratio per 5-unit increment, 1.49; 95% confidence interval, 1.11–2.00; P = .008). The observed effect was driven by internal OR door openings toward the clean instrument preparation room.
Frequent door openings during cardiac surgery were independently associated with an increased risk for SSI. This finding warrants further study to establish a potentially causal relationship between OR door openings and the occurrence of SSI.
Surgical site infections (SSIs) are associated with increased morbidity, mortality, and healthcare costs [1, 2]. The World Health Organization and several medical societies have issued guidelines to prevent SSIs, but their implementation remains challenging [3–5]. As the effect of door openings during surgery on SSI risk is ill defined but supported by potential causal mechanisms [6, 7], we aimed to estimate the effect of automatically measured operating room (OR) door openings on SSI risk in cardiac surgery.
METHODS
We performed a prospective, observational study at the University Hospital Basel (USB), a tertiary care center in Switzerland with approximately 850 cardiac surgeries performed per year. All consecutive inpatients aged ≥18 years with a cardiac surgery performed in 2 prespecified ORs from 9 June 2016 to 31 October 2017 were eligible for study inclusion. We excluded patients who had additional cardiac surgeries within 24 hours after the first cardiac surgery. We restricted the cohort to 2 ORs at the USB to mitigate confounding by different OR and surgical team characteristics. The local ethics committee approved the study as part of a continuing quality improvement program.
We analyzed door openings of the 2 main cardiac ORs at the USB (Figure 1). Beginning in June 2016, all doors of these 2 ORs were equipped with validated electronic counting devices. We defined the OR doors toward the clean perimeter corridor as “external.” The OR doors toward the clean instrument preparation room were defined as “internal.” Both ORs and the clean instrument preparation room are equipped with laminar airflow. For each included cardiac surgery, we extracted the total number of OR door openings between first incision and skin closure from a time series database.
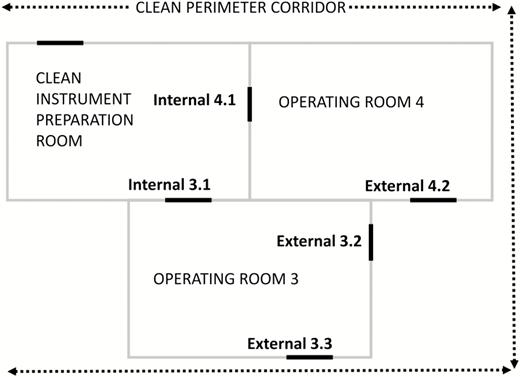
Floor plan of the two monitored cardiac operating rooms. Operating room doors are marked as bold lines.
Infection control practitioners prospectively collect SSI surveillance data as part of a national, validated surveillance program according to standard criteria [8]. In brief, they screen patients for evidence of SSI, and cases are double-checked by a board-certified infectious diseases specialist trained in surveillance. SSIs are classified according to Centers for Disease Control and Prevention definitions [9]. Standardized postdischarge SSI surveillance is conducted by telephone interviews and review of electronic medical records.
Occurrence of an SSI within 30 days after cardiac surgery was our predefined primary outcome. We prespecified the mean number of OR door openings per hour of each internal, external, and all OR doors combined as exposures of interest (mean internal and external OR door openings were very weakly correlated; r = 0.0113). We evaluated the respective exposures as a continuous variable owing to a lack of validated and clinically meaningful strata.
We assessed the respective exposure–outcome relationships by use of uni- and multivariable Cox regression models to account for loss to follow-up during the 30-day surveillance period. Follow-up was calculated from index cardiac surgery until death, loss to follow-up, SSI, or day 30, whichever occurred first. To adjust for potential confounders balanced for event predictor rates [10], we fitted multivariable models using the following covariables: nonelective/emergency surgery, preoperative American Society of Anesthesiologists classification, replacement of the ascending aorta, OR, and body mass index groups (in kg/m2, <18.5, 18.5–24.9, 25.0–29.9, 30.0–34.9, 35.0–39.9, and ≥40). Covariable selection was based on published SSI risk factors [11], an exploratory analysis, and expert consensus. Due to a low event per variable ratio and to prevent biased estimates of regression coefficients, we chose a priori to select only covariables that were correlated (in terms of effect sizes and irrespective of significance values) with both the exposure (ie, door opening frequency) and the outcome (ie, SSI incidence). These potential confounding variables were included in the multivariable models. The proportional hazards assumption was met.
As the study period was restricted due to inception of automatic OR door opening measurements in June 2016, we chose to calculate the minimal detectable hazard ratios (HRs) in the specified exposure–outcome relationship in an a priori calculation (sample size, 688; SSI proportion, 0.035; power, 0.80; 2-sided significance level, 0.05). Respective minimal detectable HRs per 5-unit increase in the mean number of door openings were estimated to be 1.57 (all OR doors combined), 1.99 (internal OR doors), and 1.82 (external OR doors). We considered these approximations of minimally detectable HRs to be suitable to asses a potential large effect of mean OR door openings on SSI risk.
In sensitivity analyses, we analyzed the respective associations by competing risk regression models and logistic regression models. We performed all analyses in Stata, version 14 (Stata Corp., College Station, TX). All P values are 2 sided.
RESULTS
Among several million monitored OR door openings, 301 594 door openings were recorded in the 2 included ORs during the study period, with 87 676 door openings being logged between incision and skin closure of included surgeries.
After excluding 32 patients with a cardiac reoperation within 24 hours, 688 individuals were included in the final analysis (Figure 2); among these, 24 (3.5%) developed an SSI within 30 days after surgery (Table 1). Among the 24 patients with an SSI, 11 superficial sternal SSIs, 12 deep sternal SSIs, and 1 organ/space SSI (ie, mediastinitis) were observed. The median follow-up in patients without an SSI was 30 days (interquartile range, 30–30 days).
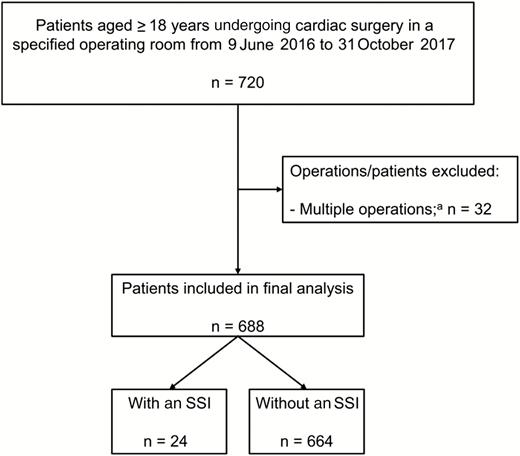
Patient selection. Abbreviation: SSI, surgical site infection. aPatients who had additional cardiac operations within 24 hours after the first cardiac operation.
| Characteristic . | No SSI (n = 664) . | SSI (n = 24) . | Overall (n = 688) . |
|---|---|---|---|
| Individual | |||
| Age in years, median (IQR) | 68 (59–74) | 70 (63–73) | 68 (59–74) |
| Female sex, n (%) | 161 (24.2) | 10 (41.6) | 171 (24.9) |
| Body mass index in kg/m2, median (IQR) | 26.2 (23.7–29.7)a | 28.5 (25.6–34.0) | 26.3 (23.7–29.7)a |
| Diabetes mellitus type 1 or 2, n (%)b | 152 (22.9) | 11 (45.8) | 163 (23.7) |
| No. of secondary diagnoses, median (IQR)c | 10 (8–13) | 15.5 (11.5–20) | 10 (8–13) |
| Days of follow-up from cardiac surgery to SSI/censoring, median (IQR) | 30.0 (30.0–30.0) | 19.5 (11.0–24.5) | 30.0 (30.0–30.0) |
| Cardiac reoperation within 30 days, n (%)d | 44 (6.6) | 3 (12.5) | 47 (6.8) |
| Crude mortality within 30 days, n (%) | 23 (3.5) | 1 (4.0) | 24 (3.5) |
| Cardiac operation | |||
| Emergency surgery, n (%)e | 102 (15.4) | 6 (25.0) | 108 (15.7) |
| Procedures, n (%)f | |||
| Coronary artery bypass grafting | 363 (54.7) | 19 (79.2) | 382 (55.5) |
| Any valve surgery | 231 (34.8) | 7 (29.2) | 238 (34.6) |
| Replacement of the ascending aorta | 56 (8.4) | 2 (8.3) | 58 (8.4) |
| Other proceduresg | 184 (27.7) | 2 (8.3) | 186 (27.0) |
| Duration of surgery in minutes, median (IQR) | 226 (190–267) | 256 (213–300) | 228 (191–269) |
| American Society of Anesthesiologists score, median (IQR) | 4 (3–4) | 4 (3–4) | 4 (3–4) |
| National nosocomial infection surveillance risk index, n (%)h | |||
| 0 | 4 (0.7) | 0 (0) | 4 (0.6) |
| 1 | 495 (80.5) | 14 (63.6) | 509 (79.9) |
| 2 | 106 (17.2) | 8 (36.3) | 114 (17.9) |
| 3 | 10 (1.6) | 0 (0) | 10 (1.6) |
| Adequate antimicrobial prophylaxis ≤60 minutes prior to incision, n (%) | 632 (95.5)i | 21 (87.5) | 653 (95.2)i |
| Operating room used during index surgery | |||
| Room 3, n (%) | 405 (61.0) | 15 (62.5) | 420 (61.0) |
| Room 4, n (%) | 259 (39.0) | 9 (37.5) | 268 (39.0) |
| Mean number of operating room door openings per hour, mean (95% confidence interval) | |||
| Internal | 17.4 (17.1–17.8) | 19.7 (18.1–21.3) | 17.4 (17.1–17.8) |
| External | 14.9 (14.6–15.3) | 17.4 (17.0–17.7) | 14.9 (14.6–15.3) |
| All | 32.3 (31.8–32.8) | 35.5 (33.3–37.7) | 32.4 (31.9–32.9) |
| Characteristic . | No SSI (n = 664) . | SSI (n = 24) . | Overall (n = 688) . |
|---|---|---|---|
| Individual | |||
| Age in years, median (IQR) | 68 (59–74) | 70 (63–73) | 68 (59–74) |
| Female sex, n (%) | 161 (24.2) | 10 (41.6) | 171 (24.9) |
| Body mass index in kg/m2, median (IQR) | 26.2 (23.7–29.7)a | 28.5 (25.6–34.0) | 26.3 (23.7–29.7)a |
| Diabetes mellitus type 1 or 2, n (%)b | 152 (22.9) | 11 (45.8) | 163 (23.7) |
| No. of secondary diagnoses, median (IQR)c | 10 (8–13) | 15.5 (11.5–20) | 10 (8–13) |
| Days of follow-up from cardiac surgery to SSI/censoring, median (IQR) | 30.0 (30.0–30.0) | 19.5 (11.0–24.5) | 30.0 (30.0–30.0) |
| Cardiac reoperation within 30 days, n (%)d | 44 (6.6) | 3 (12.5) | 47 (6.8) |
| Crude mortality within 30 days, n (%) | 23 (3.5) | 1 (4.0) | 24 (3.5) |
| Cardiac operation | |||
| Emergency surgery, n (%)e | 102 (15.4) | 6 (25.0) | 108 (15.7) |
| Procedures, n (%)f | |||
| Coronary artery bypass grafting | 363 (54.7) | 19 (79.2) | 382 (55.5) |
| Any valve surgery | 231 (34.8) | 7 (29.2) | 238 (34.6) |
| Replacement of the ascending aorta | 56 (8.4) | 2 (8.3) | 58 (8.4) |
| Other proceduresg | 184 (27.7) | 2 (8.3) | 186 (27.0) |
| Duration of surgery in minutes, median (IQR) | 226 (190–267) | 256 (213–300) | 228 (191–269) |
| American Society of Anesthesiologists score, median (IQR) | 4 (3–4) | 4 (3–4) | 4 (3–4) |
| National nosocomial infection surveillance risk index, n (%)h | |||
| 0 | 4 (0.7) | 0 (0) | 4 (0.6) |
| 1 | 495 (80.5) | 14 (63.6) | 509 (79.9) |
| 2 | 106 (17.2) | 8 (36.3) | 114 (17.9) |
| 3 | 10 (1.6) | 0 (0) | 10 (1.6) |
| Adequate antimicrobial prophylaxis ≤60 minutes prior to incision, n (%) | 632 (95.5)i | 21 (87.5) | 653 (95.2)i |
| Operating room used during index surgery | |||
| Room 3, n (%) | 405 (61.0) | 15 (62.5) | 420 (61.0) |
| Room 4, n (%) | 259 (39.0) | 9 (37.5) | 268 (39.0) |
| Mean number of operating room door openings per hour, mean (95% confidence interval) | |||
| Internal | 17.4 (17.1–17.8) | 19.7 (18.1–21.3) | 17.4 (17.1–17.8) |
| External | 14.9 (14.6–15.3) | 17.4 (17.0–17.7) | 14.9 (14.6–15.3) |
| All | 32.3 (31.8–32.8) | 35.5 (33.3–37.7) | 32.4 (31.9–32.9) |
All proportions are expressed as n patients (%).
Abbreviations: IQR, interquartile range; SSI, surgical site infection.
aData missing for 16 patients.
bPatients with a discharge diagnosis of diabetes mellitus type 1 or 2.
cNumber of secondary International Classification of Disease-10 diagnoses at discharge.
dAll cardiac surgeries between 24 hours and 30 days after index surgery; patients who had a second cardiac operation within 24 hours after index surgery were excluded at patient selection.
eAll nonelective surgeries.
fIn some patients, several procedures were performed during the index operation (the cumulative percentage exceeds 100%).
gIncluding patent foramen ovale closure, chordoplasty, pericardiectomy, cardiac tumor resection, and various cardiac device implantations.
hValid percentages; data missing for 51 patients.
iValid percentages; data missing for 2 patients.
| Characteristic . | No SSI (n = 664) . | SSI (n = 24) . | Overall (n = 688) . |
|---|---|---|---|
| Individual | |||
| Age in years, median (IQR) | 68 (59–74) | 70 (63–73) | 68 (59–74) |
| Female sex, n (%) | 161 (24.2) | 10 (41.6) | 171 (24.9) |
| Body mass index in kg/m2, median (IQR) | 26.2 (23.7–29.7)a | 28.5 (25.6–34.0) | 26.3 (23.7–29.7)a |
| Diabetes mellitus type 1 or 2, n (%)b | 152 (22.9) | 11 (45.8) | 163 (23.7) |
| No. of secondary diagnoses, median (IQR)c | 10 (8–13) | 15.5 (11.5–20) | 10 (8–13) |
| Days of follow-up from cardiac surgery to SSI/censoring, median (IQR) | 30.0 (30.0–30.0) | 19.5 (11.0–24.5) | 30.0 (30.0–30.0) |
| Cardiac reoperation within 30 days, n (%)d | 44 (6.6) | 3 (12.5) | 47 (6.8) |
| Crude mortality within 30 days, n (%) | 23 (3.5) | 1 (4.0) | 24 (3.5) |
| Cardiac operation | |||
| Emergency surgery, n (%)e | 102 (15.4) | 6 (25.0) | 108 (15.7) |
| Procedures, n (%)f | |||
| Coronary artery bypass grafting | 363 (54.7) | 19 (79.2) | 382 (55.5) |
| Any valve surgery | 231 (34.8) | 7 (29.2) | 238 (34.6) |
| Replacement of the ascending aorta | 56 (8.4) | 2 (8.3) | 58 (8.4) |
| Other proceduresg | 184 (27.7) | 2 (8.3) | 186 (27.0) |
| Duration of surgery in minutes, median (IQR) | 226 (190–267) | 256 (213–300) | 228 (191–269) |
| American Society of Anesthesiologists score, median (IQR) | 4 (3–4) | 4 (3–4) | 4 (3–4) |
| National nosocomial infection surveillance risk index, n (%)h | |||
| 0 | 4 (0.7) | 0 (0) | 4 (0.6) |
| 1 | 495 (80.5) | 14 (63.6) | 509 (79.9) |
| 2 | 106 (17.2) | 8 (36.3) | 114 (17.9) |
| 3 | 10 (1.6) | 0 (0) | 10 (1.6) |
| Adequate antimicrobial prophylaxis ≤60 minutes prior to incision, n (%) | 632 (95.5)i | 21 (87.5) | 653 (95.2)i |
| Operating room used during index surgery | |||
| Room 3, n (%) | 405 (61.0) | 15 (62.5) | 420 (61.0) |
| Room 4, n (%) | 259 (39.0) | 9 (37.5) | 268 (39.0) |
| Mean number of operating room door openings per hour, mean (95% confidence interval) | |||
| Internal | 17.4 (17.1–17.8) | 19.7 (18.1–21.3) | 17.4 (17.1–17.8) |
| External | 14.9 (14.6–15.3) | 17.4 (17.0–17.7) | 14.9 (14.6–15.3) |
| All | 32.3 (31.8–32.8) | 35.5 (33.3–37.7) | 32.4 (31.9–32.9) |
| Characteristic . | No SSI (n = 664) . | SSI (n = 24) . | Overall (n = 688) . |
|---|---|---|---|
| Individual | |||
| Age in years, median (IQR) | 68 (59–74) | 70 (63–73) | 68 (59–74) |
| Female sex, n (%) | 161 (24.2) | 10 (41.6) | 171 (24.9) |
| Body mass index in kg/m2, median (IQR) | 26.2 (23.7–29.7)a | 28.5 (25.6–34.0) | 26.3 (23.7–29.7)a |
| Diabetes mellitus type 1 or 2, n (%)b | 152 (22.9) | 11 (45.8) | 163 (23.7) |
| No. of secondary diagnoses, median (IQR)c | 10 (8–13) | 15.5 (11.5–20) | 10 (8–13) |
| Days of follow-up from cardiac surgery to SSI/censoring, median (IQR) | 30.0 (30.0–30.0) | 19.5 (11.0–24.5) | 30.0 (30.0–30.0) |
| Cardiac reoperation within 30 days, n (%)d | 44 (6.6) | 3 (12.5) | 47 (6.8) |
| Crude mortality within 30 days, n (%) | 23 (3.5) | 1 (4.0) | 24 (3.5) |
| Cardiac operation | |||
| Emergency surgery, n (%)e | 102 (15.4) | 6 (25.0) | 108 (15.7) |
| Procedures, n (%)f | |||
| Coronary artery bypass grafting | 363 (54.7) | 19 (79.2) | 382 (55.5) |
| Any valve surgery | 231 (34.8) | 7 (29.2) | 238 (34.6) |
| Replacement of the ascending aorta | 56 (8.4) | 2 (8.3) | 58 (8.4) |
| Other proceduresg | 184 (27.7) | 2 (8.3) | 186 (27.0) |
| Duration of surgery in minutes, median (IQR) | 226 (190–267) | 256 (213–300) | 228 (191–269) |
| American Society of Anesthesiologists score, median (IQR) | 4 (3–4) | 4 (3–4) | 4 (3–4) |
| National nosocomial infection surveillance risk index, n (%)h | |||
| 0 | 4 (0.7) | 0 (0) | 4 (0.6) |
| 1 | 495 (80.5) | 14 (63.6) | 509 (79.9) |
| 2 | 106 (17.2) | 8 (36.3) | 114 (17.9) |
| 3 | 10 (1.6) | 0 (0) | 10 (1.6) |
| Adequate antimicrobial prophylaxis ≤60 minutes prior to incision, n (%) | 632 (95.5)i | 21 (87.5) | 653 (95.2)i |
| Operating room used during index surgery | |||
| Room 3, n (%) | 405 (61.0) | 15 (62.5) | 420 (61.0) |
| Room 4, n (%) | 259 (39.0) | 9 (37.5) | 268 (39.0) |
| Mean number of operating room door openings per hour, mean (95% confidence interval) | |||
| Internal | 17.4 (17.1–17.8) | 19.7 (18.1–21.3) | 17.4 (17.1–17.8) |
| External | 14.9 (14.6–15.3) | 17.4 (17.0–17.7) | 14.9 (14.6–15.3) |
| All | 32.3 (31.8–32.8) | 35.5 (33.3–37.7) | 32.4 (31.9–32.9) |
All proportions are expressed as n patients (%).
Abbreviations: IQR, interquartile range; SSI, surgical site infection.
aData missing for 16 patients.
bPatients with a discharge diagnosis of diabetes mellitus type 1 or 2.
cNumber of secondary International Classification of Disease-10 diagnoses at discharge.
dAll cardiac surgeries between 24 hours and 30 days after index surgery; patients who had a second cardiac operation within 24 hours after index surgery were excluded at patient selection.
eAll nonelective surgeries.
fIn some patients, several procedures were performed during the index operation (the cumulative percentage exceeds 100%).
gIncluding patent foramen ovale closure, chordoplasty, pericardiectomy, cardiac tumor resection, and various cardiac device implantations.
hValid percentages; data missing for 51 patients.
iValid percentages; data missing for 2 patients.
In univariable analysis, an increased mean opening frequency of all OR doors combined was associated with higher risk for SSI (HR per 5-unit increment, 1.47; 95% confidence interval [CI], 1.11–1.95; P = .007; Figure 3); the observed effect was driven by internal OR door openings (HR per 5-unit increment, 2.00; 95% CI, 1.24–3.20; P = .005). There was no evidence for an association between mean opening frequencies of external OR doors and risk for SSI (HR per 5-unit increment, 1.27; 95% CI, 0.83–1.90; P = .28).
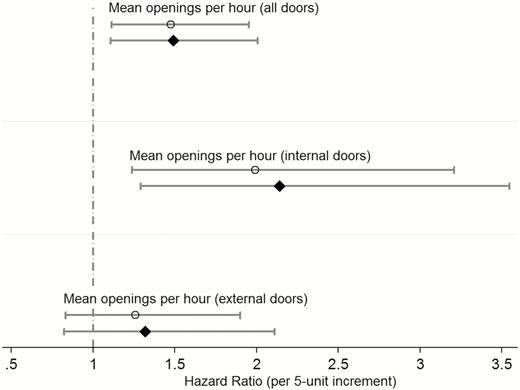
Estimated effect of door opening frequencies during cardiac surgery on surgical site infection risk (n = 688 patients). Respective crude hazard ratios (circles) and adjusted hazard ratios (diamonds) are presented with corresponding 95% confidence intervals. All multivariable models include the following covariables: nonelective (emergency) surgery, preoperative American Society of Anesthesiologists classification (groups I–V), replacement of the ascending aorta, operating room (3 or 4), and standard body mass index groups (in kg/m2, <18.5, 18.5–24.9, 25.0–29.9, 30.0–34.9, 35.0–39.9, and ≥40).
With adjustment, an increased mean opening frequency of all OR doors combined remained associated with higher risk for SSI (adjusted HR per 5-unit increment, 1.49; 95% CI, 1.11–2.00; P = .008); the observed effect was driven by internal door openings (adjusted HR per 5-unit increment, 2.14; 95% CI, 1.29–3.55; P = .003). There was no evidence of an association between mean opening frequencies of external doors and risk for SSI (adjusted HR per 5-unit increment, 1.32; 95% CI, 0.82–2.11; P = .25). Respective uni- and multivariable sensitivity analyses revealed consistent associations (see Supplementary Tables S1 and S2).
DISCUSSION
This large sample of OR door openings revealed an independent, positive association between mean OR door openings during cardiac surgery and consecutive SSI risk, mainly driven by internal door openings. Although an association between OR door openings and microbial wound contamination (ie, a surrogate marker for SSI) has been demonstrated [6, 7, 12], studies that investigate the potential effect of OR door openings on SSI risk are lacking.
There are several main explanations for the observed association between OR door openings and SSI risk. First, it may be that OR door opening frequencies are a marker for the organizational level and discipline of surgical teams [13]. Second, one could argue that OR door opening frequencies are an indicator for complex surgeries leading to higher OR traffic and therefore confounding the examined exposure–outcome relationship. However, this is unlikely, as we used multivariable regression to control for confounding effects of surgical complexity, with little change in effect sizes after adjustments. Third, OR door openings may create airflows [14] that disrupt the laminar airflow and increase the microbial concentrations at the surgical site; this was demonstrated recently by an outbreak with Mycobacterium chimaera [15, 16]. Last, an increase in OR door openings could distract the surgical team, which may lead to surgical errors and contamination.
We were surprised by the strong association between internal OR door openings and SSI risk, which was not observed for external OR doors. This may be explained by several mechanisms. First, poor coordination among surgical teams may be an independent risk factor for SSI. In our institution, additional surgical material is transported through the internal OR doors. Therefore, “poorly” organized cardiac interventions or greater complexity of specific cardiac surgeries may lead to increased internal OR door openings and potentially confound the examined OR door opening–SSI relationship. In our cohort, the reasons for specific OR door openings were not assessed, for instance, as part of a nested qualitative study. Such information could improve the assessment of the OR door opening–SSI relationship. Second, the observed differential effect of OR door openings on SSI risk could be related to differences in air pressure from the OR toward the clean perimeter corridor (0.9 Pascal) and toward the clean instrument preparation room (0.0 Pascal; data from external certification). Therefore, internal OR door openings could lead to higher airflow toward the operating field compared to external OR door openings. Third, internal OR door openings could be more disturbing for cardiac surgeons than external OR doors openings (eg, due to “social” conversations via internal doors), which may lead to surgical errors and contamination. Fourth, our study was underpowered to detect effects of small magnitude for external OR door openings; therefore, it cannot be excluded that external OR door openings had a small effect on SSI risk.
Overall, we observed a rather high mean OR door opening frequency of 32.4 openings per hour, but similar frequencies have been reported [17, 18]. In a Dutch national SSI prevention bundle [3], fewer than 10 door openings per hour was used as the surrogate marker for hygiene discipline in the OR. This surrogate marker achieved an overall compliance rate of 80% at the end of the program. Several studies have indicated that a large share of OR door openings are unnecessary and could be prevented [19, 20]. Better communication between surgeons and assistants to anticipate equipment needs may be an effective measure to reduce OR door openings. A pre–post study following an educational program emphasizing the notion of frequent door openings as a risk factor for SSI could further clarify the role of OR door openings in preventing SSI.
Our study has several limitations. First, our study results were based on a single-center cardiac surgery cohort and may not be generalizable to other healthcare settings and surgical procedures. Specifically, we observed a high frequency of OR door openings; therefore, our study findings may not be generalizable to a setting where OR door openings are less frequent. Second, the demonstrated exposure–outcome relationship between OR door opening frequencies and SSI risk may not be causal, as unmeasured factors could have confounded the respective relationship (eg, complications, surgical complexity). Third, our study was underpowered to detect effects of small size for external OR door openings.
CONCLUSIONS
Frequent door openings were independently associated with an increased risk for SSI. These findings warrant further investigation as part of a large cohort study, including a qualitative assessment of reasons for OR door openings, in order to clarify the role of OR door openings as a marker or independent risk factor for SSI.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was funded by the Division of Infectious Diseases and Hospital Epidemiology, University Hospital Basel, Switzerland. J. A. R. was partly funded by a research grant of the University Hospital Basel (“VW Pool,” Department of Medicine), Basel, Switzerland.
Potential conflicts of interest. All authors report no conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
J. A. R. and F. J. contributed equally to this work.




