-
PDF
- Split View
-
Views
-
Cite
Cite
Lilian Abbo, Bhavarth S Shukla, Amber Giles, Laura Aragon, Adriana Jimenez, Jose F Camargo, Jacques Simkins, Kathleen Sposato, Truc T Tran, Lorena Diaz, Jinnethe Reyes, Rafael Rios, Lina P Carvajal, Javier Cardozo, Maribel Ruiz, Gemma Rosello, Armando Perez Cardona, Octavio Martinez, Giselle Guerra, Thiago Beduschi, Rodrigo Vianna, Cesar A Arias, Linezolid- and Vancomycin-resistant Enterococcus faecium in Solid Organ Transplant Recipients: Infection Control and Antimicrobial Stewardship Using Whole Genome Sequencing, Clinical Infectious Diseases, Volume 69, Issue 2, 15 July 2019, Pages 259–265, https://doi.org/10.1093/cid/ciy903
Close - Share Icon Share
Abstract
Vancomycin-resistant enterococci are an important cause of healthcare-associated infections and are inherently resistant to many commonly used antibiotics. Linezolid is the only drug currently approved by the US Food and Drug Administration to treat vancomycin-resistant enterococci; however, resistance to this antibiotic appears to be increasing. Although outbreaks of linezolid- and vancomycin-resistant Enterococcus faecium (LR-VRE) in solid organ transplant recipients remain uncommon, they represent a major challenge for infection control and hospital epidemiology.
We describe a cluster of 4 LR-VRE infections among a group of liver and multivisceral transplant recipients in a single intensive care unit. Failure of treatment with linezolid in 2 cases led to a review of standard clinical laboratory methods for susceptibility determination. Testing by alternative methods including whole genome sequencing (WGS) and a comprehensive outbreak investigation including sampling of staff members and surfaces was performed.
Review of laboratory testing methods revealed a limitation in the VITEK 2 system with regard to reporting resistance to linezolid. Linezolid resistance in all cases was confirmed by E-test method. The use of WGS identified a resistant subpopulation with the G2376C mutation in the 23S ribosomal RNA. Sampling of staff members’ dominant hands as well as sampling of surfaces in the unit identified no contaminated sources for transmission.
This cluster of LR-VRE in transplant recipients highlights the possible shortcomings of standard microbiology laboratory methods and underscores the importance of WGS to identify resistance mechanisms that can inform patient care, as well as infection control and antibiotic stewardship measures.
(See the Editorial Commentary by Schuetz on pages 266–7.)
Enterococcus faecium infections are a significant cause of morbidity and mortality in critically ill patients [1]. Vancomycin-resistant enterococci (VRE) are also often resistant to ampicillin and are associated with increased mortality compared to enterococcal species that are susceptible to vancomycin [2]. VRE infections have limited treatment options; indeed, only linezolid is approved by the US Food and Drug Administration for infections caused by VRE. However, it is bacteriostatic and most authorities favor using bactericidal therapy in severe infections caused by VRE. Daptomycin, a lipopeptide antibiotic, has become first-line therapy against VRE due to its in vitro bactericidal activity, although resistance emerging during treatment has been well documented. Moreover, the usual combination of β-lactams and aminoglycosides [3, 4] has become almost obsolete for the treatment of deep-seated VRE infections as most clinical isolates are ampicillin resistant. Thus, the best therapeutic approach for these organisms is unknown [5]. Oritavancin and tedizolid are newer drugs with in vitro activity against VRE, but their role in therapy is uncertain [6].
Linezolid is an oxazolidinone antibiotic that inhibits protein synthesis by interactions with ribosomal proteins and the 23S ribosomal RNA (rRNA) of the 50S subunit [7]. Several mechanisms of resistance to linezolid have been characterized and include (1) mutations in genes encoding the 23S rRNA (the most common change observed in clinical isolates is G2576U); (2) acquisition of the cfr gene (for chloramphenicol-florfenicol resistance) encoding a rRNA methyltransferase that methylates nucleotide A2503 [8]; (3) mutations in ribosomal proteins L3 and L4 that are usually found concomitantly with 23S changes and cfr; and (4) presence of the optrA gene that encodes an adenosine triphosphate–binding cassette protein and seems to confer resistance through ribosomal protection [9].
Although nosocomial outbreaks of VRE are well documented in the literature [10], including hematologic patients [11, 12] and solid organ transplant (SOT) recipients [13], outbreaks of E. faecium resistant to both vancomycin and linezolid (LR-VRE) are less common [14]. Here, we describe a unique cluster of LR-VRE in our intensive care unit (ICU) that only affected liver and multivisceral transplant recipients and present the challenges and opportunities encountered in controlling the outbreak and managing these complex infections.
METHODS
The cluster occurred at Jackson Memorial Hospital, a 1558-licensed bed tertiary care teaching hospital in Miami, Florida. The investigation was initially conducted as part of our standard infection prevention and patient safety procedures and subsequently approved by the Institutional Review Board of the University of Miami. In April 2017, the infection control and antimicrobial stewardship departments noticed the sudden appearance of 2 patients with clinical failure to linezolid while being treated for VRE bloodstream infections within an ICU. A retrospective review of all positive cultures for VRE performed at our health system was conducted. Cases were defined as any patient between 1 January 2016 and 30 May 2017 with VRE isolated from any source that had no reported susceptibilities to linezolid using testing by VITEK 2 (bioMérieux) card AST-GP75 based on the Clinical and Laboratory Standards Institute (CLSI) breakpoint (M100-S27) of 2 μg/mL [15]. As part of the cluster investigation, all isolates were sent to the Florida Department of Health Laboratory for pulsed-field gel electrophoresis (PFGE) [16], and to the Center for Antimicrobial Resistance and Microbial Genomics at the University of Texas Health Science Center at Houston for whole genome sequencing (WGS).
Isolate Characterization
Confirmation of enterococcal species for relevant isolates was performed by polymerase chain reaction (PCR) assays, which detects several van genes and the gene encoding the D-Ala-D-Ala ligase of Enterococcus faecalis and E. faecium for species identification as previously described [17]. Minimum inhibitory concentrations (MICs) of both linezolid and daptomycin were performed by broth microdilution method using Sensititre [18] and/or E-test following manufacturer instructions (Table 1). Initial screening for the cfr and optrA genes was performed using PCR assays, as previously described with positive controls available [19, 20].
Sensitivities to Antibiotics Reported by Standard Clinical Laboratory Methods for Initial Enterococcus faecium Isolates
| Case . | VITEK 2 . | . | E-test MIC, μg/mL . | . | Sensititre . | . |
|---|---|---|---|---|---|---|
| . | Ampicillin . | Linezolid . | Linezolid . | Daptomycin . | Linezolid . | Daptomycin . |
| 1 | Resistant | Not reported | 12 | 2 | Not tested | Not tested |
| 2 | Resistant | Not reported | 12 | 2 | Not tested | >4 |
| 3 | Resistant | Not reported | 12 | 2 | Resistant | Not tested |
| 4 | Resistant | Not reported | 32 | 3 | Not tested | Not tested |
| Case . | VITEK 2 . | . | E-test MIC, μg/mL . | . | Sensititre . | . |
|---|---|---|---|---|---|---|
| . | Ampicillin . | Linezolid . | Linezolid . | Daptomycin . | Linezolid . | Daptomycin . |
| 1 | Resistant | Not reported | 12 | 2 | Not tested | Not tested |
| 2 | Resistant | Not reported | 12 | 2 | Not tested | >4 |
| 3 | Resistant | Not reported | 12 | 2 | Resistant | Not tested |
| 4 | Resistant | Not reported | 32 | 3 | Not tested | Not tested |
Abbreviation: MIC, minimum inhibitory concentration.
Sensitivities to Antibiotics Reported by Standard Clinical Laboratory Methods for Initial Enterococcus faecium Isolates
| Case . | VITEK 2 . | . | E-test MIC, μg/mL . | . | Sensititre . | . |
|---|---|---|---|---|---|---|
| . | Ampicillin . | Linezolid . | Linezolid . | Daptomycin . | Linezolid . | Daptomycin . |
| 1 | Resistant | Not reported | 12 | 2 | Not tested | Not tested |
| 2 | Resistant | Not reported | 12 | 2 | Not tested | >4 |
| 3 | Resistant | Not reported | 12 | 2 | Resistant | Not tested |
| 4 | Resistant | Not reported | 32 | 3 | Not tested | Not tested |
| Case . | VITEK 2 . | . | E-test MIC, μg/mL . | . | Sensititre . | . |
|---|---|---|---|---|---|---|
| . | Ampicillin . | Linezolid . | Linezolid . | Daptomycin . | Linezolid . | Daptomycin . |
| 1 | Resistant | Not reported | 12 | 2 | Not tested | Not tested |
| 2 | Resistant | Not reported | 12 | 2 | Not tested | >4 |
| 3 | Resistant | Not reported | 12 | 2 | Resistant | Not tested |
| 4 | Resistant | Not reported | 32 | 3 | Not tested | Not tested |
Abbreviation: MIC, minimum inhibitory concentration.
Whole Genome Sequencing and Genome Characterization
DNA was extracted using DNAEasy kit from Qiagen with lysozyme treatment of the cells after overnight culture. Library preparation was performed using a NexteraXT kit from Illumina, following manufacturer instructions, and paired-end genome sequencing was done in a MiSeq sequencer. De novo and reference-based assemblies (TX16-DO accession: NC_017960.1) were performed using CLC Genomics Workbench version 8.5. Multilocus sequence typing (MLST) was done using the MLST version 1.8 tool from CGE center [21]. Acquired resistance elements were identified using BlastX [22] against the ResFinder [23] database, selecting hits with an identity percentage >95% and coverage of at least 80% of the reference sequence. To evaluate for quinolone and linezolid mutational resistance, we performed sequence alignment with Muscle [24] against the reference sequences of the proteins GyrA (WP_002288365.1), GyrB (WP_002288364.1), ParC (WP_002296998.1), ParE (WP_002303746.1) [25], L3 (AFK57681.1), and L4 (AFK57682.1) [26]. Additionally, the reference-based assembly was used to identify the 6 copies of the 23S rRNA genes in the genome. Mutations in the position G2576C [26, 27] were manually checked in each copy of the gene. When no mutations in the 23S rRNA were found, reads were mapped against the reference assembly followed by calling of variants using the low-frequency variant detection tool from CLC Workbench version 8.5, counting the proportion of mapped reads carrying the G2576C mutation within each copy of the 23S rRNA gene.
Source Evaluation
Data were collected retrospectively from the patients’ electronic medical records, including demographics, comorbidities, antibiotic therapy, and outcomes. A list of operating room cases and providers in the ICU was created to identify commonalities. Anonymous cultures of the hands of healthcare personnel identified as having patient contact were taken including physicians, nurses, and medical technicians working the in the ICU where the cases were identified. Imprints of the dominant hand were obtained by palm, fingers, and thumb imprints for 3 seconds over the same blood agar plate. All plates were incubated at 37°C for 48 hours and screened for growth of pathogens including VRE. Initial screen of the plates was based on colony morphology and Gram stain. Final identification of pathogens was done using VITEK 2. Results of the hand cultures were shared with the surgical, nursing, and anesthesia ICU staff.
Environmental cultures of surfaces including desks, intravenous (IV) pumps, and computers were collected by swabbing areas of approximately 3 × 3 inches with premoistened cotton swabs. Single swabs were inoculated in trypticase soy broth and incubated at 37°C for 48 hours. The broths that showed turbidity after the incubation period were subcultured to BAP and McConkey agar for further identification and susceptibility testing of pathogens, including VRE.
Case Presentations
Case 1
A 49-year-old man underwent multivisceral transplant (liver, stomach, duodenum, pancreas, small bowel, and colon) due to nonalcoholic steatohepatitis cirrhosis and metastatic neuroendocrine tumor. He received perioperative prophylaxis per local protocol: vancomycin 1 g IV every 8 hours, cefepime 2 g IV every 12 hours, metronidazole 500 mg IV every 8 hours, and fluconazole. On postoperative day (POD) 11, he developed septic shock from pancreatic necrosis and underwent exploratory laparotomy as well as subsequent multiple abdominal washouts and courses of antibiotics including vancomycin, piperacillin-tazobactam, and meropenem. He was ultimately retransplanted 6 weeks later. On POD 10 of the retransplant, the patient developed enterococcal bacteremia while on therapeutic levels of vancomycin and cefepime. Enterococcus faecium positive for vanA was identified by BioFire; however, susceptibilities to linezolid and daptomycin were not reported. The patient was treated with linezolid 600 mg IV twice daily but continued to have VRE isolation from blood 4 days after the initial positive culture. The antibiotic stewardship team was made aware around the same time as case 2 as described below. After request from the treating team, susceptibility testing via E-test revealed a linezolid MIC of 12 µg/mL and daptomycin MIC of 2 µg/mL. The patient was transitioned to daptomycin 8 mg/kg IV daily and repeat blood cultures were negative, so a course of 3 weeks of therapy was completed.
Case 2
A 69-year-old man with end-stage liver disease secondary to alcoholic cirrhosis and end-stage renal disease on hemodialysis was admitted for combined liver-kidney transplantation. At approximately POD 7, the patient developed E. faecium bacteremia and septic shock, confirmed to be VRE by BioFire. The source of the infection was unclear with a differential of intra-abdominal origin vs catheter-related infection, so daptomycin 8 mg/kg IV every 24 hours as well as meropenem 1 g IV every 12 hours were started. The patient had persistent bacteremia for 4 days. After request from treating team, daptomycin and linezolid susceptibilities were assessed. Sensititre reported a daptomycin MIC of >4 µg/mL; however, E-test revealed daptomycin MIC of 2 µg/mL and a linezolid MIC of 12 µg/mL. On POD 15, piperacillin-tazobactam 3.375 g IV every 8 hours was empirically started for abdominal sepsis concomitantly with daptomycin and meropenem was discontinued. On POD 20, a central line was removed, yet 1 of 2 blood cultures isolated LR-VRE. The antibiotic regimen was again adjusted to daptomycin 10 mg/kg IV every 24 hours, ampicillin 2 g IV every 4 hours, and tigecycline 100 mg IV every 12 hours. Repeat cultures after 48 hours on triple therapy were negative. Due to the prolonged bacteremia, a decision was made to treat for 4 weeks after resolution of bacteremia with this effective combination to avoid potential relapse.
Case 3
A 62-year-old man with end-stage liver disease secondary to nonalcoholic steatohepatitis with cirrhosis and history of multiple episodes of spontaneous bacterial peritonitis was admitted for a combined liver-kidney transplant. Because of the history of chronic spontaneous bacterial peritonitis, the patient received prophylactic therapy with vancomycin 1 g IV every 24 hours and meropenem 1 g IV every 8 hours for 2 weeks after transplantation. His postoperative course was complicated by Candida glabrata abdominal collection and Stenotrophomonas maltophilia bacteremia treated with micafungin and levofloxacin, respectively. All vascular access and central lines were changed. On POD 9, the patient developed VRE bacteremia and a subhepatic collection considered not surgically feasible to drain. Daptomycin 10 mg/kg IV every 24 hours was started. Upon request by the treating team, E-test was completed and the daptomycin MIC was 2 µg/mL with linezolid MIC of 12 µg/mL. On POD 13, 1 of 4 blood cultures isolated VRE. Meropenem was changed to ampicillin-sulbactam 3 g IV every 6 hours in combination with daptomycin 12 mg/kg IV daily. Subsequent blood cultures on POD 15 and 18 isolated no organisms and the patient completed a 4-week course of treatment.
Case 4
A 68-year-old man with end-stage liver disease secondary to hemochromatosis underwent liver transplantation. The postoperative course was complicated by multiple episodes of Klebsiella pneumoniae ventilator-associated pneumonia. Three months after transplantation, the patient became hypotensive with altered mentation required intubation and transfer to the ICU. Empiric treatment for sepsis was started with vancomycin 1 g IV every 24 hours and meropenem 1 g IV every 8 hours. A urine culture from the same day isolated E. faecium. The isolate was resistant to ampicillin and vancomycin, with linezolid MIC of 32 µg/mL and daptomycin MIC of 2 µg/mL by E-test. The patient had no symptoms of a urinary tract infection and the VRE was considered a colonizer and not treated. A perihepatic collection was noted on imaging, and meropenem was continued. Two weeks later, the patient developed fever, and blood cultures isolated VRE. Treatment with daptomycin 10 mg/kg IV every 24 hours was started. All vascular access catheters were exchanged and repeat blood cultures 2 days later were negative. An E-test on the blood isolate showed a daptomycin MIC of 3 µg/mL, and a possible mitral valve vegetation was seen on a transthoracic echocardiogram. Ampicillin 2 g IV every 6 hours was added to daptomycin. The patient was successfully treated for 6 weeks with daptomycin plus ampicillin for endocarditis.
RESULTS
In total, 4 LR-VRE infections occurred between April and May 2017. All 4 screened positive for VRE colonization by rectal swab on admission to the ICU. The average length of stay from admission to positive culture was 60 days (range, 41–76). The first 2 cases were discussed by the treating physicians with the antibiotic stewardship team. Review of the electronic medical record revealed no reported susceptibilities for daptomycin or linezolid of isolates by VITEK 2.
Upon further investigation, the microbiology laboratory determined that this occurred due to a product limitation of the VITEK 2 panel, in which nonsusceptibility for both daptomycin and linezolid is not reported. Review of the package insert specified that the testing system’s ability to detect resistance is unknown for both antibiotics given lack of corresponding MIC breakpoints. Alternative testing such as broth microdilution or E-test are required to determine susceptibility to these agents, and these tests were only performed at our facility upon physician request. Since daptomycin and linezolid susceptibilities were not reported, nor was there verbal notification of resistance, the treating team erroneously assumed the organism was susceptible based on prior facility antibiograms and did not request further testing until they noted treatment failure. Furthermore, as the isolates were not reported as resistant in the electronic medical record, no electronic alert was generated to notify the infection control or antibiotic stewardship teams. Figure 1 demonstrates a summary of the timeline of events.
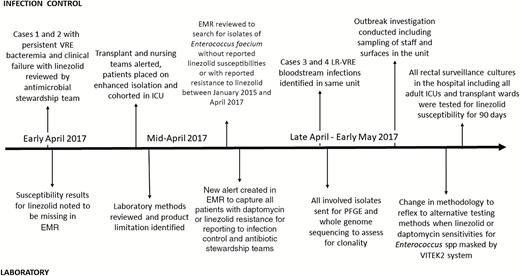
Cluster investigation timeline. Abbreviations: EMR electronic medical record; ICU, intensive care unit; LR-VRE, linezolid- and vancomycin-resistant Enterococcus faecium; PFGE, pulsed-field gel electrophoresis; VRE, vancomycin-resistant enterococci.
Subsequent retrospective review for cases missing linezolid or daptomycin susceptibilities identified 3 additional cases occurring in the same ICU. The PFGE fingerprint analysis demonstrated identical banding patterns, confirming that the isolates from 4 cases were the same strain (Figure 2). One of the cases was isolated from a cardiothoracic surgery patient in the same ICU, but it was found to be unrelated by PFGE analysis. The 4 identical isolates were confirmed to be E. faecium by identification of the ddl gene. These results suggested horizontal transmission, while case 5 was presumed to be sporadic and unrelated to the cluster in the surgical ICU. Hand cultures from ICU staff and environmental surfaces returned negative for VRE and as such the initial source or the index case was not identified.
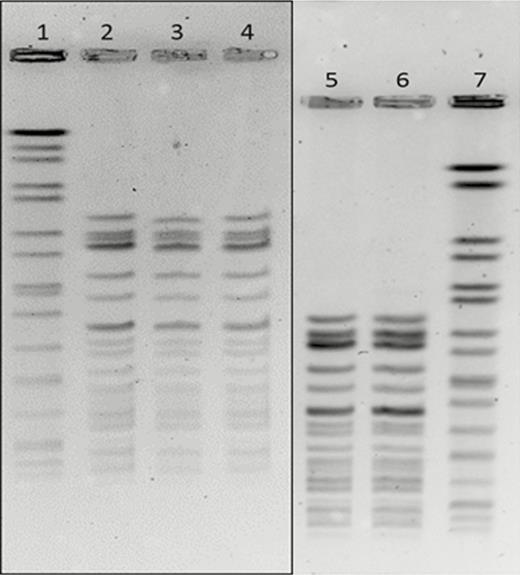
Pulsed-field gel electrophoresis results of involved isolates. Samples tested with corresponding case number: 1, standard; 2, case 2; 3, case 1; 4, case 3; 5, case 3; 6, case 4; 7, standard. The gels were run with different electrophoresis conditions to accommodate the daily workflow in the laboratory on different days; however, by running samples from case 3 along with case 4, we can see that the 2 samples match, which means that all cases have matching fingerprints, suggesting the same strain.
Multilocus sequence typing revealed that all 4 isolates also had the same sequence type, and resistome analysis showed that the isolates harbored the same genes conferring resistance to multiple types of antibiotics (Table 2). The vanA gene cluster was present in all isolates but one, and no liaFSR mutations (previously associated with daptomycin nonsusceptibility) were identified. Consistently with PCR assays, the cfr and optrA genes were not present in any of the genomes. Interestingly, we were not able to detect LR-associated mutations from the assembled genomes; however, using a reads mapping–based approach, we were able to confirm the presence of the mutation G2576C in the 6 copies of the 23S rRNA, indicating that this mechanism of resistance was consistently present in all the strains, but due to the genetic heterogeneity, it was not detectable using the assemblies.
| Case . | MLST . | Resistome . | . | . | . | . |
|---|---|---|---|---|---|---|
| . | . | Glycopeptides . | Aminoglycosides . | Trimethoprim . | MLSb . | Quinolone Mutations . |
| 1 | 117 | vanA | aadE, ant(6′)-Ia, aph(3′)-III | dfrG, dfrK | erm(B) msr(C) | GyrA(S83Y) ParC(S80I) |
| 2 | 117 | Negative | aadE, ant(6′)-Ia, aph(3′)-III | dfrG, dfrK | erm(B) msr(C) | GyrA(S83Y) ParC(S80I) |
| 3 | 117 | vanA | aadE, ant(6′)-Ia, aph(3′)-III | dfrG, dfrK | erm(B) msr(C) | GyrA(S83Y) ParC(S80I) |
| 4 | 117 | vanA | aadE, ant(6′)-Ia, aph(3′)-III | dfrG, dfrK | erm(B) msr(C) | GyrA(S83Y) ParC(S80I) |
| Case . | MLST . | Resistome . | . | . | . | . |
|---|---|---|---|---|---|---|
| . | . | Glycopeptides . | Aminoglycosides . | Trimethoprim . | MLSb . | Quinolone Mutations . |
| 1 | 117 | vanA | aadE, ant(6′)-Ia, aph(3′)-III | dfrG, dfrK | erm(B) msr(C) | GyrA(S83Y) ParC(S80I) |
| 2 | 117 | Negative | aadE, ant(6′)-Ia, aph(3′)-III | dfrG, dfrK | erm(B) msr(C) | GyrA(S83Y) ParC(S80I) |
| 3 | 117 | vanA | aadE, ant(6′)-Ia, aph(3′)-III | dfrG, dfrK | erm(B) msr(C) | GyrA(S83Y) ParC(S80I) |
| 4 | 117 | vanA | aadE, ant(6′)-Ia, aph(3′)-III | dfrG, dfrK | erm(B) msr(C) | GyrA(S83Y) ParC(S80I) |
Abbreviations: MLSb, xxx; MLST, multilocus sequence type.
| Case . | MLST . | Resistome . | . | . | . | . |
|---|---|---|---|---|---|---|
| . | . | Glycopeptides . | Aminoglycosides . | Trimethoprim . | MLSb . | Quinolone Mutations . |
| 1 | 117 | vanA | aadE, ant(6′)-Ia, aph(3′)-III | dfrG, dfrK | erm(B) msr(C) | GyrA(S83Y) ParC(S80I) |
| 2 | 117 | Negative | aadE, ant(6′)-Ia, aph(3′)-III | dfrG, dfrK | erm(B) msr(C) | GyrA(S83Y) ParC(S80I) |
| 3 | 117 | vanA | aadE, ant(6′)-Ia, aph(3′)-III | dfrG, dfrK | erm(B) msr(C) | GyrA(S83Y) ParC(S80I) |
| 4 | 117 | vanA | aadE, ant(6′)-Ia, aph(3′)-III | dfrG, dfrK | erm(B) msr(C) | GyrA(S83Y) ParC(S80I) |
| Case . | MLST . | Resistome . | . | . | . | . |
|---|---|---|---|---|---|---|
| . | . | Glycopeptides . | Aminoglycosides . | Trimethoprim . | MLSb . | Quinolone Mutations . |
| 1 | 117 | vanA | aadE, ant(6′)-Ia, aph(3′)-III | dfrG, dfrK | erm(B) msr(C) | GyrA(S83Y) ParC(S80I) |
| 2 | 117 | Negative | aadE, ant(6′)-Ia, aph(3′)-III | dfrG, dfrK | erm(B) msr(C) | GyrA(S83Y) ParC(S80I) |
| 3 | 117 | vanA | aadE, ant(6′)-Ia, aph(3′)-III | dfrG, dfrK | erm(B) msr(C) | GyrA(S83Y) ParC(S80I) |
| 4 | 117 | vanA | aadE, ant(6′)-Ia, aph(3′)-III | dfrG, dfrK | erm(B) msr(C) | GyrA(S83Y) ParC(S80I) |
Abbreviations: MLSb, xxx; MLST, multilocus sequence type.
DISCUSSION
VRE infections are a significant cause of morbidity and mortality in immunocompromised persons and SOT recipients [28]. Linezolid use in our institution has been restricted since 2003 to limit unnecessary exposure. These restrictions, including preapproval authorization and prospective monitoring, continue to be strictly enforced by the antimicrobial stewardship team.
This report highlights the need for prompt identification of resistant organisms in immunocompromised patients. Indeed, the complexities and testing variability in VRE isolates with product limitations in laboratory methods and CLSI breakpoints could delay timely appropriate therapy in critically ill patients. As a result of this cluster, our microbiology laboratory now reflexes to E-test for enterococcal isolates with no reported susceptibilities for linezolid.
Our series also highlights the benefits of using genomic approaches to identify clusters. Indeed, the use of deep sequencing data was crucial to unveil the mechanism of linezolid resistance in these strains. While prior studies have utilized WGS to identify clones and resistance elements on plasmids [29, 30], to our knowledge, this is a unique outbreak caused by strains carrying the G2576C mutation affecting all 23S rRNA copies but only present in a proportion of the bacterial population (heteroresistance). Using bioinformatics approaches, we were able to detect a mechanism of resistance that was not evident even when genome assemblies were available. The malleability of enterococcal genomes warrants careful analyses of the assemblies, and the sequencing of >1 colony recovered from the clinical sample may be needed.
In addition to hand culturing, upon identification of LR-VRE, all cases were placed in enhanced contact precautions, with lower nurse-to-patient ratio and cohorting of patients and staff to prevent further horizontal transmission. Additional strategies included strict compliance with donning and doffing of personal protective equipment and hand hygiene, dedication of patient care equipment when possible, close attention to device reprocessing for items that were shared, enhanced environmental disinfection processes, decluttering of the units, deep terminal cleaning with bleach, and ultraviolet pulse radiation of common areas.
At the time of the cluster, the ICU had recently implemented a “bare below the elbows” strategy. This practice restricts the use of white coats upon entrance to the unit and upon entering any patient room. The enhanced measures in contact precautions and hand hygiene coaching and monitoring with feedback were enforced for all healthcare providers, patients, and visitors entering the unit. Due to the case cluster, all VRE isolates from surveillance cultures were tested for linezolid susceptibility prospectively and no resistant isolates were identified up to 6 months thereafter. Based on the results of the epidemiological investigation and the genetic identity of the isolates, horizontal transmission was determined to be the mostly likely cause of the outbreak. Due to the early detection of the cases and the implementation of multiple preventive strategies, we were able to prevent transmission of and appropriately treat LR-VRE in our facility.
Multidrug-resistant organisms continue to be a real global public health threat and affect vulnerable recipients. Treatment may involve the use of unusual combinations and agents that are not approved for the specific indications. In our cases, combination therapy of daptomycin plus ampicillin (adding tigecycline in case of abdominal focus) was the most successful combination without recurrences in our patients. The mechanistic rationale for this combination is well documented [31–33] and has been shown to be effective in isolated cases [34]. Thus, collaboration with the laboratory in accordance with appropriate diagnostic methods including genome sequencing can lead to the appropriate identification of clusters and outbreaks. In turn, early identification leads to more rapid implementation of effective infection prevention and antibiotic stewardship strategies.
Notes
Acknowledgments. We thank the staff at the Florida Department of Health for initial PFGE analysis.
Disclaimer. The finding and conclusions in this report are solely the responsibility of the authors and do not necessarily represent the official views of the Association of Public Health Laboratories (APHL), the Centers for Disease Control and Prevention (CDC), or the US Department of Health and Human Services.
Financial support. C. A. A. is supported by a grant from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant number K24 AI121296).
Potential conflicts of interest. C. A. A. has received funding from UpToDate, Merck, and MeMed Diagnostics. L. Ab. has received personal fees from Achaogen, Pfizer Latin America, Merck Latin America, and Roche Diagnostics. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




