-
PDF
- Split View
-
Views
-
Cite
Cite
Cléa Melenotte, Loïc Epelboin, Matthieu Million, Sandrine Hubert, Thierry Monsec, Félix Djossou, Jean-Louis Mège, Gilbert Habib, Didier Raoult, Acute Q Fever Endocarditis: A Paradigm Shift Following the Systematic Use of Transthoracic Echocardiography During Acute Q Fever, Clinical Infectious Diseases, Volume 69, Issue 11, 1 December 2019, Pages 1987–1995, https://doi.org/10.1093/cid/ciz120
Close - Share Icon Share
Abstract
As Q fever, caused by Coxiella burnetii, is a major health challenge due to its cardiovascular complications, we aimed to detect acute Q fever valvular injury to improve therapeutic management.
In the French national reference center for Q fever, we prospectively collected data from patients with acute Q fever and valvular injury. We identified a new clinical entity, acute Q fever endocarditis, defined as valvular lesion potentially caused by C. burnetii: vegetation, valvular nodular thickening, rupture of chorda tendinae, and valve or chorda tendinae thickness. To determine whether or not the disease was superimposed on an underlying valvulopathy, patients’ physicians were contacted. Aortic bicuspidy, valvular stenosis, and insufficiency were considered as underlying valvulopathies.
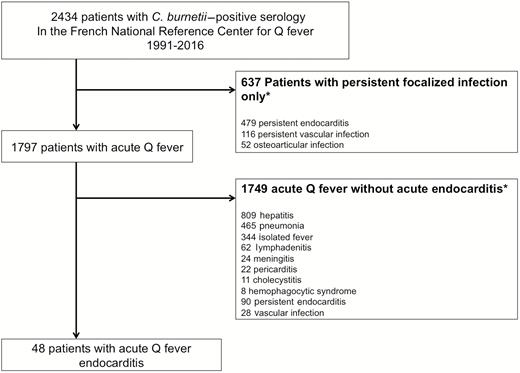
Of the 2434 patients treated in our center, 1797 had acute Q fever and 48 had acute Q fever endocarditis. In 35 cases (72%), transthoracic echocardiography (TTE) identified a valvular lesion of acute Q fever endocarditis without underlying valvulopathy. Positive anticardiolipin antibodies (>22 immunoglobulin G-type phospholipid units [GPLU]) were independently associated with acute Q fever endocarditis (odds ratio [OR], 2.7 [95% confidence interval {CI}, 1.3–5.5]; P = .004). Acute Q fever endocarditis (OR, 5.2 [95% CI, 2.6–10.5]; P < .001) and age (OR, 1.7 [95% CI, 1.1–1.9]; P = .02) were independent predictors of progression toward persistent C. burnetii endocarditis.
Systematic TTE in acute Q fever patients offers a unique opportunity for early diagnosis of acute Q fever endocarditis and for the prevention of persistent endocarditis. Transesophageal echocardiography should be proposed in men, aged >40 years, with anticardiolipin antibodies >60 GPLU when TTE is inconclusive or negative.
Coxiella burnetii endocarditis concerns <5% of patients with Q fever and, for the past 50 years, has been considered to be a chronic and fatal disease without treatment [1]. The growing interest in Q fever endocarditis and the ever-earlier use of transthoracic echocardiography (TTE) has gradually transformed the disease from a chronic fatal illness to a curable and preventable disease. Q fever endocarditis was first considered to be a slowly evolving disease, affecting men with underlying valvulopathy and characterized by marked clinical and biological manifestation [2–4]. Increased interest in Q fever endocarditis led to underlying valvulopathy being identified as a risk factor for the disease [4, 5]. TTE then became a crucial tool in the management and follow-up of the primary infection to identify any underlying valvulopathy. A preventive strategy with antibiotic prophylaxis is consequently initiated in these patients that drastically reduces the morbidity and mortality induced by C. burnetii infection [5–7]. Performed at an increasingly early stage, TTE has identified transient valve lesions associated with high levels of antiphospholipid antibodies in C. burnetii primary infections [8, 9]. Given this historical data, TTE is an indispensable tool and plays an increasingly important role in the management of acute Q fever.
Nevertheless, the systematic use of TTE remains controversial. In the Netherlands, where a dramatic Q fever outbreak was responsible for 4000 cases in 4 years, TTE has been abandoned during the acute phase of the disease [10, 11]. Four hundred forty-nine patients with a “chronic” C. burnetii infection have been reported to date, with a mortality rate reaching 27% [12]. Despite this, echocardiography is no longer recommended in case of acute Q fever, insofar de Lange et al showed that valvulopathy was not significantly associated with “chronic” Q fever and was considered too costly and unnecessary [11, 13]. Nonetheless, in a recent Australian study, 92% of patients with C. burnetii endocarditis had underlying valvulopathy [14].
To clarify the interest of TTE during the acute phase of the disease, we searched the Q fever database from the French National Reference Center (NRC). We identified 48 cases of acute Q fever with a new valvular injury. We have defined acute Q fever endocarditis as a new clinical entity and propose new diagnostic criteria and therapeutic strategy to facilitate clinical practice.
MATERIALS AND METHODS
Q Fever Clinical Data
Since 1991, the French NRC for Q fever received >200 000 serum samples tested for C. burnetii from various countries around the world. Sex, age, past medical history, underlying valvulopathy, and echocardiographic data were prospectively collected by phone from 1991 to 2016 and stored in a computerized database. Patients gave their oral consent and the local ethics committee (Comité d’éthique Institut Hospitalo-Universitaire Méditerranée infection) approved the study under registration number 12–016.
Q Fever Definition
Acute Q fever was defined by the association of clinical symptoms (fever and/or hepatitis and/or pneumonia) with serologic criteria for phase II immunoglobulin G (IgG) levels ≥200 and phase II immunoglobulin M (IgM) levels ≥50 with seroconversion or positive polymerase chain reaction (PCR) (IS1111 and IS30a), as previously described during the 3 months after the onset of symptoms [15]. When the C. burnetii–specific PCR was positive, blood was cultured on L929 cells to isolate living bacteria. Coxiella burnetii persistent focalized infection diagnostic criteria were recently published for endocarditis, vascular infection, and osteoarticular infections [1]. This includes evidence of the presence of the bacteria (positive microbiological test) in addition to persistence of clinical symptoms for >3 months with the identification of an infectious focus [16].
Transthoracic Findings
Transthoracic echocardiography was systematically advised as soon as possible within the 3 months following the diagnosis of Q fever, and transesophageal echocardiography (TEE) was performed at the discretion of the physician [9, 16, 17]. A centralized reviewing of the pathological TTE imaging was performed. Transthoracic and transesophageal echocardiographic studies were performed using state-of-the-art echocardiographic ultrasound systems (GE Vivid E9, Vingmed Ultrasound, Horten, Norway; and/or iE33, Philips Medical Systems, Andover, Massachusetts), with a standardized methodology, as previously described [18–20]. Echocardiographic data included the presence and maximal length of vegetations.
Acute Q Fever Endocarditis Definition
We define acute Q fever endocarditis as the presence of a new and acquired abnormal valvular image lesion within 3 months of a primary C. burnetii infection (Table 1). Acute Q fever endocarditis is confirmed when a valvular lesion, such as vegetation, a valvular nodular thickening (irregular round nodule >3 mm), or valvular chorda tendinea rupture is present in addition to one of the microbiological criteria (positive serology, PCR, or culture). It is considered possible when the valve is thickened (diastolic thickening >5 mm), or when the chorda tendinea is thickened in addition to one of the microbiological criteria (Table 1). According to the recommended treatment previously described, patients with acute Q fever endocarditis were treated with doxycycline and hydroxychloroquine for an 18-month period, as with patients with persistent C. burnetii endocarditis [1].
| Diagnostic Criteria for Acute Coxiella burnetii Endocarditis . |
|---|
| Major criteria within 3 mo of the onset of symptoms |
| • Microbiological criteria |
| IgG levels ≥200 and IgM levels ≥50 for phase II |
| OR |
| Positive PCR on blood sample |
| OR/AND |
| Positive culture on blood sample |
| • Echocardiographic criteria (TTE or TEE) |
| Valvular vegetation or valvular nodular thickening |
| Chorda tendinea rupture |
| Minor criteria echocardiographic criteria (TTE or TEE) within 3 mo of the onset of symptoms |
| • Valve thickening |
| • Chorda tendinea thickening |
| C. burnetii acute endocarditis is definite |
| Two major criteria are fulfilled: 1 microbiological and 1 echocardiography criterion |
| C. burnetii acute endocarditis is possible |
| One major microbiological criterion and 1 minor criterion based on echocardiographic results |
| Diagnostic Criteria for Acute Coxiella burnetii Endocarditis . |
|---|
| Major criteria within 3 mo of the onset of symptoms |
| • Microbiological criteria |
| IgG levels ≥200 and IgM levels ≥50 for phase II |
| OR |
| Positive PCR on blood sample |
| OR/AND |
| Positive culture on blood sample |
| • Echocardiographic criteria (TTE or TEE) |
| Valvular vegetation or valvular nodular thickening |
| Chorda tendinea rupture |
| Minor criteria echocardiographic criteria (TTE or TEE) within 3 mo of the onset of symptoms |
| • Valve thickening |
| • Chorda tendinea thickening |
| C. burnetii acute endocarditis is definite |
| Two major criteria are fulfilled: 1 microbiological and 1 echocardiography criterion |
| C. burnetii acute endocarditis is possible |
| One major microbiological criterion and 1 minor criterion based on echocardiographic results |
Abbreviations: IgG, immunoglobulin G; IgM, immunoglobulin M; PCR, polymerase chain reaction; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
| Diagnostic Criteria for Acute Coxiella burnetii Endocarditis . |
|---|
| Major criteria within 3 mo of the onset of symptoms |
| • Microbiological criteria |
| IgG levels ≥200 and IgM levels ≥50 for phase II |
| OR |
| Positive PCR on blood sample |
| OR/AND |
| Positive culture on blood sample |
| • Echocardiographic criteria (TTE or TEE) |
| Valvular vegetation or valvular nodular thickening |
| Chorda tendinea rupture |
| Minor criteria echocardiographic criteria (TTE or TEE) within 3 mo of the onset of symptoms |
| • Valve thickening |
| • Chorda tendinea thickening |
| C. burnetii acute endocarditis is definite |
| Two major criteria are fulfilled: 1 microbiological and 1 echocardiography criterion |
| C. burnetii acute endocarditis is possible |
| One major microbiological criterion and 1 minor criterion based on echocardiographic results |
| Diagnostic Criteria for Acute Coxiella burnetii Endocarditis . |
|---|
| Major criteria within 3 mo of the onset of symptoms |
| • Microbiological criteria |
| IgG levels ≥200 and IgM levels ≥50 for phase II |
| OR |
| Positive PCR on blood sample |
| OR/AND |
| Positive culture on blood sample |
| • Echocardiographic criteria (TTE or TEE) |
| Valvular vegetation or valvular nodular thickening |
| Chorda tendinea rupture |
| Minor criteria echocardiographic criteria (TTE or TEE) within 3 mo of the onset of symptoms |
| • Valve thickening |
| • Chorda tendinea thickening |
| C. burnetii acute endocarditis is definite |
| Two major criteria are fulfilled: 1 microbiological and 1 echocardiography criterion |
| C. burnetii acute endocarditis is possible |
| One major microbiological criterion and 1 minor criterion based on echocardiographic results |
Abbreviations: IgG, immunoglobulin G; IgM, immunoglobulin M; PCR, polymerase chain reaction; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
Underlying Valvulopathy Definition
Underlying valvulopathy was defined as the presence of a non-infection-related valve abnormality, such as aortic bicuspidy, mitral insufficiency, aortic stenosis, rheumatic valve, and prosthetic valve [20].
The underlying valvulopathy is considered to be “known” when its presence was identified before the diagnosis of Q fever and “unknown” when it was identified at the time of Q fever diagnosis. Patients with known underlying valvulopathy were not included if no additional valvular lesion (vegetation, valvular nodular thickening, valvular chorda tendinea rupture, valve thickness, and chorda tendinea thickness) was identified in this way. These patients were considered as having an acute Q fever with an underlying valvulopathy, which is a risk for developing a persistent infection, but were not considered as having acute Q fever endocarditis in this study.
To identify whether an underlying valvulopathy was “known” or “unknown,” we contacted the referring hospital, the patient’s cardiologist, or the patient’s family doctor to ask whether a TTE had been performed previously. Patients for whom we were unable to contact the corresponding physicians regarding their valvular history were excluded from the analysis.
Anticardiolipin Antibody Test
When C. burnetii serology was positive, testing for G-isotype anticardiolipin antibodies (IgG aCLs) was performed as previously described on first positive serum samples [8]. IgG aCLs were defined as positive when >22 immunoglobulin G-type phospholipid units (GPLU).
Statistical Analysis
To compare the distribution of continuous and dichotomous variables between 2 groups, we used the χ2 or the 2-sided Fisher exact test, respectively. All tests were 2-sided and P < .05 was considered significant. Indicative factors for acute Q fever endocarditis were determined using the logistic regression model. Multivariate models were adjusted for sex and age at baseline. Variables were calculated using SPSS version 22 Statistical Software.
RESULTS
Clinical Manifestations
Of the 2434 patients with positive C. burnetii serology consistent with Q fever, 1797 had acute Q fever, 1302 had TTE in the acute phase of the disease, and among them, 351 had acute Q fever with an underlying valvulopathy. Forty-eight patients were diagnosed with acute Q fever endocarditis (Figure 1). All of them had a first TTE, which identified valvular injury in 37 cases, whereas the TEE performed in 23 cases diagnosed 11 patients with valvular additional lesions not identified with TTE. Of these 11 patients, 10 were male and all were >40 years of age. Anticardiolipin antibodies were available for 10 of these 11 patients, and were positive and higher than 60 GPLU in all cases. Among the 48 patients included, 37 patients were men (77%) and the mean age was 56.8 years (standard deviation, 14 years). Half of the patients presented with hepatitis (n = 24), 13 had pneumonia, 3 had lymphadenitis, and 2 had an acute thrombotic event (1 pulmonary embolism, 1 renal arterial thrombosis) (Table 2). Eight patients had positive C. burnetii–specific PCR on serum sample and 1 patient had a positive blood culture for C. burnetii (Figure 2). The genome was sequenced (CSUR UR CB M AC/ACH 207).
| Characteristic . | No. . | (%) . |
|---|---|---|
| Age, mean (SD) | 56.8±14 | |
| Sex, male/female | 37/11 | (77/23) |
| Diabetes mellitus | 6 | (12) |
| Valvular predisposition | ||
| No underlying valvulopathy | 35 | (72) |
| Underlying valvulopathy | 13 | (27) |
| Known underlying valvulopathy | 9 | (18) |
| Aortic bicuspidy | 1 | (2) |
| Mitral insufficiency | 6 | (12) |
| Rheumatic valvulopathy | 1 | (2) |
| Tricuspid insufficiency | 1 | (2) |
| Unknown underlying valvulopathy | 4 | (8) |
| Aortic bicuspidy | 1 | (2) |
| Aortic stenosis | 1 | (2) |
| Mitral insufficiency | 2 | (4) |
| Definite | 17 | (35) |
| Valvular vegetation | 14 | (29) |
| Valvular nodular thickening | 2 | (4) |
| Chorda tendinea rupture | 1 | (2) |
| Possible | 31 | (64) |
| Thickness | 30 | (62) |
| Chorda tendinea thickness | 1 | (2) |
| Valve involved | ||
| Mitral | 25 | (52) |
| Aortic | 22 | (46) |
| Tricuspid | 1 | (2) |
| Pulmonic | 0 | (0) |
| Positive serology, IgG phase I | ||
| First serology, median (IQR) | 200 | (50–800) |
| Highest serology, median (IQR) | 800 | (400–1600) |
| <400 | 10 | (20) |
| 400–800 | 18 | (37) |
| >800 | 20 | (41, 6) |
| Positive serology, phase II, median (IQR) | ||
| First serology IgM | 200 | (100–800) |
| First serology IgG | 400 | (200–1600) |
| Anticardiolipin antibodies | ||
| Available data | 41 | (85) |
| Positive (>22 GPLU) | 28 | (68) |
| High level (>90 GPLU) | 22 | (53) |
| PCR (IS1111 IS30A) positive | 8 | (19) |
| Positive culture | 1 | (2) |
| Treatment | ||
| Doxycycline and hydroxychloroquine | 36 | (75) |
| Time of follow-up | ||
| >12 mo | 25 | (52) |
| <12 mo | 23 | (48) |
| Characteristic . | No. . | (%) . |
|---|---|---|
| Age, mean (SD) | 56.8±14 | |
| Sex, male/female | 37/11 | (77/23) |
| Diabetes mellitus | 6 | (12) |
| Valvular predisposition | ||
| No underlying valvulopathy | 35 | (72) |
| Underlying valvulopathy | 13 | (27) |
| Known underlying valvulopathy | 9 | (18) |
| Aortic bicuspidy | 1 | (2) |
| Mitral insufficiency | 6 | (12) |
| Rheumatic valvulopathy | 1 | (2) |
| Tricuspid insufficiency | 1 | (2) |
| Unknown underlying valvulopathy | 4 | (8) |
| Aortic bicuspidy | 1 | (2) |
| Aortic stenosis | 1 | (2) |
| Mitral insufficiency | 2 | (4) |
| Definite | 17 | (35) |
| Valvular vegetation | 14 | (29) |
| Valvular nodular thickening | 2 | (4) |
| Chorda tendinea rupture | 1 | (2) |
| Possible | 31 | (64) |
| Thickness | 30 | (62) |
| Chorda tendinea thickness | 1 | (2) |
| Valve involved | ||
| Mitral | 25 | (52) |
| Aortic | 22 | (46) |
| Tricuspid | 1 | (2) |
| Pulmonic | 0 | (0) |
| Positive serology, IgG phase I | ||
| First serology, median (IQR) | 200 | (50–800) |
| Highest serology, median (IQR) | 800 | (400–1600) |
| <400 | 10 | (20) |
| 400–800 | 18 | (37) |
| >800 | 20 | (41, 6) |
| Positive serology, phase II, median (IQR) | ||
| First serology IgM | 200 | (100–800) |
| First serology IgG | 400 | (200–1600) |
| Anticardiolipin antibodies | ||
| Available data | 41 | (85) |
| Positive (>22 GPLU) | 28 | (68) |
| High level (>90 GPLU) | 22 | (53) |
| PCR (IS1111 IS30A) positive | 8 | (19) |
| Positive culture | 1 | (2) |
| Treatment | ||
| Doxycycline and hydroxychloroquine | 36 | (75) |
| Time of follow-up | ||
| >12 mo | 25 | (52) |
| <12 mo | 23 | (48) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: GPLU, immunoglobulin G-type phospholipid units; IgG, immunoglobulin G; IgM, immunoglobulin M; IQR, interquartile range; PCR, polymerase chain reaction; SD, standard deviation.
| Characteristic . | No. . | (%) . |
|---|---|---|
| Age, mean (SD) | 56.8±14 | |
| Sex, male/female | 37/11 | (77/23) |
| Diabetes mellitus | 6 | (12) |
| Valvular predisposition | ||
| No underlying valvulopathy | 35 | (72) |
| Underlying valvulopathy | 13 | (27) |
| Known underlying valvulopathy | 9 | (18) |
| Aortic bicuspidy | 1 | (2) |
| Mitral insufficiency | 6 | (12) |
| Rheumatic valvulopathy | 1 | (2) |
| Tricuspid insufficiency | 1 | (2) |
| Unknown underlying valvulopathy | 4 | (8) |
| Aortic bicuspidy | 1 | (2) |
| Aortic stenosis | 1 | (2) |
| Mitral insufficiency | 2 | (4) |
| Definite | 17 | (35) |
| Valvular vegetation | 14 | (29) |
| Valvular nodular thickening | 2 | (4) |
| Chorda tendinea rupture | 1 | (2) |
| Possible | 31 | (64) |
| Thickness | 30 | (62) |
| Chorda tendinea thickness | 1 | (2) |
| Valve involved | ||
| Mitral | 25 | (52) |
| Aortic | 22 | (46) |
| Tricuspid | 1 | (2) |
| Pulmonic | 0 | (0) |
| Positive serology, IgG phase I | ||
| First serology, median (IQR) | 200 | (50–800) |
| Highest serology, median (IQR) | 800 | (400–1600) |
| <400 | 10 | (20) |
| 400–800 | 18 | (37) |
| >800 | 20 | (41, 6) |
| Positive serology, phase II, median (IQR) | ||
| First serology IgM | 200 | (100–800) |
| First serology IgG | 400 | (200–1600) |
| Anticardiolipin antibodies | ||
| Available data | 41 | (85) |
| Positive (>22 GPLU) | 28 | (68) |
| High level (>90 GPLU) | 22 | (53) |
| PCR (IS1111 IS30A) positive | 8 | (19) |
| Positive culture | 1 | (2) |
| Treatment | ||
| Doxycycline and hydroxychloroquine | 36 | (75) |
| Time of follow-up | ||
| >12 mo | 25 | (52) |
| <12 mo | 23 | (48) |
| Characteristic . | No. . | (%) . |
|---|---|---|
| Age, mean (SD) | 56.8±14 | |
| Sex, male/female | 37/11 | (77/23) |
| Diabetes mellitus | 6 | (12) |
| Valvular predisposition | ||
| No underlying valvulopathy | 35 | (72) |
| Underlying valvulopathy | 13 | (27) |
| Known underlying valvulopathy | 9 | (18) |
| Aortic bicuspidy | 1 | (2) |
| Mitral insufficiency | 6 | (12) |
| Rheumatic valvulopathy | 1 | (2) |
| Tricuspid insufficiency | 1 | (2) |
| Unknown underlying valvulopathy | 4 | (8) |
| Aortic bicuspidy | 1 | (2) |
| Aortic stenosis | 1 | (2) |
| Mitral insufficiency | 2 | (4) |
| Definite | 17 | (35) |
| Valvular vegetation | 14 | (29) |
| Valvular nodular thickening | 2 | (4) |
| Chorda tendinea rupture | 1 | (2) |
| Possible | 31 | (64) |
| Thickness | 30 | (62) |
| Chorda tendinea thickness | 1 | (2) |
| Valve involved | ||
| Mitral | 25 | (52) |
| Aortic | 22 | (46) |
| Tricuspid | 1 | (2) |
| Pulmonic | 0 | (0) |
| Positive serology, IgG phase I | ||
| First serology, median (IQR) | 200 | (50–800) |
| Highest serology, median (IQR) | 800 | (400–1600) |
| <400 | 10 | (20) |
| 400–800 | 18 | (37) |
| >800 | 20 | (41, 6) |
| Positive serology, phase II, median (IQR) | ||
| First serology IgM | 200 | (100–800) |
| First serology IgG | 400 | (200–1600) |
| Anticardiolipin antibodies | ||
| Available data | 41 | (85) |
| Positive (>22 GPLU) | 28 | (68) |
| High level (>90 GPLU) | 22 | (53) |
| PCR (IS1111 IS30A) positive | 8 | (19) |
| Positive culture | 1 | (2) |
| Treatment | ||
| Doxycycline and hydroxychloroquine | 36 | (75) |
| Time of follow-up | ||
| >12 mo | 25 | (52) |
| <12 mo | 23 | (48) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: GPLU, immunoglobulin G-type phospholipid units; IgG, immunoglobulin G; IgM, immunoglobulin M; IQR, interquartile range; PCR, polymerase chain reaction; SD, standard deviation.
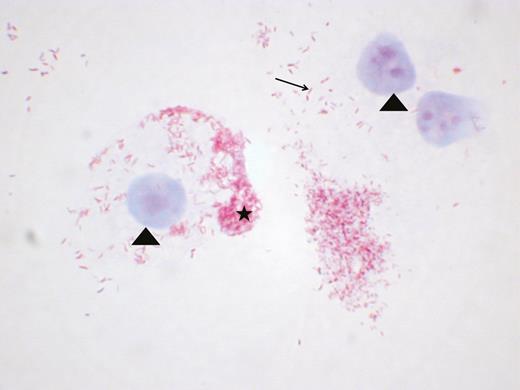
Positive culture of Coxiella burnetii in acute Q fever endocarditis from a blood sample. Coxiella burnetii is a gram-negative fastidious intracellular bacterium that needs cells to multiply; the blood was therefore seeded directly onto L929 cells in a Biosafety Level 3 laboratory and culture was started 3 weeks later. L929 cells and nucleus are colored with the malachite green colorant (arrowhead). Coxiella burnetii bacteria are pink-colored by the fuchsine colorant. Coxiella burnetii is identified as a unique bacterium (arrow) and is concentrated in intracytoplasmic vacuoles (star). Gimenez coloration, magnification ×100.
According to the diagnostic criteria presented here, 17 (34%) patients had definite acute endocarditis and 31 (64%) had possible acute endocarditis (Table 2). Follow-up lasted a mean of 16 months (standard deviation, 19 months).
Of the 48 patients included, 35 (70%) had no underlying valvulopathy at the time of Q fever diagnosis, 5 had normal TTE before Q fever diagnosis, and 30 had no underlying valvulopathy on the date of diagnosis of acute Q fever. Fourteen patients had a history of TTE exploration prior to Q fever diagnosis. Among them, 9 had an underlying valvulopathy diagnosed before the diagnosis of Q fever (known underlying valvulopathy), whereas 4 were discovered at the time of acute Q fever endocarditis (unknown underlying valvulopathy): 1 aortic bicuspidy, 1 mitral insufficiency, 1 aortic stenosis, and 1 mitral insufficiency with aortic stenosis. Finally, 13 patients (26%) presented acute Q fever on an underlying valvulopathy: 2 aortic bicuspidy, 8 mitral insufficiency (1 of them had also an aortic stenosis), 1 aortic stenosis, 1 rheumatic aortic valvulopathy, and 1 tricuspid insufficiency (Table 2). Figure 3 illustrates acute Q fever endocarditis on TTE and confirmed by TEE in a patient without underlying valvulopathy. Figure 4 illustrates acute Q fever endocarditis on an underlying bicuspid aortic valvulopathy.
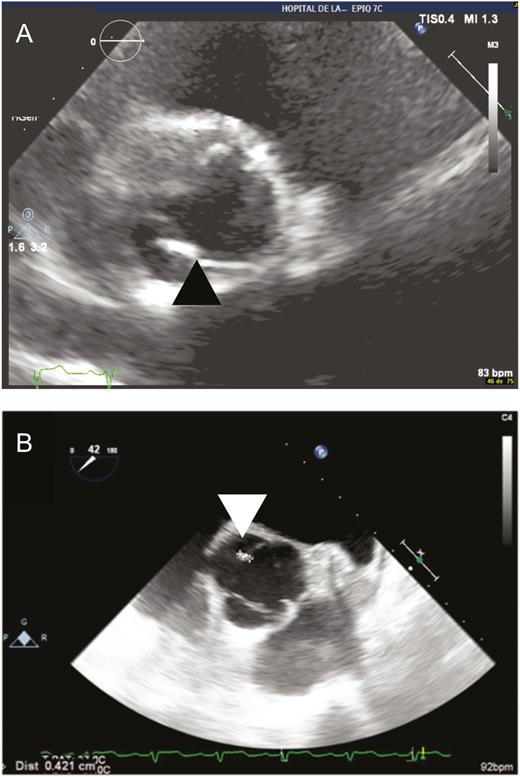
Acute Q fever endocarditis. A, Parasternal transthoracic echocardiography zooms in on the aortic valve, showing a tricuspid aortic valve with the presence of a hyperechogenic valvular nodular thickening lesion on the noncoronary sigmoid valve (black arrowhead). B, Transesophageal echocardiography (45°) zooms in on the aortic valve, confirming the presence of a 4-mm nodular lesion on the noncoronary sigmoid (white arrowhead).

Acute Q fever endocarditis on aortic bicuspidy. A, Transthoracic echocardiography in 5 cavities; a nodular lesion is present on the aortic valve (black arrowhead). B, Transesophageal echocardiography (TEE) 0°: a nodular hyperechogenic additional slightly vibratile lesion measuring 6 mm was observed on the cusp (white arrowhead). C, TEE 56°: aortic bicuspidy type 1 (double arrow): a nodular hyperechogenic additional vibratile lesion measuring 6 mm was observed on the cusp (white arrowhead).
For 13 patients (26%), the disease developed into a persistent C. burnetii infection. Of the 12 patients who developed persistent Q fever endocarditis, 3 had underlying valvulopathy (1 aortic bicuspidy, 1 tricuspid insufficiency, and 1 rheumatic valvulopathy); additional lesions were vegetation in 1 case, and mitral thickness and aortic thickness in the other cases others. One patient developed a persistent C. burnetii vascular infection.
One patient with persistent C. burnetii endocarditis died. He was a 54-year-old man, with diabetes and ischemic heart disease. He presented with definite acute Q fever endocarditis with a valvular vegetation on the aortic valve, whereas Q fever serology showed low serological titers (phase I: IgG 200, IgM 100, immunoglobulin A [IgA] 0; phase II: IgG 400, IgM 200, IgA 0). The patient was immediately treated with doxycycline and hydroxyplaquenil. He died of a mesenteric infarction 1 year later, which could hypothetically be linked to the infection.
C. burnetii Transthoracic Echocardiography Results
In 17 patients with confirmed endocarditis, 14 had valvular vegetation, 2 had valvular nodular thickening, and 1 patient had a tricuspid chorda tendinea rupture. In 9 cases, this involved the aortic valve, in 7 cases the mitral valve, and the tricuspid valve in 1 case. One patient presented a rupture of the mitral chorda tendinea. Five of these patients with confirmed acute Q fever endocarditis presented an underlying valvulopathy. Six (41%) patients with definite acute Q fever endocarditis went on to develop persistent C. burnetii infection (5 endocarditis and 1 vascular infection).
Of the 31 patients with possible acute Q fever endocarditis, 30 presented with a thickened valve and 1 patient with a thickening of the chorda tendinea. Eight of them had an underlying valvulopathy and 7 went on to develop persistent endocarditis (Table 2).
C. burnetii Diagnosis and Immune Response
At the time of diagnosis, the median phase I IgG titers were low (200 [interquartile range {IQR}, 50–800]) and the highest-phase I IgG (800 [IQR, 400–1600]) was reached a mean of 1.5 months (standard deviation, 2.5 months) after the first positive serology (Table 2). IgG anticardiolipin antibodies were tested and available for 40 patients. Among them, 28 patients presented positive aCLs (70%), whereas 22 patients (55%) presented elevated (>90 GPLU) positive aCLs (Table 2).
Factor Associated With Acute Q Fever Endocarditis
By comparing the group of patients with acute Q fever without endocarditis to the group of patients with acute Q fever endocarditis, we identified that positive (>22 GPLU) and high levels (>90 GPLU) of aCLs and thrombosis were significantly more frequent in the group of patients with acute endocarditis (P < .05) (Table 3).
Patients With Acute Q Fever Endocarditis and Progression to Persistent Q Fever Endocarditis
| Acute Q Fever Manifestation . | Acute Q Fever Without Endocarditis (n = 1749) . | Acute Endocarditis (n = 48) . | P Value . |
|---|---|---|---|
| Sex, male | 1183 (67) | 37 (77) | .16a |
| Age, y, mean (SD) | 51.8 (17.5) | 56.8 (14) | .098b |
| Severe immunosuppression | 62 (3.5) | 2 (4) | .8a |
| Fever only | 344 (19.7) | 4 (8) | .039c |
| Pneumonia | 465 (26.4) | 13 (27) | .93c |
| Hepatitis | 810 (46) | 24 (50) | .61c |
| Meningitis | 24 (1.36) | 1 (2) | .50a |
| Cholecystitis | 11 (0.62) | 0 (0) | .58a |
| Pericarditis | 22 (1.25) | 1 (2) | .61a |
| Lymphadenitis | 62 (3.53) | 3 (6) | .32a |
| Hemophagocytic syndrome | 8 (0.45) | 1 (2) | .11a |
| Thrombosis | 14 (0.74) | 2 (4) | .03a |
| Persistent focalized Coxiella burnetii infection | 117 (6.66) | 13 (27) | < .001c |
| Persistent endocarditis | 91 (5.2) | 12 (25) | < .001c |
| Persistent vascular infection | 28 (1.6) | 1 (2) | .56a |
| Osteoarticular infection | 5 (0.28) | 0 (0) | .99a |
| C. burnetii first phase I antibodies, IgG >800 | 233 (13.3) | 8 (16) | .5c |
| Positive aCLs (>22 GPLU) | 436 (24.9) | 28 (58) | .002c |
| Positive aCLs (>75 GPLU) | 254 (14.5) | 23 (47) | < .001c |
| Positive aCLs (>90 GPLU) | 216 (12.3) | 22 (46) | < .001c |
| Acute Q Fever Manifestation . | Acute Q Fever Without Endocarditis (n = 1749) . | Acute Endocarditis (n = 48) . | P Value . |
|---|---|---|---|
| Sex, male | 1183 (67) | 37 (77) | .16a |
| Age, y, mean (SD) | 51.8 (17.5) | 56.8 (14) | .098b |
| Severe immunosuppression | 62 (3.5) | 2 (4) | .8a |
| Fever only | 344 (19.7) | 4 (8) | .039c |
| Pneumonia | 465 (26.4) | 13 (27) | .93c |
| Hepatitis | 810 (46) | 24 (50) | .61c |
| Meningitis | 24 (1.36) | 1 (2) | .50a |
| Cholecystitis | 11 (0.62) | 0 (0) | .58a |
| Pericarditis | 22 (1.25) | 1 (2) | .61a |
| Lymphadenitis | 62 (3.53) | 3 (6) | .32a |
| Hemophagocytic syndrome | 8 (0.45) | 1 (2) | .11a |
| Thrombosis | 14 (0.74) | 2 (4) | .03a |
| Persistent focalized Coxiella burnetii infection | 117 (6.66) | 13 (27) | < .001c |
| Persistent endocarditis | 91 (5.2) | 12 (25) | < .001c |
| Persistent vascular infection | 28 (1.6) | 1 (2) | .56a |
| Osteoarticular infection | 5 (0.28) | 0 (0) | .99a |
| C. burnetii first phase I antibodies, IgG >800 | 233 (13.3) | 8 (16) | .5c |
| Positive aCLs (>22 GPLU) | 436 (24.9) | 28 (58) | .002c |
| Positive aCLs (>75 GPLU) | 254 (14.5) | 23 (47) | < .001c |
| Positive aCLs (>90 GPLU) | 216 (12.3) | 22 (46) | < .001c |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: aCL, anticardiolipin antibodies; GPLU, immunoglobulin G-type phospholipid units; SD, standard deviation.
aFisher exact test (mid P test).
bMann-Whitney test.
cχ2 test for 2 groups.
Patients With Acute Q Fever Endocarditis and Progression to Persistent Q Fever Endocarditis
| Acute Q Fever Manifestation . | Acute Q Fever Without Endocarditis (n = 1749) . | Acute Endocarditis (n = 48) . | P Value . |
|---|---|---|---|
| Sex, male | 1183 (67) | 37 (77) | .16a |
| Age, y, mean (SD) | 51.8 (17.5) | 56.8 (14) | .098b |
| Severe immunosuppression | 62 (3.5) | 2 (4) | .8a |
| Fever only | 344 (19.7) | 4 (8) | .039c |
| Pneumonia | 465 (26.4) | 13 (27) | .93c |
| Hepatitis | 810 (46) | 24 (50) | .61c |
| Meningitis | 24 (1.36) | 1 (2) | .50a |
| Cholecystitis | 11 (0.62) | 0 (0) | .58a |
| Pericarditis | 22 (1.25) | 1 (2) | .61a |
| Lymphadenitis | 62 (3.53) | 3 (6) | .32a |
| Hemophagocytic syndrome | 8 (0.45) | 1 (2) | .11a |
| Thrombosis | 14 (0.74) | 2 (4) | .03a |
| Persistent focalized Coxiella burnetii infection | 117 (6.66) | 13 (27) | < .001c |
| Persistent endocarditis | 91 (5.2) | 12 (25) | < .001c |
| Persistent vascular infection | 28 (1.6) | 1 (2) | .56a |
| Osteoarticular infection | 5 (0.28) | 0 (0) | .99a |
| C. burnetii first phase I antibodies, IgG >800 | 233 (13.3) | 8 (16) | .5c |
| Positive aCLs (>22 GPLU) | 436 (24.9) | 28 (58) | .002c |
| Positive aCLs (>75 GPLU) | 254 (14.5) | 23 (47) | < .001c |
| Positive aCLs (>90 GPLU) | 216 (12.3) | 22 (46) | < .001c |
| Acute Q Fever Manifestation . | Acute Q Fever Without Endocarditis (n = 1749) . | Acute Endocarditis (n = 48) . | P Value . |
|---|---|---|---|
| Sex, male | 1183 (67) | 37 (77) | .16a |
| Age, y, mean (SD) | 51.8 (17.5) | 56.8 (14) | .098b |
| Severe immunosuppression | 62 (3.5) | 2 (4) | .8a |
| Fever only | 344 (19.7) | 4 (8) | .039c |
| Pneumonia | 465 (26.4) | 13 (27) | .93c |
| Hepatitis | 810 (46) | 24 (50) | .61c |
| Meningitis | 24 (1.36) | 1 (2) | .50a |
| Cholecystitis | 11 (0.62) | 0 (0) | .58a |
| Pericarditis | 22 (1.25) | 1 (2) | .61a |
| Lymphadenitis | 62 (3.53) | 3 (6) | .32a |
| Hemophagocytic syndrome | 8 (0.45) | 1 (2) | .11a |
| Thrombosis | 14 (0.74) | 2 (4) | .03a |
| Persistent focalized Coxiella burnetii infection | 117 (6.66) | 13 (27) | < .001c |
| Persistent endocarditis | 91 (5.2) | 12 (25) | < .001c |
| Persistent vascular infection | 28 (1.6) | 1 (2) | .56a |
| Osteoarticular infection | 5 (0.28) | 0 (0) | .99a |
| C. burnetii first phase I antibodies, IgG >800 | 233 (13.3) | 8 (16) | .5c |
| Positive aCLs (>22 GPLU) | 436 (24.9) | 28 (58) | .002c |
| Positive aCLs (>75 GPLU) | 254 (14.5) | 23 (47) | < .001c |
| Positive aCLs (>90 GPLU) | 216 (12.3) | 22 (46) | < .001c |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: aCL, anticardiolipin antibodies; GPLU, immunoglobulin G-type phospholipid units; SD, standard deviation.
aFisher exact test (mid P test).
bMann-Whitney test.
cχ2 test for 2 groups.
The logistic regression model was used to identify independent predictors of acute Q fever endocarditis, including sex, age, thrombosis, and IgG aCLs. Using for each different models, we identified that positive aCLs (>22 GPLU) (odds ratio [OR], 2.7 [95% confidence interval {CI}, 1.3–5.5]; P = .004) and high IgG aCL levels (>90 GPLU) (OR, 3.4 [95% CI, 2.0–7.5]; P < .001) were independently associated with acute Q fever endocarditis. In a receiver operating characteristic analysis, a dose-dependent relationship was observed between IgG aCL levels and acute Q fever endocarditis (area under the curve, 0.6 [95% CI, .5–.7]; P = .007).
Furthermore, among patients with acute Q fever, both acute Q fever endocarditis (OR, 5.2 [95% CI, 2.6–10.5]; P < .001) and age (OR, 1.7 [95% CI, 1.1–1.9]; P = .02) were independent predictors of progression toward persistent C. burnetii endocarditis.
DISCUSSION
In this study, we describe 48 cases of a new clinical entity, acute Q fever endocarditis, which affects 2.8% of patients with acute Q fever. Seventy percent, more than two-thirds of patients, had no underlying valvulopathy and presented an acquired valvular lesion highly suspected to be C. burnetii induced.
Supporting the paradigm shift seen in the 21st century regarding Q fever endocarditis, it should first be noted that the clinical presentation of acute Q fever endocarditis does not meet the usual manifestations proposed in the Duke criteria [21]. Indeed, fever, hepatitis, and pneumonia were the primary clinical manifestations of acute Q fever endocarditis (Table 2) [17, 22].
This paradigm change involved an even more underlying valvulopathy. Only 26% of patients with acute Q fever endocarditis had an underlying valvulopathy, whereas it concerns 95% of patients with persistent C. burnetii infection [5, 14, 17]. In accordance with the recent literature reports, mitral regurgitation was the most frequent native valve predisposing factor [21]. TTE was previously recommended to detect underlying valvulopathy and prevent the progression from acute Q fever to persistent C. burnetii endocarditis (a risk that is estimated at 40%) [5]. This diagnosis tool is now recommended, above all, to detect acute Q fever endocarditis; then to identify unknown underlying valvulopathies; and, finally, to confirm a known valvulopathy [5, 23]. One-third of patients with acute Q fever endocarditis having a previous TTE before the diagnosis of Q fever presented no underlying valvulopathy. Unfortunately, no imaging of these TTEs was saved because they were considered normal and because they were performed in peripheral centers.
Furthermore, the presence of positive PCR results on blood samples and the positive C. burnetii blood culture on L929 cells (Table 2 and Figure 2) attest to the circulation of the bacteria in blood at the early stages of the disease and in cases of valvular injury. This phenomenon of positive blood culture in the course of endocarditis is part of the Duke criteria, but is rarely observed during endocarditis induced by intracellular bacteria. Indeed, C. burnetii, as a fastidious bacterium, requires time and a Biosafety Level 3 laboratory to be cultivated. Coxiella burnetii is therefore on the list of bacteria responsible for “blood culture–negative endocarditis” [21, 24].
In addition, as described for a decade, primary C. burnetii infections could be responsible for the explosive secretion of several autoantibodies, especially aCLs [25]. The increase in aCLs has been described in association with an increased risk of acute Q fever complications and as a predictive factor of acute Q fever endocarditis [8, 9, 16, 26]. With a particular tropism for the endocardial endothelium and the heart valve, aCLs may be responsible for valve injuries [27]. Valve thickness, irregular nodules, fibrinous vegetations, and calcification are described as acquired valvular heart lesions in Libman-Sacks endocarditis [28]. Our report is consistent with the previous results described in the literature, with respect to valvular injury, the increased level of aCLs, and the high risk of thrombosis. In addition, in 1959, Evans et al reported the first case of Q fever endocarditis, which was possibly an acute Q fever endocarditis occurring 3 weeks after the onset of a flulike syndrome [3]. Postmortem examination showed a cardiac valve vegetation (an organized overlying thrombus with large mononuclear cells) and a thrombus of the left coronary artery [3]. Although aCLs have not been reported in this observation, this clinical case may provide evidence that acute Q fever endocarditis with valvular thrombus may be accompanied by thrombotic phenomena that could be enhanced by the secretion of aCL.
Our study reports that acute Q fever endocarditis was an independent predictive factor with a 6-fold increased risk of progressing to persistent endocarditis among patients with acute Q fever, making acute endocarditis a serious disease.
Finally, we highlight the need for performing TTE at an early stage on all patients with Q fever. While 81% of patients currently diagnosed with acute Q fever endocarditis had no known underlying valvulopathy, we support the crucial role that TTE plays in the immediate diagnosis of acute valvular lesions induced by C. burnetii. In men >40 years of age, with aCLs >60 GPLU and negative or inconclusive TTE, TEE should be proposed for the diagnosis of acute Q fever endocarditis (Figure 5). This diagnosis strategy would reduce the incidence of C. burnetii persistent endocarditis and its complications by appropriately treating patients with valvular damage [1, 8]. In terms of possible or definite acute Q fever endocarditis, we recommend a therapeutic treatment with doxycycline and hydroxychloroquine over an 18-month period. However, further studies are necessary to clarify, optimize, and possibly shorten the duration of the treatment of acute Q fever endocarditis. TTE and clinical and serological tests should be proposed every 3 months in case of acute Q fever endocarditis to ensure disappearance of clinical symptomatology, toleration of treatment, disappearance of valvular lesions, and decrease of serological levels. Antibiotic doses in the blood could be suggested monthly to ensure compliance with the regulations.
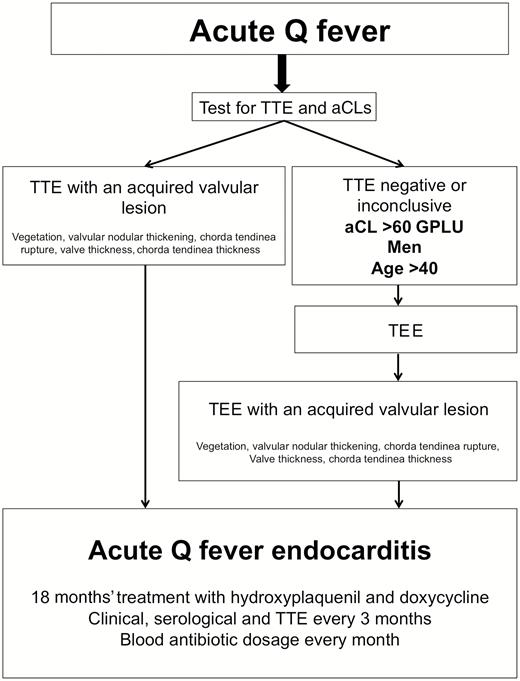
Diagram for diagnosis and management of acute Q fever endocarditis. Abbreviations: aCLs, anticardiolipin antibodies; GPLU, immunoglobulin G-type phospholipid units; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
In conclusion, clinical manifestations of acute Q fever endocarditis are nonspecific; underlying valvulopathy is, in most cases, unknown or absent; and echocardiographic valvular presentations could be similar to noninfectious valvular degenerative damage. The immediate use of TTE will optimize the detection of valvular injury in case of acute Q fever endocarditis. Further investigations are necessary to learn whether early doxycycline associated with hydroxychloroquine treatment could improve the clinical outcome of these patients.
Notes
Acknowledgments.The authors thank Nathalie Duclos, Emeline Vial, and Clio Grimaldier for the culture of Coxiella burnetii specimens in the Biosafety Level 3 routine laboratory and the photography performed for Figure 4. They also thank Claudie Makarawiez for clinical data.
Financial support. This work was supported by the French government under the Investments for the Future program managed by the French National Agency for Research (agence régionale de Recherche, ANR) (reference number Méditerranée infection ANR-10-IAHU-03). This work was also supported by Région Provence-Alpes-Côte d’Azur and European funding through Fonds Européen de Développement Régional (European Regional Development Fund PA0000320)—Plateformes de Recherche et d’Innovation Mutualisées Méditerranée Infection.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




