-
PDF
- Split View
-
Views
-
Cite
Cite
S M Hosseini-Moghaddam, M Shokoohi, G Singh, S F Dufresne, A Boucher, A Jevnikar, G V R Prasad, A Shoker, D Kabbani, M J Hebert, H Cardinal, I Houde, A Humar, D Kumar, A Multicenter Case-control Study of the Effect of Acute Rejection and Cytomegalovirus Infection on Pneumocystis Pneumonia in Solid Organ Transplant Recipients, Clinical Infectious Diseases, Volume 68, Issue 8, 15 April 2019, Pages 1320–1326, https://doi.org/10.1093/cid/ciy682
Close - Share Icon Share
Abstract
Pneumocystis pneumonia (PCP) is associated with morbidity and mortality in solid organ transplant (SOT) recipients. In this case-control study, we determined the association between posttransplant PCP and 3 variables: cytomegalovirus (CMV) infection, allograft rejection, and prophylaxis.
Eight transplant centers participated. For each case (SOT recipient with PCP), 3–5 controls (SOT recipients without PCP) were included. Controls were matched to the cases based on transplant center, type of allograft, and date of transplantation (±6 months).
We enrolled 53 cases and 209 controls. Transplant types included kidney (n = 198), heart (n = 30), liver (n = 15), kidney-pancreas (n = 14), and lung (n = 5). PCP occurred beyond 12 months after transplantation in 43 (81.1%) cases. Thirty-four cases (64.1%) required admission to the intensive care unit, and 28 (52.8%) had mechanical ventilation. Allograft failure occurred in 20 (37.7%) cases, and 14 (26.9%) died. No patient developed PCP prophylaxis breakthrough. The proportion of female sex (P = .009), kidney dysfunction (P = .001), cardiac diseases (P = .005), diabetes mellitus (P = .03), allograft rejection (P = .001), CMV infection (P = .001), and severe lymphopenia (P = .001) were significantly higher in cases. In the logistic regression model, CMV infection (adjusted odds ratio [aOR], 4.6 [95% confidence interval {CI}, 2.0–10.5]) and allograft rejection (aOR, 3.0 [95% CI, 1.5–6.1]) significantly increased the likelihood of PCP.
PCP was mostly a late-onset disease occurring after complete course of prophylaxis, particularly among patients with CMV infection or allograft rejection. PCP is associated with significant allograft loss. Extended prophylaxis targeting recipients with allograft rejection or CMV infection may reduce the risk of PCP.
Pneumocystis jirovecii is an opportunistic fungal microorganism that causes pneumocystis pneumonia (PCP) in immunocompromised patients [1]. PCP is associated with a significant morbidity and mortality in solid organ transplant (SOT) recipients [2–4]. To prevent PCP, chemoprophylaxis is routinely recommended during the first year after transplantation for all SOT recipients except for lung transplant recipients, who receive lifelong prophylaxis [5]. In the absence of prophylaxis, up to 24% of transplant recipients may develop PCP, with mortality in this group ranging from 5% to 49% [6].
Numerous reports have indicated that PCP is a reemerging infectious disease in transplantation [4, 7–12]. This is evidenced by several reports of PCP presenting as a late-onset disease occurring beyond the first year following transplantation despite 6–12 months of prophylaxis [9, 10, 12]. Posttransplant PCP generally presents as a severe pneumonia and may cause nosocomial outbreaks [9, 13–17]. Late-onset PCP is associated with graft loss [18, 19] and considerable mortality [13, 20].
Several factors have been reported to be associated with posttransplant PCP. Older age [13, 19], sex [21, 22], type of transplantation [17, 22], cytomegalovirus (CMV) infection [15, 17, 23], allograft rejection [18, 24], and immunosuppression [17, 24] are variables that have been demonstrated to increase the risk of infection. However, these findings have generally been noted in single-center studies. In addition, the majority of data on posttransplant PCP originates from Europe and Australia, whereas North American data on the topic are limited [7, 16, 17, 25, 26]. In this multicenter case-control study, we determined the effect size estimate of CMV infection, allograft rejection, and PCP prophylaxis considering the simultaneous effects of other variables that have been demonstrated to increase the likelihood of PCP in single-center studies.
METHODS
We performed a case-control study of posttransplant PCP in SOT recipients across 8 transplant centers in Canada. All participating centers reviewed their local data, microbiology, and pathology diagnoses to find patients with posttransplant PCP who were diagnosed between 2010 and 2017. All centers obtained local institutional ethics board approval. Patients with pneumonia and typical radiographic findings including diffuse, bilateral, ground glass opacities, interstitial infiltrates, pulmonary nodules, and/or atypical presentation such as lobar infiltration, consolidation, and pneumothorax were considered as cases only if the diagnosis of PCP was achieved by microscopy with staining (direct fluorescent antibody, Gram-Wright, Wright-Giemsa, or modified Papanicolaou) or histopathology. No patient with possible diagnosis based on β-d-glucan assay was included. The specimens were taken from bronchoalveolar lavage, bronchoscopy, or tissue biopsy. The reference day for the patients in the case group was defined as the day when microbiological evidence of pneumocystis infection was obtained (D0). Cases were followed for 6 months after D0. Late-onset PCP was defined as PCP occurring beyond 1 year after transplantation. Each case was matched with 3–5 controls. Controls were identified at the same medical center as the respective cases and defined as SOT patients without PCP. Controls were matched for type of transplant and were transplanted within 6 months of the cases. Controls were followed for the same posttransplant duration as their matched cases.
Data related to demographic information, underlying chronic illnesses, donor status, organ transplantation, induction, maintenance immunosuppression, posttransplant pneumocystis prophylaxis, allograft rejection, lymphocyte count, and CMV infection were collected for both cases and controls. CMV infection was defined as new-onset CMV viremia <6 months before D0 detected by quantitative polymerase chain reaction (qPCR) [25]. We included data related to allograft rejection only if the diagnosis was biopsy proven and occurred <6 months before D0. We defined allograft failure as requirement for retransplantation or returning for dialysis (only for renal transplant recipients).
Patients with biopsy-proven allograft rejection were treated with augmented immunosuppression. Treatment of patients with higher grades of allograft rejection (ie, Banff grade II or III) included pulse high-dose intravenous glucocorticoids with or without rabbit antithymocyte globulin. Patients with histopathologic evidence of antibody-mediated rejection or mixed cell–mediated and antibody-mediated rejection received high-dose glucocorticoids plus plasma exchange or intravenous immunoglobulin [27].
Statistical Analysis
Cases and controls were compared using the independent t test for continuous variables and χ2 test for categorical variables. Crude and adjusted logistic regression models were applied to assess the association between PCP and main independent variables including acute allograft rejection, CMV infection, and PCP prophylaxis. Therefore, we reported crude odds ratios (ORs) and adjusted ORs (aORs) associated with 95% confidence intervals (CIs). The adjusted regression models aimed to control the confounding influence of those covariates that were available in the present study and were identified as a confounder in the literature as follows: age, sex, retransplantation, history of diabetes mellitus, posttransplant renal dysfunction (defined as estimated glomerular filtration rate <60 mL/minute/1.73 m2 for >3 months regardless of the cause), and history of cardiac disease (defined as any documented history of coronary artery disease or congestive heart failure). Although lymphopenia have been reported as a risk factor for PCP [25, 28], we did not include this variable in multivariate analysis due to missing data in >10% of cases. We further adjusted for matching variables (ie, type of organ transplantation and medical center) [29]. Additionally, we created a combined measure of allograft rejection and CMV infection as follows: no allograft rejection and no CMV infection (reference category); either allograft rejection or CMV infection; and both allograft rejection and CMV infection. All analyses were performed using Stata version 15 software (StataCorp).
RESULTS
Clinical Characteristics and Outcomes
A total of 53 SOT patients with PCP were identified (cases) along with 209 controls. Center recruitment of cases and controls was as follows: London Health Sciences Centre (n = 75), Toronto General Hospital (n = 25), St Michael’s Hospital (n = 20), St Paul’s Hospital, Saskatoon (n = 20), Centre hospitalier de l’Université de Montréal (n = 48), Centre hospitalier universitaire de Québec-Université Laval (CHUQ; n = 5), Hôpital Notre-Dame (n = 39), and University of Alberta Hospital (n = 30). CHUQ initially reported 6 cases and 24 controls. However, 5 cases and their controls were excluded due to occurrence of the disease before 2010.
The majority of cases were kidney transplant recipients (75.5%), followed by heart (11.3%), liver (5.7%), kidney-pancreas (5.7%), and lung transplants (1.9%). Of the 53 cases, 43 (81.1%) developed late-onset disease. Table 1 demonstrates the clinical and radiologic findings, and the onset of the disease in patients with PCP. Figure 1 demonstrates the interval between transplantation and the onset of PCP.
Clinical Findings, Radiographic Information, and Disease Onset in Patients With Pneumocystis Pneumonia (n = 53)
| Variable . | No. (%) . |
|---|---|
| Fevera surrounding diagnosis date (n = 52) | 30 (57.7) |
| Shortness of breath surrounding diagnosis date (n = 53) | 46 (86.8) |
| Duration (n = 43) | |
| <7 d | 22 (51.2) |
| 7–14 d | 15 (34.9) |
| >14 d | 6 (13.9) |
| Productive cough surrounding diagnosis date (n = 53) | 15 (28.3) |
| Duration (n = 18) | |
| <7 d | 6 (42.9) |
| 7–14 d | 6 (42.9) |
| >14 d | 2 (14.2) |
| Dry cough surrounding diagnosis date (n = 53) | 29 (54.7) |
| Duration (n = 32) | |
| <7 d | 15 (51.7) |
| 7–14 d | 9 (31.0) |
| >14 d | 5 (17.2) |
| Chills surrounding diagnosis date (n = 52) | 8 (15.4) |
| Duration (n = 7) | |
| <7 d | 5 (71.4) |
| 7–14 d | 1 (14.3) |
| >14 d | 1 (14.3) |
| Major radiographic findings including CXR or CT scan (n = 53) | |
| Diffuse interstitial infiltrates | 43 (81.1) |
| Nodules | 18 (34.0) |
| Consolidation | 18 (34.0) |
| Pneumatocele, pneumothorax | 3 (5.7) |
| PCP onset time, posttransplant years (n = 53) | |
| Median (IQR) | 3.3 (1.3–11.1) |
| Early-onset PCP (≤1 y after transplantation) | 10 (18.9) |
| Late-onset PCP (>1 y after transplantation) | 43 (81.1) |
| 2nd year | 9 |
| 3rd year | 3 |
| 4th year | 6 |
| 5th year | 1 |
| 6th–10th year | 10 |
| >10th y | 14 |
| Variable . | No. (%) . |
|---|---|
| Fevera surrounding diagnosis date (n = 52) | 30 (57.7) |
| Shortness of breath surrounding diagnosis date (n = 53) | 46 (86.8) |
| Duration (n = 43) | |
| <7 d | 22 (51.2) |
| 7–14 d | 15 (34.9) |
| >14 d | 6 (13.9) |
| Productive cough surrounding diagnosis date (n = 53) | 15 (28.3) |
| Duration (n = 18) | |
| <7 d | 6 (42.9) |
| 7–14 d | 6 (42.9) |
| >14 d | 2 (14.2) |
| Dry cough surrounding diagnosis date (n = 53) | 29 (54.7) |
| Duration (n = 32) | |
| <7 d | 15 (51.7) |
| 7–14 d | 9 (31.0) |
| >14 d | 5 (17.2) |
| Chills surrounding diagnosis date (n = 52) | 8 (15.4) |
| Duration (n = 7) | |
| <7 d | 5 (71.4) |
| 7–14 d | 1 (14.3) |
| >14 d | 1 (14.3) |
| Major radiographic findings including CXR or CT scan (n = 53) | |
| Diffuse interstitial infiltrates | 43 (81.1) |
| Nodules | 18 (34.0) |
| Consolidation | 18 (34.0) |
| Pneumatocele, pneumothorax | 3 (5.7) |
| PCP onset time, posttransplant years (n = 53) | |
| Median (IQR) | 3.3 (1.3–11.1) |
| Early-onset PCP (≤1 y after transplantation) | 10 (18.9) |
| Late-onset PCP (>1 y after transplantation) | 43 (81.1) |
| 2nd year | 9 |
| 3rd year | 3 |
| 4th year | 6 |
| 5th year | 1 |
| 6th–10th year | 10 |
| >10th y | 14 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CT, computed tomography; CXR, chest radiograph; IQR, interquartile range; PCP, pneumocystis pneumonia.
aTemperature >37.8°C.
Clinical Findings, Radiographic Information, and Disease Onset in Patients With Pneumocystis Pneumonia (n = 53)
| Variable . | No. (%) . |
|---|---|
| Fevera surrounding diagnosis date (n = 52) | 30 (57.7) |
| Shortness of breath surrounding diagnosis date (n = 53) | 46 (86.8) |
| Duration (n = 43) | |
| <7 d | 22 (51.2) |
| 7–14 d | 15 (34.9) |
| >14 d | 6 (13.9) |
| Productive cough surrounding diagnosis date (n = 53) | 15 (28.3) |
| Duration (n = 18) | |
| <7 d | 6 (42.9) |
| 7–14 d | 6 (42.9) |
| >14 d | 2 (14.2) |
| Dry cough surrounding diagnosis date (n = 53) | 29 (54.7) |
| Duration (n = 32) | |
| <7 d | 15 (51.7) |
| 7–14 d | 9 (31.0) |
| >14 d | 5 (17.2) |
| Chills surrounding diagnosis date (n = 52) | 8 (15.4) |
| Duration (n = 7) | |
| <7 d | 5 (71.4) |
| 7–14 d | 1 (14.3) |
| >14 d | 1 (14.3) |
| Major radiographic findings including CXR or CT scan (n = 53) | |
| Diffuse interstitial infiltrates | 43 (81.1) |
| Nodules | 18 (34.0) |
| Consolidation | 18 (34.0) |
| Pneumatocele, pneumothorax | 3 (5.7) |
| PCP onset time, posttransplant years (n = 53) | |
| Median (IQR) | 3.3 (1.3–11.1) |
| Early-onset PCP (≤1 y after transplantation) | 10 (18.9) |
| Late-onset PCP (>1 y after transplantation) | 43 (81.1) |
| 2nd year | 9 |
| 3rd year | 3 |
| 4th year | 6 |
| 5th year | 1 |
| 6th–10th year | 10 |
| >10th y | 14 |
| Variable . | No. (%) . |
|---|---|
| Fevera surrounding diagnosis date (n = 52) | 30 (57.7) |
| Shortness of breath surrounding diagnosis date (n = 53) | 46 (86.8) |
| Duration (n = 43) | |
| <7 d | 22 (51.2) |
| 7–14 d | 15 (34.9) |
| >14 d | 6 (13.9) |
| Productive cough surrounding diagnosis date (n = 53) | 15 (28.3) |
| Duration (n = 18) | |
| <7 d | 6 (42.9) |
| 7–14 d | 6 (42.9) |
| >14 d | 2 (14.2) |
| Dry cough surrounding diagnosis date (n = 53) | 29 (54.7) |
| Duration (n = 32) | |
| <7 d | 15 (51.7) |
| 7–14 d | 9 (31.0) |
| >14 d | 5 (17.2) |
| Chills surrounding diagnosis date (n = 52) | 8 (15.4) |
| Duration (n = 7) | |
| <7 d | 5 (71.4) |
| 7–14 d | 1 (14.3) |
| >14 d | 1 (14.3) |
| Major radiographic findings including CXR or CT scan (n = 53) | |
| Diffuse interstitial infiltrates | 43 (81.1) |
| Nodules | 18 (34.0) |
| Consolidation | 18 (34.0) |
| Pneumatocele, pneumothorax | 3 (5.7) |
| PCP onset time, posttransplant years (n = 53) | |
| Median (IQR) | 3.3 (1.3–11.1) |
| Early-onset PCP (≤1 y after transplantation) | 10 (18.9) |
| Late-onset PCP (>1 y after transplantation) | 43 (81.1) |
| 2nd year | 9 |
| 3rd year | 3 |
| 4th year | 6 |
| 5th year | 1 |
| 6th–10th year | 10 |
| >10th y | 14 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CT, computed tomography; CXR, chest radiograph; IQR, interquartile range; PCP, pneumocystis pneumonia.
aTemperature >37.8°C.
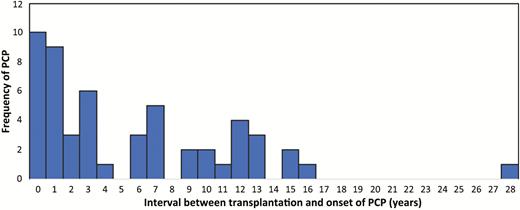
Onset of pneumocystis pneumonia, by time of transplantation. Abbreviation: PCP, pneumocystis pneumonia.
Of 10 patients who developed PCP in the first year after transplantation (early-onset disease), 7 did not receive prophylaxis and 3 received prophylaxis for only 3 months. In patients with early-onset PCP (n = 10), 6 (60%) developed PCP in the first 6 months following transplantation. On the other hand, 6 of 9 patients who developed PCP in the second year after transplantation received at least 6 months prophylaxis (66.7%). In 24 patients (45.3%), the PCP occurred beyond 5 years after transplantation. No patient with PCP was receiving effective PCP prophylaxis at the time of diagnosis.
The diagnosis, treatment, course of illness, and the outcome of PCP are given in Table 2. The diagnosis of PCP was mainly made by microscopy with staining. More than 90% of patients with posttransplantation PCP admitted to hospital and the majority required a long-term hospital stay. More than 60% of patients were admitted to intensive care unit (ICU) and >50% of patients required mechanical ventilation. Most patients with PCP stayed in ICU for >3 weeks. Kidney dysfunction was a common complication and >40% of patients needed hemodialysis after PCP.
Diagnosis, Treatment Regimen, Course of Illness, and the Outcome of Pneumocystis Pneumonia (n = 53)
| Variable . | No. (%) . |
|---|---|
| PCP diagnosis (n = 52) | |
| Microscopy with staining | 51 (98.1) |
| Tissue biopsy | 1 (1.9) |
| Treatment for PCP | |
| TMP/SMX (n = 53) | 50 (94.3) |
| Trimethoprim/dapsone (n = 52) | 1 (1.9) |
| Primaquine/clindamycin (n = 53) | 24 (45.3) |
| Atovaquone (n = 52) | 9 (17.3) |
| Pentamidine (n = 53) | 4 (7.5) |
| Caspofungin, micafungin, or anidulafungin (n = 53) | 6 (11.3) |
| High-dose prednisone (40–80 mg/d) (n = 53) | 39 (73.6) |
| Hospital admission (n = 53) | 50 (94.3) |
| Hospital admission duration, d (n = 49) | |
| Median (IQR) | 28 (12–41) |
| Admission to ICU (n = 53) | 34 (64.1) |
| ICU admission duration, d (n = 32) | |
| Median (IQR) | 21 (12–33) |
| Mechanical ventilation (n = 53) | 28 (52.8) |
| Intubation duration, d (n = 27) | |
| Median (IQR) | 19 (12–29) |
| Hypotension requiring vasopressorsa (n = 53) | 22 (41.5) |
| Dialysis during admission (n = 53) | 23 (43.4) |
| Allograft failure (n = 53) | 20 (37.7) |
| Requirement for permanent hemodialysis after PCP (n = 48) | 11 (22.9) |
| Deathb (n = 52) | 14 (26.9) |
| Variable . | No. (%) . |
|---|---|
| PCP diagnosis (n = 52) | |
| Microscopy with staining | 51 (98.1) |
| Tissue biopsy | 1 (1.9) |
| Treatment for PCP | |
| TMP/SMX (n = 53) | 50 (94.3) |
| Trimethoprim/dapsone (n = 52) | 1 (1.9) |
| Primaquine/clindamycin (n = 53) | 24 (45.3) |
| Atovaquone (n = 52) | 9 (17.3) |
| Pentamidine (n = 53) | 4 (7.5) |
| Caspofungin, micafungin, or anidulafungin (n = 53) | 6 (11.3) |
| High-dose prednisone (40–80 mg/d) (n = 53) | 39 (73.6) |
| Hospital admission (n = 53) | 50 (94.3) |
| Hospital admission duration, d (n = 49) | |
| Median (IQR) | 28 (12–41) |
| Admission to ICU (n = 53) | 34 (64.1) |
| ICU admission duration, d (n = 32) | |
| Median (IQR) | 21 (12–33) |
| Mechanical ventilation (n = 53) | 28 (52.8) |
| Intubation duration, d (n = 27) | |
| Median (IQR) | 19 (12–29) |
| Hypotension requiring vasopressorsa (n = 53) | 22 (41.5) |
| Dialysis during admission (n = 53) | 23 (43.4) |
| Allograft failure (n = 53) | 20 (37.7) |
| Requirement for permanent hemodialysis after PCP (n = 48) | 11 (22.9) |
| Deathb (n = 52) | 14 (26.9) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ICU, intensive care unit; IQR, interquartile range; PCP, pneumocystis pneumonia; TMP/SMX, trimethoprim/sulfamethoxazole.
aDopamine, dobutamine, norepinephrine, or amrinone.
bAll-cause mortality.
Diagnosis, Treatment Regimen, Course of Illness, and the Outcome of Pneumocystis Pneumonia (n = 53)
| Variable . | No. (%) . |
|---|---|
| PCP diagnosis (n = 52) | |
| Microscopy with staining | 51 (98.1) |
| Tissue biopsy | 1 (1.9) |
| Treatment for PCP | |
| TMP/SMX (n = 53) | 50 (94.3) |
| Trimethoprim/dapsone (n = 52) | 1 (1.9) |
| Primaquine/clindamycin (n = 53) | 24 (45.3) |
| Atovaquone (n = 52) | 9 (17.3) |
| Pentamidine (n = 53) | 4 (7.5) |
| Caspofungin, micafungin, or anidulafungin (n = 53) | 6 (11.3) |
| High-dose prednisone (40–80 mg/d) (n = 53) | 39 (73.6) |
| Hospital admission (n = 53) | 50 (94.3) |
| Hospital admission duration, d (n = 49) | |
| Median (IQR) | 28 (12–41) |
| Admission to ICU (n = 53) | 34 (64.1) |
| ICU admission duration, d (n = 32) | |
| Median (IQR) | 21 (12–33) |
| Mechanical ventilation (n = 53) | 28 (52.8) |
| Intubation duration, d (n = 27) | |
| Median (IQR) | 19 (12–29) |
| Hypotension requiring vasopressorsa (n = 53) | 22 (41.5) |
| Dialysis during admission (n = 53) | 23 (43.4) |
| Allograft failure (n = 53) | 20 (37.7) |
| Requirement for permanent hemodialysis after PCP (n = 48) | 11 (22.9) |
| Deathb (n = 52) | 14 (26.9) |
| Variable . | No. (%) . |
|---|---|
| PCP diagnosis (n = 52) | |
| Microscopy with staining | 51 (98.1) |
| Tissue biopsy | 1 (1.9) |
| Treatment for PCP | |
| TMP/SMX (n = 53) | 50 (94.3) |
| Trimethoprim/dapsone (n = 52) | 1 (1.9) |
| Primaquine/clindamycin (n = 53) | 24 (45.3) |
| Atovaquone (n = 52) | 9 (17.3) |
| Pentamidine (n = 53) | 4 (7.5) |
| Caspofungin, micafungin, or anidulafungin (n = 53) | 6 (11.3) |
| High-dose prednisone (40–80 mg/d) (n = 53) | 39 (73.6) |
| Hospital admission (n = 53) | 50 (94.3) |
| Hospital admission duration, d (n = 49) | |
| Median (IQR) | 28 (12–41) |
| Admission to ICU (n = 53) | 34 (64.1) |
| ICU admission duration, d (n = 32) | |
| Median (IQR) | 21 (12–33) |
| Mechanical ventilation (n = 53) | 28 (52.8) |
| Intubation duration, d (n = 27) | |
| Median (IQR) | 19 (12–29) |
| Hypotension requiring vasopressorsa (n = 53) | 22 (41.5) |
| Dialysis during admission (n = 53) | 23 (43.4) |
| Allograft failure (n = 53) | 20 (37.7) |
| Requirement for permanent hemodialysis after PCP (n = 48) | 11 (22.9) |
| Deathb (n = 52) | 14 (26.9) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ICU, intensive care unit; IQR, interquartile range; PCP, pneumocystis pneumonia; TMP/SMX, trimethoprim/sulfamethoxazole.
aDopamine, dobutamine, norepinephrine, or amrinone.
bAll-cause mortality.
Management of PCP
The most frequent first-line antibiotic regimen that was used for the management of PCP was trimethoprim/sulfamethoxazole (TMP/SMX). However, 45% of patients received combination of primaquine and clindamycin due to poor response to treatment with TMP/SMX or side effects of this medication. The frequencies of the other treatment regimens are given in Table 2. Approximately, three-quarters of patients received adjunctive glucocorticoid therapy with high-dose prednisone (>40 mg/day) for the management of severe pneumonitis. Patients with severe PCP were more likely to receive treatment with prednisone. Twenty-nine of 34 patients who were admitted to ICU (85.3%) and 10 of 19 (52.6%) patients who did not require ICU admission received adjunctive glucocorticoid therapy (P = .01). Similarly, 24 of 28 (85.7%) patients with mechanical ventilation and 15 of 25 (60.0%) patients who did not need mechanical ventilation received adjunctive glucocorticoid therapy (P = .03).
Despite treatment, 20 patients with PCP lost their allografts (37.7%), including 15 renal, 2 liver, 2 kidney-pancreas, and 1 heart transplant recipients. Of these, 12 patients (60%) including 9 renal, 2 liver, and 1 heart transplant recipients died. In total, PCP was associated with >25% mortality (Table 2). It should be noted that although patients with severe pneumonitis were more likely to receive adjunctive glucocorticoid therapy, this treatment did not affect the mortality. Eleven of 39 patients (28.2%) who received glucocorticoid therapy and 3 of 14 (21.4%) patients who did not receive this treatment died (P = .59).
Case-control Study
Baseline characteristics including age, induction, and maintenance immunosuppression of cases and controls were similar (Table 3). However, cases were more likely to be female (OR, 2.2 [95% CI, 1.2–4.2]) and to have underlying diabetes mellitus (OR, 1.95 [95% CI, 1.06–3.6]), posttransplant renal dysfunction (OR, 4.6 [95% CI, 2.4–8.8]), and history of cardiac disease (OR, 2.5 [95% CI, 1.3–4.7]). Cases were also more likely to have had allograft rejection (P < .001) or CMV infection (P < .001) within the 6 months prior to the diagnosis of PCP. Although all patients with allograft rejection were treated with augmented immunosuppression, receiving rabbit antithymocyte globulin as treatment of rejection was significantly associated with PCP (7 [13.2%] cases vs 8 [3.8%] controls; OR, 3.8 [95% CI, 1.32–11.07]; P = .01). The presence of severe lymphopenia, defined as an absolute lymphocyte count of <500 cells/μL, was also a risk factor for development of PCP. Posttransplant PCP prophylaxis for at least 6 months did not significantly decrease the risk of PCP.
Baseline Characteristics of Solid Organ Transplant Recipients With and Without Pneumocystis Pneumonia
| Variable . | Cases (n = 53) . | Controls (n = 209) . | P Value . |
|---|---|---|---|
| Age at the time of transplantation, mean (SD) | 49.4 (15.3) | 47.0 (14.1) | .275a |
| Sex | .009 | ||
| Female | 31 (58.5) | 81 (38.8) | |
| Male | 22 (41.5) | 128 (61.2) | |
| Transplant organ | 1.00 | ||
| Kidney | 40 (75.5) | 158 (75.6) | |
| Heart | 6 (11.3) | 24 (11.5) | |
| Lung | 1 (1.9) | 4 (1.9) | |
| Liver | 3 (5.7) | 12 (5.7) | |
| Kidney and pancreas | 3 (5.7) | 11 (5.3) | |
| Donor status | .682 | ||
| Deceased | 41 (77.4) | 167 (79.9) | |
| Living | 12 (22.6) | 42 (20.1) | |
| Retransplantation | 8 (15.1) | 15 (7.2) | .070 |
| Underlying medical illness | |||
| Diabetes mellitus | 26 (49.1) | 69 (33.0) | .030 |
| On insulin | 18 (69.2) | 42 (66.7) | .814 |
| COPD | 2 (3.8) | 8 (3.8) | .985 |
| Asthma | 5 (9.4) | 14 (6.6) | .493 |
| Other chronic lung disease | 8 (15.1) | 20 (9.6) | .245 |
| Kidney dysfunction after transplantation | 24 (45.3) | 32 (15.4) | <.001 |
| Heart disease | 20 (37.7) | 41 (19.6) | .005 |
| Cancer treatment after transplantation | 4 (7.7) | 20 (9.7) | .661 |
| Cigarette smoking after transplantation | 5 (9.4) | 23 (11.1) | .733 |
| Induction with antithymocyte globulin | 46 (86.8) | 170 (81.3) | .351 |
| Maintenance immunosuppression | |||
| Prednisone | 47 (88.7) | 169 (80.9) | .182 |
| Cyclosporine | 2 (3.8) | 24 (11.8) | .086 |
| Tacrolimus, extended-release formulation | 27 (51.9) | 100 (48.1) | .620 |
| Tacrolimus, immediate-release formulation | 21 (39.6) | 70 (33.6) | .416 |
| Sirolimus | 3 (5.9) | 12 (5.8) | .981 |
| Everolimus | 0 (0) | 1 (0.5) | .612 |
| Azathioprine | 1 (1.9) | 9 (4.3) | .406 |
| Mycophenolic acid, delayed release | 24 (46.1) | 116 (56.0) | .201 |
| Mycophenolate mofetil | 23 (43.4) | 93 (44.7) | .863 |
| Allograft rejection ≤6 mo before D0 | 28 (52.8) | 45 (21.5) | <.001 |
| Once | 23 | 35 | |
| More than once | 5 | 10 | |
| CMV infection ≤6 mo before D0 | 19 (35.8) | 20 (9.6) | <.001 |
| Combined allograft rejection and CMV | <.001 | ||
| Neither is present | 20 (37.7) | 147 (70.3) | |
| Only 1 is present | 19 (35.8) | 59 (28.2) | |
| Both are present | 14 (26.4) | 3 (1.4) | |
| Posttransplant PCP prophylaxis for at least 6 mo | 38 (71.7) | 167 (79.9) | .196 |
| Lymphopenia | <.001a | ||
| Severe lymphopenia (<500 cells/µL) | 33 (63.4) | 49 (25.8) | |
| Mild to moderate lymphopenia (500–1000 cells/µL) | 14 (26.9) | 41 (21.6) | |
| Normal lymphocyte count (>1000 cells/µL) | 5 (9.6) | 100 (52.6) | |
| Variable . | Cases (n = 53) . | Controls (n = 209) . | P Value . |
|---|---|---|---|
| Age at the time of transplantation, mean (SD) | 49.4 (15.3) | 47.0 (14.1) | .275a |
| Sex | .009 | ||
| Female | 31 (58.5) | 81 (38.8) | |
| Male | 22 (41.5) | 128 (61.2) | |
| Transplant organ | 1.00 | ||
| Kidney | 40 (75.5) | 158 (75.6) | |
| Heart | 6 (11.3) | 24 (11.5) | |
| Lung | 1 (1.9) | 4 (1.9) | |
| Liver | 3 (5.7) | 12 (5.7) | |
| Kidney and pancreas | 3 (5.7) | 11 (5.3) | |
| Donor status | .682 | ||
| Deceased | 41 (77.4) | 167 (79.9) | |
| Living | 12 (22.6) | 42 (20.1) | |
| Retransplantation | 8 (15.1) | 15 (7.2) | .070 |
| Underlying medical illness | |||
| Diabetes mellitus | 26 (49.1) | 69 (33.0) | .030 |
| On insulin | 18 (69.2) | 42 (66.7) | .814 |
| COPD | 2 (3.8) | 8 (3.8) | .985 |
| Asthma | 5 (9.4) | 14 (6.6) | .493 |
| Other chronic lung disease | 8 (15.1) | 20 (9.6) | .245 |
| Kidney dysfunction after transplantation | 24 (45.3) | 32 (15.4) | <.001 |
| Heart disease | 20 (37.7) | 41 (19.6) | .005 |
| Cancer treatment after transplantation | 4 (7.7) | 20 (9.7) | .661 |
| Cigarette smoking after transplantation | 5 (9.4) | 23 (11.1) | .733 |
| Induction with antithymocyte globulin | 46 (86.8) | 170 (81.3) | .351 |
| Maintenance immunosuppression | |||
| Prednisone | 47 (88.7) | 169 (80.9) | .182 |
| Cyclosporine | 2 (3.8) | 24 (11.8) | .086 |
| Tacrolimus, extended-release formulation | 27 (51.9) | 100 (48.1) | .620 |
| Tacrolimus, immediate-release formulation | 21 (39.6) | 70 (33.6) | .416 |
| Sirolimus | 3 (5.9) | 12 (5.8) | .981 |
| Everolimus | 0 (0) | 1 (0.5) | .612 |
| Azathioprine | 1 (1.9) | 9 (4.3) | .406 |
| Mycophenolic acid, delayed release | 24 (46.1) | 116 (56.0) | .201 |
| Mycophenolate mofetil | 23 (43.4) | 93 (44.7) | .863 |
| Allograft rejection ≤6 mo before D0 | 28 (52.8) | 45 (21.5) | <.001 |
| Once | 23 | 35 | |
| More than once | 5 | 10 | |
| CMV infection ≤6 mo before D0 | 19 (35.8) | 20 (9.6) | <.001 |
| Combined allograft rejection and CMV | <.001 | ||
| Neither is present | 20 (37.7) | 147 (70.3) | |
| Only 1 is present | 19 (35.8) | 59 (28.2) | |
| Both are present | 14 (26.4) | 3 (1.4) | |
| Posttransplant PCP prophylaxis for at least 6 mo | 38 (71.7) | 167 (79.9) | .196 |
| Lymphopenia | <.001a | ||
| Severe lymphopenia (<500 cells/µL) | 33 (63.4) | 49 (25.8) | |
| Mild to moderate lymphopenia (500–1000 cells/µL) | 14 (26.9) | 41 (21.6) | |
| Normal lymphocyte count (>1000 cells/µL) | 5 (9.6) | 100 (52.6) | |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CMV, cytomegalovirus; COPD, chronic obstructive pulmonary disease; D0, day when microbiological evidence of pneumocystis infection was obtained; PCP, pneumocystis pneumonia; SD, standard deviation.
aStudent t test.
Baseline Characteristics of Solid Organ Transplant Recipients With and Without Pneumocystis Pneumonia
| Variable . | Cases (n = 53) . | Controls (n = 209) . | P Value . |
|---|---|---|---|
| Age at the time of transplantation, mean (SD) | 49.4 (15.3) | 47.0 (14.1) | .275a |
| Sex | .009 | ||
| Female | 31 (58.5) | 81 (38.8) | |
| Male | 22 (41.5) | 128 (61.2) | |
| Transplant organ | 1.00 | ||
| Kidney | 40 (75.5) | 158 (75.6) | |
| Heart | 6 (11.3) | 24 (11.5) | |
| Lung | 1 (1.9) | 4 (1.9) | |
| Liver | 3 (5.7) | 12 (5.7) | |
| Kidney and pancreas | 3 (5.7) | 11 (5.3) | |
| Donor status | .682 | ||
| Deceased | 41 (77.4) | 167 (79.9) | |
| Living | 12 (22.6) | 42 (20.1) | |
| Retransplantation | 8 (15.1) | 15 (7.2) | .070 |
| Underlying medical illness | |||
| Diabetes mellitus | 26 (49.1) | 69 (33.0) | .030 |
| On insulin | 18 (69.2) | 42 (66.7) | .814 |
| COPD | 2 (3.8) | 8 (3.8) | .985 |
| Asthma | 5 (9.4) | 14 (6.6) | .493 |
| Other chronic lung disease | 8 (15.1) | 20 (9.6) | .245 |
| Kidney dysfunction after transplantation | 24 (45.3) | 32 (15.4) | <.001 |
| Heart disease | 20 (37.7) | 41 (19.6) | .005 |
| Cancer treatment after transplantation | 4 (7.7) | 20 (9.7) | .661 |
| Cigarette smoking after transplantation | 5 (9.4) | 23 (11.1) | .733 |
| Induction with antithymocyte globulin | 46 (86.8) | 170 (81.3) | .351 |
| Maintenance immunosuppression | |||
| Prednisone | 47 (88.7) | 169 (80.9) | .182 |
| Cyclosporine | 2 (3.8) | 24 (11.8) | .086 |
| Tacrolimus, extended-release formulation | 27 (51.9) | 100 (48.1) | .620 |
| Tacrolimus, immediate-release formulation | 21 (39.6) | 70 (33.6) | .416 |
| Sirolimus | 3 (5.9) | 12 (5.8) | .981 |
| Everolimus | 0 (0) | 1 (0.5) | .612 |
| Azathioprine | 1 (1.9) | 9 (4.3) | .406 |
| Mycophenolic acid, delayed release | 24 (46.1) | 116 (56.0) | .201 |
| Mycophenolate mofetil | 23 (43.4) | 93 (44.7) | .863 |
| Allograft rejection ≤6 mo before D0 | 28 (52.8) | 45 (21.5) | <.001 |
| Once | 23 | 35 | |
| More than once | 5 | 10 | |
| CMV infection ≤6 mo before D0 | 19 (35.8) | 20 (9.6) | <.001 |
| Combined allograft rejection and CMV | <.001 | ||
| Neither is present | 20 (37.7) | 147 (70.3) | |
| Only 1 is present | 19 (35.8) | 59 (28.2) | |
| Both are present | 14 (26.4) | 3 (1.4) | |
| Posttransplant PCP prophylaxis for at least 6 mo | 38 (71.7) | 167 (79.9) | .196 |
| Lymphopenia | <.001a | ||
| Severe lymphopenia (<500 cells/µL) | 33 (63.4) | 49 (25.8) | |
| Mild to moderate lymphopenia (500–1000 cells/µL) | 14 (26.9) | 41 (21.6) | |
| Normal lymphocyte count (>1000 cells/µL) | 5 (9.6) | 100 (52.6) | |
| Variable . | Cases (n = 53) . | Controls (n = 209) . | P Value . |
|---|---|---|---|
| Age at the time of transplantation, mean (SD) | 49.4 (15.3) | 47.0 (14.1) | .275a |
| Sex | .009 | ||
| Female | 31 (58.5) | 81 (38.8) | |
| Male | 22 (41.5) | 128 (61.2) | |
| Transplant organ | 1.00 | ||
| Kidney | 40 (75.5) | 158 (75.6) | |
| Heart | 6 (11.3) | 24 (11.5) | |
| Lung | 1 (1.9) | 4 (1.9) | |
| Liver | 3 (5.7) | 12 (5.7) | |
| Kidney and pancreas | 3 (5.7) | 11 (5.3) | |
| Donor status | .682 | ||
| Deceased | 41 (77.4) | 167 (79.9) | |
| Living | 12 (22.6) | 42 (20.1) | |
| Retransplantation | 8 (15.1) | 15 (7.2) | .070 |
| Underlying medical illness | |||
| Diabetes mellitus | 26 (49.1) | 69 (33.0) | .030 |
| On insulin | 18 (69.2) | 42 (66.7) | .814 |
| COPD | 2 (3.8) | 8 (3.8) | .985 |
| Asthma | 5 (9.4) | 14 (6.6) | .493 |
| Other chronic lung disease | 8 (15.1) | 20 (9.6) | .245 |
| Kidney dysfunction after transplantation | 24 (45.3) | 32 (15.4) | <.001 |
| Heart disease | 20 (37.7) | 41 (19.6) | .005 |
| Cancer treatment after transplantation | 4 (7.7) | 20 (9.7) | .661 |
| Cigarette smoking after transplantation | 5 (9.4) | 23 (11.1) | .733 |
| Induction with antithymocyte globulin | 46 (86.8) | 170 (81.3) | .351 |
| Maintenance immunosuppression | |||
| Prednisone | 47 (88.7) | 169 (80.9) | .182 |
| Cyclosporine | 2 (3.8) | 24 (11.8) | .086 |
| Tacrolimus, extended-release formulation | 27 (51.9) | 100 (48.1) | .620 |
| Tacrolimus, immediate-release formulation | 21 (39.6) | 70 (33.6) | .416 |
| Sirolimus | 3 (5.9) | 12 (5.8) | .981 |
| Everolimus | 0 (0) | 1 (0.5) | .612 |
| Azathioprine | 1 (1.9) | 9 (4.3) | .406 |
| Mycophenolic acid, delayed release | 24 (46.1) | 116 (56.0) | .201 |
| Mycophenolate mofetil | 23 (43.4) | 93 (44.7) | .863 |
| Allograft rejection ≤6 mo before D0 | 28 (52.8) | 45 (21.5) | <.001 |
| Once | 23 | 35 | |
| More than once | 5 | 10 | |
| CMV infection ≤6 mo before D0 | 19 (35.8) | 20 (9.6) | <.001 |
| Combined allograft rejection and CMV | <.001 | ||
| Neither is present | 20 (37.7) | 147 (70.3) | |
| Only 1 is present | 19 (35.8) | 59 (28.2) | |
| Both are present | 14 (26.4) | 3 (1.4) | |
| Posttransplant PCP prophylaxis for at least 6 mo | 38 (71.7) | 167 (79.9) | .196 |
| Lymphopenia | <.001a | ||
| Severe lymphopenia (<500 cells/µL) | 33 (63.4) | 49 (25.8) | |
| Mild to moderate lymphopenia (500–1000 cells/µL) | 14 (26.9) | 41 (21.6) | |
| Normal lymphocyte count (>1000 cells/µL) | 5 (9.6) | 100 (52.6) | |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CMV, cytomegalovirus; COPD, chronic obstructive pulmonary disease; D0, day when microbiological evidence of pneumocystis infection was obtained; PCP, pneumocystis pneumonia; SD, standard deviation.
aStudent t test.
Subsequently, the association between the main exposure variables (allograft rejection, PCP prophylaxis for at least 6 months, and CMV infection) and posttransplant PCP was analyzed using a logistic regression model. In this model, acute allograft rejection (aOR, 3.0 [95% CI, 1.5–6.1]; P = .002) and CMV infection (aOR, 4.6 [95% CI, 2.0–10.5]; P < .001) significantly increased the risk of PCP. The presence of both CMV infection and allograft rejection was significantly associated with development of PCP (aOR, 25.3 [95% CI, 5.8–109.8]; P ≤ .001). On the other hand, PCP prophylaxis for 6–12 months did not significantly prevent PCP (aOR, 1.8 [95% CI, .82–4.0]; P = .14).
DISCUSSION
Posttransplant PCP is an emerging problem worldwide [7, 18, 30]. PCP is associated with significant morbidity and mortality [7, 12, 19]. In our study, >90% of SOT patients required hospital admission. Approximately, 60% of patients were admitted to ICU and more than half of SOT patients with PCP required mechanical ventilation. PCP was also associated with considerable risk of allograft failure. In our study, 38% of the recipients with PCP lost their allografts. Allograft loss in patients with PCP may occur due to multiple contributing factors including end organ damage as a complication of severe pneumosepsis, treatment with nephrotoxic medications, reducing of immunosuppression and adverse effects of TMP/SMX. Since 41 of 43 (95.3%) of kidney or kidney-pancreas transplant recipients received TMP/SMX, we were not able to demonstrate the potential role of renal adverse effects of TMP/SMX as a contributing factor in kidney allograft failure. Overall, graft loss appears to be unexpectedly high in posttransplant PCP [7, 19, 30] and further prospective studies are required to determine the roles of contributing factors in detail.
Our data also showed that PCP is associated with considerable mortality. More than 25% of patients with posttransplant PCP died despite appropriate care and treatment. This is similar to the findings of previous studies showing graft loss rate ranging from 26% to 35.2% and mortality rate of 15.6% to 50% [7, 19, 22, 31].
In this case-control study, patients with severe PCP were more likely to receive adjunctive glucocorticoid therapy. More than 85% of patients who were admitted to ICU or required mechanical ventilation received high-dose prednisone. Although adjunctive glucocorticoid therapy was more frequently used for patients with severe disease, this treatment did not affect mortality (28.2% vs 21.4%; P = .59). Our finding may support the results of a recent retrospective cohort study that showed adjunctive steroid therapy does not provide benefit in mortality of human immunodeficiency virus–negative immunocompromised patients with PCP [32]. Further clinical trials are needed to demonstrate the effect of adjunctive glucocorticoid therapy in the management of posttransplant PCP.
The diagnosis of PCP requires a high index of suspicion and delay in diagnosis may be associated with severe adverse outcome [33]. In this study, 40% of patients with PCP were afebrile. Nonproductive cough and shortness of breath were the 2 most frequent symptoms at the time of clinical presentation. Diffuse interstitial infiltration appears to be the most common radiographic finding at the time of diagnosis. Detection of the microorganism in respiratory specimens was most commonly achieved by microscopy with staining of bronchoalveolar lavage fluid. Polymerase chain reaction or microscopy with staining is necessary because this microorganism cannot be cultured [25, 34].
More than 75% of patients with PCP were renal transplant recipients. Although recipients of renal allograft seem to be more susceptible to PCP, this finding could be due to a higher frequency of renal transplantation compared to other SOTs. Unfortunately, a considerable number of patients with PCP required renal replacement therapy. More than 20% of renal transplant patients with PCP returned to permanent hemodialysis. All these findings showed the serious effect of PCP on allograft function, specifically in renal transplant recipients.
Posttransplant PCP is usually a severe infectious disease requiring careful preventive strategies. The American Society of Transplantation guideline suggests universal prophylaxis in the first 6–12 months after transplantation except in lung transplant recipients, in whom lifelong prophylaxis is recommended [35]. However, time of the onset of PCP is not limited to the first year after transplantation [12]. PCP may occur after complete course of 6–12 months’ prophylaxis [12, 36]. Several outbreaks of delayed-onset PCP have been reported [7, 9, 12, 37, 38]. Our data showed that this disease may occur with a considerable interval after transplantation. The median interval between the time of transplantation and PCP was >3 years. As we showed in Table 3, current prophylaxis strategy may not significantly reduce the risk of posttransplant PCP. Due to an increased frequency of late-onset PCP, some centers have opted to place their transplant recipients on lifelong prophylaxis. However, universal lifelong prophylaxis of all SOT recipients may lead to increased cost and toxicity [17, 35]. The other prophylactic strategy is targeted prophylaxis. Extended duration of prophylaxis targeting high-risk recipients may be a more cost-effective strategy to reduce the rate of late-onset disease.
In this case-control study, we showed that allograft rejection considerably increases the risk of posttransplant PCP. Allograft rejection has been shown to be associated with late-onset PCP in SOT recipients [15, 16, 22, 26]. This effect could be related to treatment regimens of rejection, which usually include intravenous glucocorticoids and augmentation of immunosuppression. It should be also noted that augmented immunosuppression is not the only reason that rejection increases the risk of PCP. Allograft dysfunction has been also shown to increase the risk of PCP as an independent risk factor [17, 39]. SOT recipients who have recently experienced allograft rejection should be considered high-risk for late-onset PCP. Targeted prophylaxis of patients with allograft rejection may provide a protective effect and reduce the risk.
We also showed CMV infection (CMV viremia as measured by a qPCR assay) is an independent risk factor for posttransplant PCP. In previous single-center studies, the variable of “detectable CMV viremia” has been frequently used to demonstrate the effect of CMV infection as a risk factor on posttransplant PCP [15, 17, 24, 25, 28, 40]. Similarly, in this multicenter study, we did not limit CMV infection to patients with symptomatic or tissue-invasive disease. Due to variability in frequencies of qPCR monitoring in transplant centers, different viremia treatment thresholds, and the fact that CMV can present with nonspecific symptoms, we only used new-onset CMV viremia as our variable of interest.
CMV infection and allograft rejection have a bidirectional association [41]. As a result, CMV infection directly and indirectly increases the risk of PCP. The association between CMV infection and posttransplant PCP is biologically plausible considering the immunomodulatory effect of this viral infection. Patients who develop CMV infection after transplantation appear to require prophylaxis to decrease the risk of PCP.
Strengths of our study include its multicenter nature and relatively large number of cases, without which multivariate analyses would not be possible. Having 3–5 controls for each case also improved the statistical power to determine the associations. This study was also associated with some limitations. We were not able to generate incidence data due to unknown denominators at some centers. It is possible some cases of PCP were not detected if they presented to peripheral hospitals. However, all medical centers at these sites used electronic medical record systems and generally have good follow-up of their transplant recipients. We were also not able to definitively determine if cases at an individual center represented person-to-person transmission. Although the exposure of interest in this study was CMV infection and allograft rejection within the 6-month period before the diagnosis of PCP, this interval in some patients was 3 months or less. Due to limitations of case-control design, we were not able to determine the exact temporal relationship between CMV infection or allograft rejection and PCP. Further prospective cohort studies and randomized clinical trials are required to determine this temporal association.
In summary, our study showed that in the current era, PCP mostly occurs as a late-onset disease beyond the first year after transplantation. Extended prophylaxis targeting recipients with allograft rejection or CMV infection may reduce the frequency of PCP.
Notes
Acknowledgments. We thank and acknowledge all colleagues including physicians, nurses, and research assistants who participated in this multicenter study.
Financial support. The Canadian National Transplant Research Program provided meeting facilities for face-to-face meetings, financial support for data collection, and administrative support.
Potential conflicts of interest. A. H. has received grants and personal fees from Shire, Merck, and Chimerix, and grants from Roche. D. K. has received grants and personal fees from Shire and Merck and grants from Roche. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




