-
PDF
- Split View
-
Views
-
Cite
Cite
Patricia Escandón, Nancy A Chow, Diego H Caceres, Lalitha Gade, Elizabeth L Berkow, Paige Armstrong, Sandra Rivera, Elizabeth Misas, Carolina Duarte, Heather Moulton-Meissner, Rory M Welsh, Claudia Parra, Luz Angela Pescador, Nohora Villalobos, Soraya Salcedo, Indira Berrio, Carmen Varón, Andrés Espinosa-Bode, Shawn R Lockhart, Brendan R Jackson, Anastasia P Litvintseva, Mauricio Beltran, Tom M Chiller, Molecular Epidemiology of Candida auris in Colombia Reveals a Highly Related, Countrywide Colonization With Regional Patterns in Amphotericin B Resistance, Clinical Infectious Diseases, Volume 68, Issue 1, 1 January 2019, Pages 15–21, https://doi.org/10.1093/cid/ciy411
Close - Share Icon Share
Abstract
Candida auris is a multidrug-resistant yeast associated with hospital outbreaks worldwide. During 2015–2016, multiple outbreaks were reported in Colombia. We aimed to understand the extent of contamination in healthcare settings and to characterize the molecular epidemiology of C. auris in Colombia.
We sampled patients, patient contacts, healthcare workers, and the environment in 4 hospitals with recent C. auris outbreaks. Using standardized protocols, people were swabbed at different body sites. Patient and procedure rooms were sectioned into 4 zones and surfaces were swabbed. We performed whole-genome sequencing (WGS) and antifungal susceptibility testing (AFST) on all isolates.
Seven of the 17 (41%) people swabbed were found to be colonized. Candida auris was isolated from 37 of 322 (11%) environmental samples. These were collected from a variety of items in all 4 zones. WGS and AFST revealed that although isolates were similar throughout the country, isolates from the northern region were genetically distinct and more resistant to amphotericin B (AmB) than the isolates from central Colombia. Four novel nonsynonymous mutations were found to be significantly associated with AmB resistance.
Our results show that extensive C. auris contamination can occur and highlight the importance of adherence to appropriate infection control practices and disinfection strategies. Observed genetic diversity supports healthcare transmission and a recent expansion of C. auris within Colombia with divergent AmB susceptibility.
Candida auris is an emerging yeast that has been reported worldwide as a cause of severe invasive infections in healthcare settings [1]. Unlike many Candida species, C. auris is capable of healthcare transmission and is often multidrug resistant, with instances of resistance to all 3 major classes of antifungals [2]. Mechanisms of C. auris drug resistance remain unclear.
Molecular methods, notably whole-genome sequencing (WGS), have been used to explore its population structure [3, 4]. WGS and epidemiologic data suggest independent, nearly simultaneous emergence of 4 populations on 3 continents. Specifically, WGS revealed a profound phylogeographic structure with 4 geographically isolated, genetically distinct clades (East Asian, South Asian, African, and South American) [2]. To date, it is unclear whether and how C. auris spreads between countries and between healthcare facilities within a country.
Although public health officials are aware that C. auris is capable of healthcare transmission, its modes of spread within hospitals and between patients are poorly understood. A previous investigation of a C. auris outbreak in Europe used amplified fragment-length polymorphism to describe the genetic diversity of C. auris isolates collected and concluded that a single introduction occurred within the hospital [5]. Additionally, the authors hypothesized that both colonization of healthcare workers and the persistence of C. auris in the environment significantly contributed to transmission. Other studies have shown that the yeast can persist on surfaces for weeks [6, 7], and its ability to form biofilms on surfaces has been described [8]. These findings have highlighted the need for examinations of C. auris diversity among patients, healthcare workers, and environmental surfaces within and between healthcare facilities.
In this report, we describe an investigation of 4 hospitals in Colombia that had reported C. auris outbreaks, in which 51 isolates from blood cultures were identified during February 2015–August 2016. To aid infection control efforts, we conducted an extensive environmental sampling in each of the hospitals in September 2016 and provided a description of C. auris contamination. We performed WGS and antifungal susceptibility testing (AFST) on all environmental and clinical isolates to identify transmission clusters among patients, healthcare workers, and environmental surfaces. Finally, we assessed differences in genetic diversity and drug resistance between isolates in the northern and central regions in an effort to describe the overall molecular epidemiology of C. auris in Colombia.
METHODS
Site Locations
Environmental and body swabs were collected at 4 hospitals (hospitals A–D) that had reported C. auris outbreaks. Hospitals A and B are approximately 70 km away from each other and located in adjacent states in Colombia’s north coast region. Both are approximately 700 km away from the central region of Bogotá where hospitals C and D are located. Hospital A is a pediatric intensive care unit, and hospitals B, C, and D are large tertiary-care hospitals. This investigation was determined to constitute nonresearch public health practice by the US Centers for Disease Control and Prevention (CDC) and the Instituto Nacional de Salud in Colombia, and therefore it was not subject to institutional review board review requirements.
Environmental Sampling
Sampling protocols described by the CDC’s Division of Healthcare Quality and Promotion [9] were adapted for this study. Environmental samples were taken with 3M Sponge-Sticks (Fisher Scientific, Pittsburg, Pennsylvania). Samples from sink spouts were taken with EnviroMax Plus swabs (Fisher Scientific).
A sampling plan was established by reviewing medical records of patients with C. auris infections who had either previously stayed or were currently in the hospital with an aim to identify rooms where prolonged stays occurred. Uninhabited patient rooms were also sampled. We grouped surfaces into 3 or 4 zones (Supplementary Figure 1). Zone 1 consisted of the patient bed and the adjacent environment, including floors and items in contact with the bed (eg, bedrails, pillows, catheters). Zone 2 consisted of surfaces near zone 1 with infrequent patient contact but frequent healthcare worker contact; these surfaces included medical devices (eg, cardiac monitors, ventilators). Zone 3 consisted of surfaces beyond zone 2 with little to no patient contact and infrequent healthcare worker contact (eg, windows, cabinets, floors further than the immediate vicinity of the patient bed). Zone 4 consisted of surfaces in a bathroom adjacent to the patient room; if existent, surfaces such as the toilet and sink were sampled. Additional zone 4 surfaces consisted of hallway items (transport stretchers, mobile storage cabinets, cleaning equipment).
Body Swabs of Patients, Patient Contacts, and Healthcare Workers
A patient and their contacts were swabbed if the patient had a diagnosis of C. auris infection or was residing in a room previously inhabited by a C. auris–infected patient. Patient contacts included family members and visitors. Fisherfinest Transport Swabs containing Amies charcoal (Fisher Scientific) were rolled on an area no larger than 26 cm2 in both horizontal and vertical sweeps for approximately 1 minute. The following body sites were swabbed using 1 swab per site: right axilla, left axilla, right groin, left groin, right nostril, left nostril, right ear, left ear, mouth, and rectum. For healthcare workers and patient contacts who preferred, the following sites were swabbed as composite sites: axillae, groin, nostrils, and ears. Additionally, composite swabs of healthcare workers’ hands were taken.
Yeast Isolation and Identification
Samples were processed using a salt Sabouraud dextrose enrichment broth as previously described [6]. Recovered yeast isolates were typed using the matrix-assisted laser desorption–ionization (MALDI) time-of-flight mass spectrometry technology (BD Bruker MALDI Biotyper).
Candida auris Isolates for WGS and Antifungal Susceptibility Testing
Candida auris isolates recovered from environmental and body swabs are described in Supplementary Table 1. Forty clinical isolates were collected from hospitals A, B, and D from the February 2015–August 2016 outbreaks, and 36 are described in Supplementary Table 1. WGS sequences from isolates from Japan, India, Pakistan, South Africa, and Venezuela were previously described [2]. Sequences were deposited in the National Center for Biotechnology Information Sequence Read Archive (BioProject ID: PRJNA470683).
WGS and Single-Nucleotide Polymorphism Calling
DNA was extracted using the ZR Fungal/Bacterial DNA MiniPrep kit (Zymo Research, Irvine, California). Genomic libraries were constructed using the NEBNext Ultra DNA Library Prep kit for Illumina (New England Biolabs, Ipswich, Massachusetts) and sequenced on Illumina HiSeq 2500 (Illumina, San Diego, California) using the HiSeq Rapid SBS Kit v2 500-cycle (Illumina). Samples were only used that had at least 50× coverage. FastQC and PRINSEQ [10] were used to assess quality of read data and perform read filtering. Read data were aligned against a publically available genome sequenced on PacBio RS II [2] using BWA [11]. Single-nucleotide polymorphism (SNP) variants were identified using SAMtools [12] and filtered using the publicly available SNP analysis pipeline NASP [13] to remove positions that had <10× coverage, <90% variant allele calls, or that were identified by Nucmer [14] as being within duplicated regions in the reference. Phylogenetic analysis was performed on SNP matrices using Mega [15], and bootstrapping was performed with 1000 iterations.
Antifungal Susceptibility Testing
AFST was performed on isolates as outlined by the Clinical and Laboratory Standards Institute guidelines [16] using microdilution plates (Trek Diagnostics, Oakwood Village, Ohio) for azoles and echinocandins. Amphotericin B (AmB) susceptibilities were measured using Etest (bioMérieux, Marcy l’Etoile, France). Interpretive breakpoints for C. auris were defined based on those breakpoints established for other closely related Candida species. Resistance to fluconazole was set at a minimum inhibitory concentration (MIC) of ≥32 µg/mL, AmB at ≥2.0 µg/mL, anidulafungin and micafungin at ≥4 µg/mL, and caspofungin at ≥2 µg/mL. A MIC of 1.5 for AmB using Etest was rounded to 2.0.
Analysis of Genomic SNPs Associated With AmB Resistance
Of the variable positions identified, SNPs that occurred in at least 10 strains were selected. Then the presence of these SNPs was compared with MIC values for AmB. For each SNP position, we compared the MIC values between the mutate isolates and the wild-type isolates by performing a Mann-Whitney test using Real Statistics Resource Pack. For the 5 SNPs in protein-coding regions that had a significant association with AmB resistance, a 501-nucleotide sequence was extracted that contained the SNP and was characterized using BLAST (Basic Local Alignment Search Tool).
RESULTS
Clinical isolates recovered from blood cultures during the February 2015–August 2016 outbreaks clustered with isolates from Venezuela as compared to those from other countries (Figure 1A). Isolates from Colombia and Venezuela were separated by approximately 140 SNPs, and a maximum pairwise difference of 40 SNPs was found between any 2 Colombian isolates (Figure 1B). The high degree of similarity among isolates in Colombia and in each hospital supports reports of a widespread C. auris outbreak.
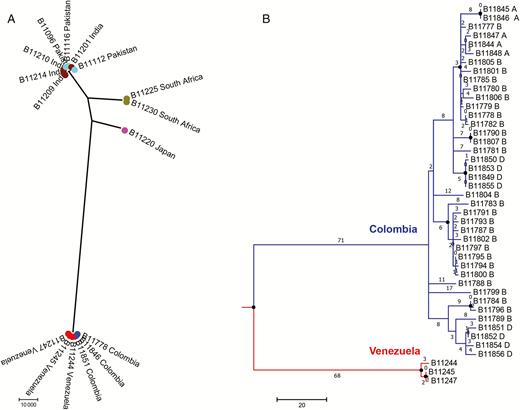
Phylogenetic analysis of clinical Candida auris isolates from Colombia and other countries reporting C. auris cases. A, Maximum parsimony tree of isolates from South Asian (Indian and Pakistani), African, Japanese, and South American (Venezuelan and Colombian) C. auris cases. Scale represents 10000 single-nucleotide polymorphisms (SNPs). Each circle represents a single isolate. B, Maximum parsimony tree of isolates from Venezuelan (red) and Colombian (blue) C. auris cases. Scale represents 20 SNPs. Numbers represent SNPs. A, B, and D indicate hospitals A, B, and D. Circles at nodes indicate separations with a bootstrap value ≥90%.
Of the environmental samples collected, 37 (11%) were positive for C. auris. Positive samples were represented in all 4 zones (Table 1) and from a variety of items (Supplementary Table 1). From zone 1, C. auris was found on bedrails, a bed hand controller, a cellular phone, and floors. Positive samples from zone 2 were taken from chairs, bed trays, and medical equipment. From zone 3, C. auris was found on closet cabinets, door handles, alcohol gel dispensers, and from zone 4, items included sink basins, bedpans, and mop buckets. Overall, the highest positivity rates were at hospital A (18%) and hospital D (26%) (Table 1). All 107 samples collected from hospital B were negative for C. auris. Hospital D was the only hospital where C. auris was found on surfaces from all 4 zones.
Candida auris-positive Environmental Samples Collected From Colombian Medical Facilities
| Hospital . | Zone 1 (Positive/Total) . | Zone 2 (Positive/Total) . | Zone 3 (Positive/Total) . | Zone 4 (Positive/Total) . | Total . |
|---|---|---|---|---|---|
| A | 7/20 | 5/22 | 0/20 | 0/6 | 12/68 (18%) |
| B | 0/28 | 0/23 | 0/15 | 0/41 | 0/107 (0) |
| C | 0/12 | 1/10 | 1/7 | 0/30 | 2/59 (3%) |
| D | 7/25 | 5/21 | 6/16 | 5/26 | 23/88 (26%) |
| Total | 14/85 (16%) | 11/76 (14%) | 7/58 (12%) | 5/103 (5%) | 37/322 (11%) |
| Hospital . | Zone 1 (Positive/Total) . | Zone 2 (Positive/Total) . | Zone 3 (Positive/Total) . | Zone 4 (Positive/Total) . | Total . |
|---|---|---|---|---|---|
| A | 7/20 | 5/22 | 0/20 | 0/6 | 12/68 (18%) |
| B | 0/28 | 0/23 | 0/15 | 0/41 | 0/107 (0) |
| C | 0/12 | 1/10 | 1/7 | 0/30 | 2/59 (3%) |
| D | 7/25 | 5/21 | 6/16 | 5/26 | 23/88 (26%) |
| Total | 14/85 (16%) | 11/76 (14%) | 7/58 (12%) | 5/103 (5%) | 37/322 (11%) |
“Positive” indicates environmental samples from which Candida auris was isolated, and “total” indicates the number of samples collected.
Candida auris-positive Environmental Samples Collected From Colombian Medical Facilities
| Hospital . | Zone 1 (Positive/Total) . | Zone 2 (Positive/Total) . | Zone 3 (Positive/Total) . | Zone 4 (Positive/Total) . | Total . |
|---|---|---|---|---|---|
| A | 7/20 | 5/22 | 0/20 | 0/6 | 12/68 (18%) |
| B | 0/28 | 0/23 | 0/15 | 0/41 | 0/107 (0) |
| C | 0/12 | 1/10 | 1/7 | 0/30 | 2/59 (3%) |
| D | 7/25 | 5/21 | 6/16 | 5/26 | 23/88 (26%) |
| Total | 14/85 (16%) | 11/76 (14%) | 7/58 (12%) | 5/103 (5%) | 37/322 (11%) |
| Hospital . | Zone 1 (Positive/Total) . | Zone 2 (Positive/Total) . | Zone 3 (Positive/Total) . | Zone 4 (Positive/Total) . | Total . |
|---|---|---|---|---|---|
| A | 7/20 | 5/22 | 0/20 | 0/6 | 12/68 (18%) |
| B | 0/28 | 0/23 | 0/15 | 0/41 | 0/107 (0) |
| C | 0/12 | 1/10 | 1/7 | 0/30 | 2/59 (3%) |
| D | 7/25 | 5/21 | 6/16 | 5/26 | 23/88 (26%) |
| Total | 14/85 (16%) | 11/76 (14%) | 7/58 (12%) | 5/103 (5%) | 37/322 (11%) |
“Positive” indicates environmental samples from which Candida auris was isolated, and “total” indicates the number of samples collected.
Of 17 people swabbed at hospitals A, B, and D, 7 (41%) were found to be colonized; these included 5 of 7 (71%) patients and 2 of 6 (33%) healthcare workers (Table 2). Among patients, the axilla, groin, and rectum had the highest positivity rate (2/7 [28%] each; Figure 2). Candida auris was isolated from the hands of 2 healthcare workers and the groin of 1 healthcare worker. None of the 4 patient contacts swabbed were positive for C. auris. Body swabs were not collected at hospital C because patients with C. auris infections or presumed C. auris infections were not present at the time of sampling. As with the environmental samples, the highest positivity rates were at hospital A (3/5 [60%]) and hospital D (3/7 [43%]).
Results of Candida auris Screening in Colombian Medical Facilities With Previously Reported Outbreaks of C. auris, February 2015–August 2016
| Hospital . | Patients (Positive/Total) . | Contactsa (Positive/Total) . | Medical Staff (Positive/Total) . | Total . |
|---|---|---|---|---|
| A | 1/2 | … | 2/3 | 3/5 (60%) |
| B | 1/2 | 0/2 | 0/1 | 1/5 (20%) |
| D | 3/3 | 0/2 | 0/2 | 3/7 (43%) |
| Total | 5/7 (71%) | 0/4 (0) | 2/6 (33%) | 7/17 (41%) |
| Hospital . | Patients (Positive/Total) . | Contactsa (Positive/Total) . | Medical Staff (Positive/Total) . | Total . |
|---|---|---|---|---|
| A | 1/2 | … | 2/3 | 3/5 (60%) |
| B | 1/2 | 0/2 | 0/1 | 1/5 (20%) |
| D | 3/3 | 0/2 | 0/2 | 3/7 (43%) |
| Total | 5/7 (71%) | 0/4 (0) | 2/6 (33%) | 7/17 (41%) |
“Positive” indicates samples from anatomical sites from which Candida auris was isolated, and “total” indicates the number of samples collected. Multiple samples were collected from each individual.
aContacts were visitors or family members of the patient.
Results of Candida auris Screening in Colombian Medical Facilities With Previously Reported Outbreaks of C. auris, February 2015–August 2016
| Hospital . | Patients (Positive/Total) . | Contactsa (Positive/Total) . | Medical Staff (Positive/Total) . | Total . |
|---|---|---|---|---|
| A | 1/2 | … | 2/3 | 3/5 (60%) |
| B | 1/2 | 0/2 | 0/1 | 1/5 (20%) |
| D | 3/3 | 0/2 | 0/2 | 3/7 (43%) |
| Total | 5/7 (71%) | 0/4 (0) | 2/6 (33%) | 7/17 (41%) |
| Hospital . | Patients (Positive/Total) . | Contactsa (Positive/Total) . | Medical Staff (Positive/Total) . | Total . |
|---|---|---|---|---|
| A | 1/2 | … | 2/3 | 3/5 (60%) |
| B | 1/2 | 0/2 | 0/1 | 1/5 (20%) |
| D | 3/3 | 0/2 | 0/2 | 3/7 (43%) |
| Total | 5/7 (71%) | 0/4 (0) | 2/6 (33%) | 7/17 (41%) |
“Positive” indicates samples from anatomical sites from which Candida auris was isolated, and “total” indicates the number of samples collected. Multiple samples were collected from each individual.
aContacts were visitors or family members of the patient.

Screening for Candida auris in patients. Results of 7 patients screened for Candida auris. Body sites (ear, nose, mouth, axilla, groin, and rectum) were swabbed. Black represents body sites negative for C. auris and red represents positive body sites. A, B, and D indicate hospitals A, B, and D.
To better understand modes of transmission, we examined C. auris isolates recovered from environmental surfaces (n = 37) and colonized people (n = 15) using WGS. Maximum pairwise SNP difference of 13 SNPs (Figure 3A) was observed. Strikingly, isolates clustered by geography at the regional level, thereby forming 2 clades (northern and central). Isolates from hospitals in the northern region (hospitals A and B) clustered with the northern clade and isolates from hospitals in the central regions (hospitals C and D) clustered with the central clade. Genetically, isolates within clades were more similar than to those between clades. The separation of the northern and central clades had a bootstrap value of 93% (Figure 3A), indicating a highly supported separation.

Phylogenetic analysis and amphotericin B (AmB) susceptibility testing of Candida auris isolates recovered from hospitals A–D (indicated by A, B, C, and D). A, Maximum parsimony tree of isolates recovered from the environment (n = 37), patients (n = 12), and healthcare workers (n = 3). Scale represents 0.5 single-nucleotide polymorphisms. Circles at nodes indicate separations with a bootstrap value ≥90%. B, Distribution of AmB minimum inhibitory concentrations (MICs) for isolates recovered from the environment (n = 35), patients (n = 12), and healthcare workers (n = 3). Dotted line indicates the AmB breakpoint of 2. ****P < .0001, Mann-Whitney test. Brown indicates isolates from the northern region and green from the central region.
Clusters of genetically identical isolates (0 SNPs difference) were found in hospitals A, B, and D (Figure 3A). For example, isolates from the floor (B12317) and bed rails (B12325) within a patient room in hospital A were found to be genetically identical to an isolate from a stretcher (B12326) located in the hallway adjacent to the patient room. Additionally, in hospital A, isolates from a patient (B12328), the bed mattress of the patient (B12323), and body swabs (B12329, groin composite; B12330, hands composite; B12331, hands composite) from 2 healthcare workers providing care to the patient were found to be genetically identical (Figure 3A). No such clusters were found when comparing isolates between hospitals.
AFST of clinical blood isolates and isolates recovered from environmental and body swabs revealed that all isolates had low MICs to voriconazole, itraconazole, isavuconazole, and echinocandins. Ten of 87 (14%) isolates were resistant to fluconazole (Supplementary Table 1), and 27 (31%) were resistant to AmB (Supplementary Table 1). Of the isolates recovered from environmental and body swabs, resistance to AmB varied by region; specifically, isolates from hospitals in the northern region had significantly higher MICs (P ≤ .0001) to AmB (Figure 3B) than isolates from hospitals in the central region. From the northern region, 7 of 11 (64%) environmental isolates were resistant to AmB in contrast to 0 of 24 (0%) environmental isolates from the central region. A similar finding was observed among the 15 isolates recovered from 7 persons screened. We recovered AmB-resistant C. auris from all 4 colonized persons in the northern region (2 patients and 2 healthcare workers) and only AmB-susceptible C. auris from the 3 colonized patients in the central region.
To identify potential mechanisms of AmB resistance, we identified SNPs that had a strong association with AmB resistance (P ≤ .001). These included 5 SNPs located in different genomic loci (utg4_968953: T/C, utg5_821828: C/T, utg4_160118 (G/A), utg4_352365 (G/A), utg3_385439 (G/A) [contig_position: susceptible/resistant]). All but utg3_385439 (G/A) were identified as nonsynonymous mutations in protein-coding regions. Notably, mutation utg5_821828 (C/T) resulted in the amino acid change serine to asparagine in a gene predicted to encode for a transcription factor similar to FLO8 in Candida albicans. Mutation utg4_968953 (T/C) resulted in a change from the hydrophobic amino acid isoleucine to the polar amino acid threonine in a gene predicted to encode for a membrane transporter.
DISCUSSION
Investigation of C. auris in Colombia revealed widespread environmental contamination and colonization among patients and healthcare workers. By utilizing WGS, we observed that C. auris throughout the country is genetically highly related. We found genetically identical isolates within hospitals that connected patients to environmental surfaces and healthcare workers. These findings suggest that C. auris was recently introduced into healthcare centers and that healthcare transmission has occurred within hospitals and between hospitals at the regional level. Additionally, a regional difference in AmB resistance was observed, and, from these results, 4 novel nonsynonymous mutations were found to be significantly associated with AmB resistance.
Overall, these findings indicate that C. auris became widespread and persistent in certain healthcare environments in Colombia, suggesting a need for intensified infection control and environmental cleaning and disinfection. We found that C. auris contaminates surfaces at varying distances from the patient bed. Schelenz et al documented a C. auris outbreak in London and recovered the yeast from bed surfaces associated with infected patients. They concluded that colonized patients can spread C. auris into their close environment, thereby becoming a risk for continuous transmission [5]. Supporting these findings, we observed that samples from zone 1 had the highest positivity rate (Table 1). However, we also found C. auris on surfaces from zones 2–4, which included many items not predicted to be frequently touched by patients, and in the case of hospital D, surfaces from zone 3 had the highest positivity rate. We found C. auris on multiple floor surfaces and floor-related surfaces such as the sole of a healthcare worker’s shoe and a nondisposable mop used to disinfect floors in patient rooms. Indeed, we found C. auris on items frequently touched by healthcare workers alone and by cleaning staff alone.
Our findings from screening patients, patient contacts, and healthcare workers mirrored findings from environmental sampling in that hospitals A and D had the highest positivity rates. This further supports the idea that both environmental contamination and colonization of patients is promoting continual transmission. Ultimately, adherence to appropriate infection control and environmental disinfection is critical for limiting transmission. These include transmission-based precautions such as placing the patient with C. auris in a single room and using contact precautions, increased emphasis on hand hygiene on the unit where a patient with C. auris resides, and environmental disinfection using an Environmental Protection Agency–registered hospital-grade disinfectant effective against Clostridium difficile spores. Daily and terminal cleaning and disinfection of patient rooms and areas where a patient with C. auris resides or receives care are necessary. Comprehensive recommendations are found at https://www.cdc.gov/fungal/diseases/candidiasis/c-auris-infection-control.html.
Limiting the spread of C. auris is crucial considering the degree of multidrug resistance that has been observed in other countries. Here, the proportion resistant to azoles and echinocandins was lower than to those from other countries examined in previous studies [2]. However, we observed a cluster of isolates from a patient and the patient’s room resistant to fluconazole, suggesting that fluconazole resistance can develop and subsequently be transmitted in healthcare settings. The difference in AmB resistance between the northern and central regions might be explained by differences in patient populations and AmB use between hospitals in the northern and central regions. Alternatively, this difference might reflect transmission of a resistant strain that emerged similarly to the fluconazole-resistant strain described above. The CDC recommends AmB deoxycholate as the initial treatment of choice for neonates and infants <2 months of age with C. auris infection. For adults and children >2 months of age, an echinocandin drug is recommended as the initial treatment of choice, and if the patient is clinically unresponsive, switching to liposomal AmB can be considered. CDC recommendations for treatment are found at https://www.cdc.gov/fungal/diseases/candidiasis/c-auris-treatment.html.
AmB resistance in Candida species is rare, and mechanisms of action are poorly understood. The drug is known to bind to the sterol ergosterol in the fungal cell membrane, induce the formation of pores, and cause cell lysis. Recent studies have reported that mutations in genes involved in ergosterol biosynthesis are associated with AmB resistance [17, 18]. However, alternative mechanisms are of interest. We identified 4 novel nonsynonymous mutations significantly associated with AmB resistance, suggesting their involvement in mechanisms of AmB resistance. One mutation, utg5_821828 (C/T), is located in a gene predicted to encode for a transcription factor similar to FLO8. In yeast, FLO8 has been shown to function as a transcriptional activator of genes linked to biofilm formation and has been linked to AmB resistance in C. albicans [19, 20]. As such, a mutation like utg5_821828 (C/T) could affect the transcriptional regulation of downstream targets that ultimately impact the potency of AmB. The other mutation, utg4_968953 (T/C), is located in a gene predicted to encode for a membrane transporter. Whether membrane transporters play a role in mechanisms of AmB resistance has yet to be shown. Future studies that dissect these mutations and other SNPs identified from comparing resistant and susceptible isolates may help uncover mechanisms of AmB resistance in C. auris.
Notes
Acknowledgments. We thank the staff of Hospital Militar Central, Clínica Los Nogales, Fundación Unidad de Cuidados Intensivos Doña Pilar, and Clínica General del Norte for providing assistance during the collection of the samples; members from the Instituto Nacional de Salud (Jorge Díaz, Adriana Gómez, and Norida Vélez) and the Centers for Disease Control and Prevention (CDC) (Cary Hilbert and Matthew Stuckey) for their assistance in collecting data from clinical files and environmental samples in each of the 4 institutions; and José Munoz for bioinformatics support. We also thank the Genome Sequencing Laboratory at the CDC for support in WGS. Finally, we would like to acknowledge the Epidemic Intelligence Service program and acknowledge the Oak Ridge Institute for Science and Education.
Disclaimer. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the CDC.
Financial support. This work was supported by the US CDC and Instituto Nacional de Salud, Colombia.
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
P. E. and N. A. C. contributed equally to this work.
M. B. and T. M. C. contributed equally to this work.




