-
PDF
- Split View
-
Views
-
Cite
Cite
Torgny Sunnerhagen, Amanda Törnell, Maria Vikbrant, Bo Nilson, Magnus Rasmussen, HANDOC: A Handy Score to Determine the Need for Echocardiography in Non-β-Hemolytic Streptococcal Bacteremia, Clinical Infectious Diseases, Volume 66, Issue 5, 1 March 2018, Pages 693–698, https://doi.org/10.1093/cid/cix880
Close - Share Icon Share
Abstract
Non-β-hemolytic streptococci (NBHS) can cause infective endocarditis (IE). Echocardiography is used to diagnose IE, but it is not known which patients with NBHS bacteremia should undergo echocardiography.
Medical records of patients with NBHS bacteremia in southern Sweden from 2012 to 2014 were studied retrospectively. The patients were divided into 2 cohorts. In the first, correlations between the reported data and IE were studied. These variables were used to construct the HANDOC score, which was then validated in the second cohort.
Three hundred thirty-nine patients with NBHS bacteremia were included in the first cohort, of whom 26 fulfilled the criteria for IE. Several factors differed significantly between the patients with IE and those without. Among these variables, the presence of Heart murmur or valve disease; Aetiology with the groups of Streptococcus mutans, Streptococcus bovis, Streptococcus sanguinis, or Streptococcus anginosus; Number of positive blood cultures ≥2; Duration of symptoms of 7 days or more; Only 1 species growing in blood cultures; and Community-acquired infection were chosen to form the HANDOC score. With a cutoff between 2 and 3 points, HANDOC had a sensitivity of 100% and specificity of 73% in the first cohort. When tested in the validation cohort (n = 399), the sensitivity was 100% and the specificity 76%.
HANDOC can be used in to identify patients with NBHS bacteremia who have a risk of IE so low that echocardiography can be omitted; therefore, its implementation might reduce the use of echocardiography.
Infective endocarditis (IE) is a difficult-to-diagnose condition with diverse and unspecific symptomatology [1, 2]. Non-β-hemolytic streptococci (NBHS) have been the dominant cause of IE historically, and are still responsible for a large proportion (13%–44%) of cases [3–8]. Species determination of NBHS has been difficult, but the introduction of matrix-assisted laser desorption/ionization–time of flight mass spectrometry (MALDI-TOF MS) has provided a tool for secure determination, at least to the group level [9–11]. Among NBHS, the Streptococcus mitis group has been reported to be the most common cause of IE; the Streptococcus mutans and Streptococcus bovis groups are less common, although overrepresented in IE compared to all-cause bacteremia; and the Streptococcus salivarius and Streptococcus anginosus groups are underrepresented as causes of IE [8, 12, 13]. Blood cultures and transesophageal echocardiography (TEE) are the cornerstones in the diagnosis of IE [14, 15].
In addition to IE, NBHS are known to cause other types of invasive infections such as abscesses [13, 16, 17], neutropenic fever [18, 19], and bacteremia in neonates [20]. The risk factors for IE in patients with NBHS bacteremia have not been studied systematically, although prior dental surgery has been associated with a higher risk of IE and neutropenia with a lower likelihood of IE [21]. In bacteremia caused by Staphylococcus aureus, persistent bacteremia, community-acquired infection, and the presence of prosthetic valves or cardiac implantable devices are associated with IE [22, 23] and these features have been employed to form scoring systems, such as PREDICT and VIRSTA, which help to determine the need for echocardiography [22, 24]. For enterococcal bacteremia, a scoring system termed NOVA can guide the use of TEE [25], and an adapted form of the NOVA score has been validated [26]. There are no scoring systems available to help clinicians determine whether or not to perform TEE when presented with a patient with NBHS bacteremia. To rectify this, we conducted a retrospective survey to establish the risk factors for IE in patients with NBHS bacteremia and formulate a risk stratification score for IE.
METHODS
Study Design
Two cohorts of patients with NBHS bacteremia were studied retrospectively. A list of blood cultures positive for NBHS from 977 individual, consecutive patients was received from the Department of Clinical Microbiology in Lund, Sweden. The laboratory is the only clinical microbiology laboratory in a geographically defined administrative region, with 1.3 million inhabitants. Patients <18 years of age, those with inaccessible patient charts, or those with neutropenia were excluded. The inclusion was made into 1 of 2 cohorts, the first with patients cultured between 1 January 2012 and 30 June 2013, the second with patients cultured between 1 July 2013 and 31 December 2014. The first group was used to assess general patient characteristics and outcomes, and to generate the scoring system. The second group of patients was used to validate the scoring system. The BacT/Alert blood culture system (bioMérieux, Marcy l’Etoile, France) was used and identification of the bacteria was done to the group level [27] using MALDI-TOF MS (Bruker Daltonics, Bremen, Germany) as described previously [12]. The bacteria were categorized into 7 groups: the Streptococcus anginosus group, the Streptococcus bovis group, the Streptococcus sanguinis group, the Streptococcus mitis group, the Streptococcus mutans group, the Streptococcus salivarius group, and other NBHS [28, 29] (for details see Supplementary Data 2). Streptococcus pneumoniae, though a member of the S. mitis group, was not included in this study. Bacterial isolates that were reported as NBHS without species or group (n = 130) were reassessed with Ultraflextreme MALDI-TOF MS, using the MALDI Biotyper version 3.1 software with MBT Compass Library, DB-6903 MSP (Bruker Daltonics, Bremen, Germany) on stored isolates. A score of ≥2.0 was required for group identification [9, 12].
Assessment of Medical Records
The medical records of the first cohort were reviewed according to a predefined protocol (Supplementary Appendix 1). The procedure was approved by the local committee for research ethics (2013/13). Patients were considered to have IE if they fulfilled the modified Duke criteria [30] or were diagnosed with IE at autopsy. Patients were placed in the negative group if (1) TEE had been performed without signs of IE; (2) if they received <14 days of intravenous antibiotics or 21 days of antibiotics in total and survived for at least 6 months without relapse of bacteremia; or (3) had no signs of IE at autopsy. Patients who did not meet the criteria for the positive or negative group fell into the unknown category.
Validation Cohort
Data from the patients in the validation cohort was gathered after the score was finalized. The number of parameters included was limited to general patient demographics, the variables included in the chosen risk stratification model, and the data necessary to confirm or deny the presence of IE.
Data Analysis
Statistical analysis was performed using SPSS Statistics 24 (IBM) and MedCalc (MedCalc software bvba). Patients with confirmed IE or confirmed absence of IE were compared using Mann-Whitney U test or Fisher exact test. Because the testing was made to generate candidate variables for the odds ratio testing, no correction for multiple testing was done. Univariable odds ratio calculations were performed on variables that were candidates for the scoring systems.
RESULTS
Main Cohort
Between 1 January 2012 and 30 June 2013, blood cultures from 446 patients with growth of NBHS were recorded. After excluding persons <18 years of age (n = 54), neutropenic patients (n = 31) and those where medical records were not accessible (n = 13), 348 patients remained. Nine isolates that had not previously been identified to the species level were excluded as they were not NBHS. When analyzing the remaining patients, 26 cases of IE and 197 cases of non-IE were identified; the remainder were unknown (Figure 1).
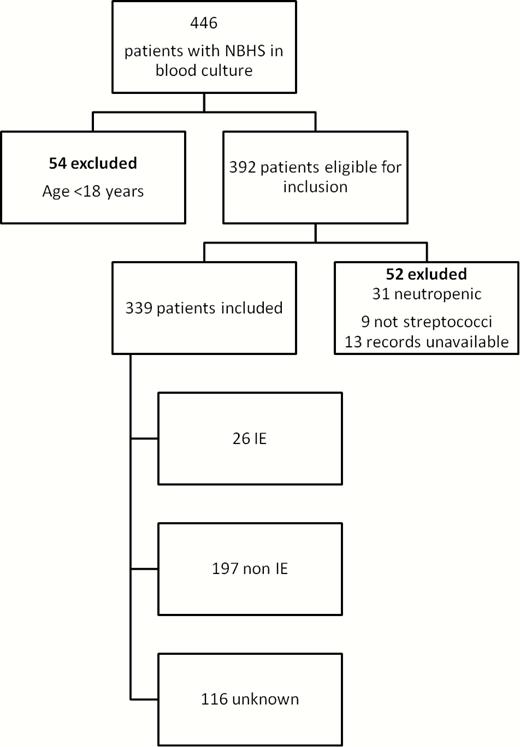
Flowchart of inclusion and exclusion in the first cohort. Abbreviations: IE, infective endocarditis; NBHS, non-β-hemolytic streptococci.
Demographics and Diagnoses
Demographic variables are presented in Table 1. Patients with IE had experienced symptoms for a significantly longer period at the time when the blood culture was taken (P < .0001). Some factors were significantly more common in the group with IE, including community-acquired infection (P = .02), preexisting heart valve disease (P < .001), and heart murmur upon auscultation (P < .001). Embolic events were more common in the IE group, but this difference was not significant (P = .2). The presence of fever was similar in those with confirmed or excluded IE.
| Characteristic . | All Cases (N = 339) . | IE Confirmed (n = 26) . | IE Excluded (n = 197) . | P Value, IE Confirmed vs Excluded . |
|---|---|---|---|---|
| Age, median, y (range) | 74 (20–101) | 65 (24–91) | 73 (20–96) | .1 |
| Sex, male | 190 (56) | 21 (81) | 116 (59) | .03 |
| Charlson score [31], median (range) | 2 (0–11) | 1.5 (0–7) | 2 (0–11) | .8 |
| Community acquired | 145 (43) | 19 (73) | 94 (48) | .02 |
| Healthcare associated | 143 (42) | 4 (15) | 76 (39) | .03 |
| Nosocomial | 51 (15) | 3 (12) | 27 (14) | 1.0 |
| Duration of symptoms, d, median (range) | 1 (0–114) | 16.5 (0–114) | 1 (0–61) | <.001 |
| Previous IE | 3 (1) | 1 (4) | 0 (0) | .1 |
| Pacemaker | 18 (5) | 4 (15) | 10 (5) | .07 |
| Heart valve disease | 46 (14) | 15 (58) | 18 (9) | <.001 |
| Heart murmur | 70 (21) | 16 (62) | 33 (15) | <.001 |
| Fever | 229 (68) | 21 (81) | 145 (74) | .6 |
| Embolization | 8 (2) | 2 (8) | 3 (2) | .05 |
| Characteristic . | All Cases (N = 339) . | IE Confirmed (n = 26) . | IE Excluded (n = 197) . | P Value, IE Confirmed vs Excluded . |
|---|---|---|---|---|
| Age, median, y (range) | 74 (20–101) | 65 (24–91) | 73 (20–96) | .1 |
| Sex, male | 190 (56) | 21 (81) | 116 (59) | .03 |
| Charlson score [31], median (range) | 2 (0–11) | 1.5 (0–7) | 2 (0–11) | .8 |
| Community acquired | 145 (43) | 19 (73) | 94 (48) | .02 |
| Healthcare associated | 143 (42) | 4 (15) | 76 (39) | .03 |
| Nosocomial | 51 (15) | 3 (12) | 27 (14) | 1.0 |
| Duration of symptoms, d, median (range) | 1 (0–114) | 16.5 (0–114) | 1 (0–61) | <.001 |
| Previous IE | 3 (1) | 1 (4) | 0 (0) | .1 |
| Pacemaker | 18 (5) | 4 (15) | 10 (5) | .07 |
| Heart valve disease | 46 (14) | 15 (58) | 18 (9) | <.001 |
| Heart murmur | 70 (21) | 16 (62) | 33 (15) | <.001 |
| Fever | 229 (68) | 21 (81) | 145 (74) | .6 |
| Embolization | 8 (2) | 2 (8) | 3 (2) | .05 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviation: IE, infective endocarditis.
| Characteristic . | All Cases (N = 339) . | IE Confirmed (n = 26) . | IE Excluded (n = 197) . | P Value, IE Confirmed vs Excluded . |
|---|---|---|---|---|
| Age, median, y (range) | 74 (20–101) | 65 (24–91) | 73 (20–96) | .1 |
| Sex, male | 190 (56) | 21 (81) | 116 (59) | .03 |
| Charlson score [31], median (range) | 2 (0–11) | 1.5 (0–7) | 2 (0–11) | .8 |
| Community acquired | 145 (43) | 19 (73) | 94 (48) | .02 |
| Healthcare associated | 143 (42) | 4 (15) | 76 (39) | .03 |
| Nosocomial | 51 (15) | 3 (12) | 27 (14) | 1.0 |
| Duration of symptoms, d, median (range) | 1 (0–114) | 16.5 (0–114) | 1 (0–61) | <.001 |
| Previous IE | 3 (1) | 1 (4) | 0 (0) | .1 |
| Pacemaker | 18 (5) | 4 (15) | 10 (5) | .07 |
| Heart valve disease | 46 (14) | 15 (58) | 18 (9) | <.001 |
| Heart murmur | 70 (21) | 16 (62) | 33 (15) | <.001 |
| Fever | 229 (68) | 21 (81) | 145 (74) | .6 |
| Embolization | 8 (2) | 2 (8) | 3 (2) | .05 |
| Characteristic . | All Cases (N = 339) . | IE Confirmed (n = 26) . | IE Excluded (n = 197) . | P Value, IE Confirmed vs Excluded . |
|---|---|---|---|---|
| Age, median, y (range) | 74 (20–101) | 65 (24–91) | 73 (20–96) | .1 |
| Sex, male | 190 (56) | 21 (81) | 116 (59) | .03 |
| Charlson score [31], median (range) | 2 (0–11) | 1.5 (0–7) | 2 (0–11) | .8 |
| Community acquired | 145 (43) | 19 (73) | 94 (48) | .02 |
| Healthcare associated | 143 (42) | 4 (15) | 76 (39) | .03 |
| Nosocomial | 51 (15) | 3 (12) | 27 (14) | 1.0 |
| Duration of symptoms, d, median (range) | 1 (0–114) | 16.5 (0–114) | 1 (0–61) | <.001 |
| Previous IE | 3 (1) | 1 (4) | 0 (0) | .1 |
| Pacemaker | 18 (5) | 4 (15) | 10 (5) | .07 |
| Heart valve disease | 46 (14) | 15 (58) | 18 (9) | <.001 |
| Heart murmur | 70 (21) | 16 (62) | 33 (15) | <.001 |
| Fever | 229 (68) | 21 (81) | 145 (74) | .6 |
| Embolization | 8 (2) | 2 (8) | 3 (2) | .05 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviation: IE, infective endocarditis.
Microbiology
Table 2 summarizes the microbiological findings. Streptococci of the S. sanguinis group were the most common cause of IE (11 of the 26 confirmed cases), followed by S. bovis group (5 cases), S. mutans group (4 cases), S. mitis group (4 cases), and S. salivarius group (2 cases). No IE cases in this cohort were caused by S. anginosus group isolates. Compared to the non-IE group, S. sanguinis (P = .001), S. bovis (P = .03), and S. mutans (P = .007) group streptococci were overrepresented in the IE group, and S. anginosus group streptococci were underrepresented (P < .001). A detailed account of group and species distribution is given in Supplementary Appendix 3. Having a single bacterial species in the blood culture was more common in the IE group (P < .001). The number of positive blood cultures was higher in the IE group (P < .001), with a median of 2 positive compared with 1 in the non-IE group. The presence of continuous bacteremia was also significantly higher (P = .002) in the group with confirmed IE.
| Streptococcal Species . | Overall (N = 339) . | IE Confirmed (n = 26) . | IE Excluded (n = 197) . | P Value, Difference Between IE Confirmed and Excluded . |
|---|---|---|---|---|
| S. mitis group | 102 (30) | 4 (15) | 61 (31) | .1 |
| S. sanguinis group | 52 (15) | 11 (42) | 28 (14) | .001 |
| S. bovis group | 27 (8) | 5 (19) | 11 (6) | .03 |
| S. anginosus group | 105 (31) | 0 (0) | 64 (33) | <.001 |
| S. mutans group | 9 (3) | 4 (15) | 4 (2) | .007 |
| S. salivarius group | 35 (10) | 2 (8) | 25 (13) | .8 |
| Other NBHS | 19 (6) | 1 (4) | 12 (6) | 1.0 |
| No. of positive cultures, median (range) | 2 (1–8) | 2 (1–7) | 1 (1–8) | <.001 |
| Continous bacteremiaa | 12 (4) | 5 (19) | 5 (3) | .002 |
| Only 1 species in culture | 213 (63) | 25 (96) | 123 (62) | <.001 |
| Streptococcal Species . | Overall (N = 339) . | IE Confirmed (n = 26) . | IE Excluded (n = 197) . | P Value, Difference Between IE Confirmed and Excluded . |
|---|---|---|---|---|
| S. mitis group | 102 (30) | 4 (15) | 61 (31) | .1 |
| S. sanguinis group | 52 (15) | 11 (42) | 28 (14) | .001 |
| S. bovis group | 27 (8) | 5 (19) | 11 (6) | .03 |
| S. anginosus group | 105 (31) | 0 (0) | 64 (33) | <.001 |
| S. mutans group | 9 (3) | 4 (15) | 4 (2) | .007 |
| S. salivarius group | 35 (10) | 2 (8) | 25 (13) | .8 |
| Other NBHS | 19 (6) | 1 (4) | 12 (6) | 1.0 |
| No. of positive cultures, median (range) | 2 (1–8) | 2 (1–7) | 1 (1–8) | <.001 |
| Continous bacteremiaa | 12 (4) | 5 (19) | 5 (3) | .002 |
| Only 1 species in culture | 213 (63) | 25 (96) | 123 (62) | <.001 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: IE, infective endocarditis; NBHS, non-β-hemolytic streptococci.
aContinuous bacteremia was defined as the finding of the same bacterial isolate during the episode at least 1 day after the first culture taken.
| Streptococcal Species . | Overall (N = 339) . | IE Confirmed (n = 26) . | IE Excluded (n = 197) . | P Value, Difference Between IE Confirmed and Excluded . |
|---|---|---|---|---|
| S. mitis group | 102 (30) | 4 (15) | 61 (31) | .1 |
| S. sanguinis group | 52 (15) | 11 (42) | 28 (14) | .001 |
| S. bovis group | 27 (8) | 5 (19) | 11 (6) | .03 |
| S. anginosus group | 105 (31) | 0 (0) | 64 (33) | <.001 |
| S. mutans group | 9 (3) | 4 (15) | 4 (2) | .007 |
| S. salivarius group | 35 (10) | 2 (8) | 25 (13) | .8 |
| Other NBHS | 19 (6) | 1 (4) | 12 (6) | 1.0 |
| No. of positive cultures, median (range) | 2 (1–8) | 2 (1–7) | 1 (1–8) | <.001 |
| Continous bacteremiaa | 12 (4) | 5 (19) | 5 (3) | .002 |
| Only 1 species in culture | 213 (63) | 25 (96) | 123 (62) | <.001 |
| Streptococcal Species . | Overall (N = 339) . | IE Confirmed (n = 26) . | IE Excluded (n = 197) . | P Value, Difference Between IE Confirmed and Excluded . |
|---|---|---|---|---|
| S. mitis group | 102 (30) | 4 (15) | 61 (31) | .1 |
| S. sanguinis group | 52 (15) | 11 (42) | 28 (14) | .001 |
| S. bovis group | 27 (8) | 5 (19) | 11 (6) | .03 |
| S. anginosus group | 105 (31) | 0 (0) | 64 (33) | <.001 |
| S. mutans group | 9 (3) | 4 (15) | 4 (2) | .007 |
| S. salivarius group | 35 (10) | 2 (8) | 25 (13) | .8 |
| Other NBHS | 19 (6) | 1 (4) | 12 (6) | 1.0 |
| No. of positive cultures, median (range) | 2 (1–8) | 2 (1–7) | 1 (1–8) | <.001 |
| Continous bacteremiaa | 12 (4) | 5 (19) | 5 (3) | .002 |
| Only 1 species in culture | 213 (63) | 25 (96) | 123 (62) | <.001 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: IE, infective endocarditis; NBHS, non-β-hemolytic streptococci.
aContinuous bacteremia was defined as the finding of the same bacterial isolate during the episode at least 1 day after the first culture taken.
Management and Outcome
Table 3 shows patient outcome and clinical management. Neither the 30-day all-cause mortality nor the 6-month all-cause mortality differed significantly between cases with confirmed or excluded IE. A higher proportion of patients in the IE group had undergone (TEE) or trans-thoracic echocardiography (TTE).
| Outcome . | All Cases (N = 339) . | IE Confirmed (n = 26) . | IE Excluded (n = 197) . | P Value, IE Confirmed vs Excluded . |
|---|---|---|---|---|
| Death within 30 d | 48 (14) | 1 (4) | 5 (3) | .5 |
| Death within 6 mo | 97 (29) | 4 (15) | 11 (6) | .08 |
| Days hospitalized, median (range) | 9 (0–139) | 21 (0–45) | 8 (0–139) | <.001 |
| Length of antibiotic treatment, median (range) | 13 (0–150) | 28 (0–95) | 12 (0–150) | <.001 |
| TTE performed | 118 (35) | 24 (92) | 70 (36) | <.001 |
| TEE performed | 72 (12) | 22 (85) | 49 (25) | <.001 |
| Outcome . | All Cases (N = 339) . | IE Confirmed (n = 26) . | IE Excluded (n = 197) . | P Value, IE Confirmed vs Excluded . |
|---|---|---|---|---|
| Death within 30 d | 48 (14) | 1 (4) | 5 (3) | .5 |
| Death within 6 mo | 97 (29) | 4 (15) | 11 (6) | .08 |
| Days hospitalized, median (range) | 9 (0–139) | 21 (0–45) | 8 (0–139) | <.001 |
| Length of antibiotic treatment, median (range) | 13 (0–150) | 28 (0–95) | 12 (0–150) | <.001 |
| TTE performed | 118 (35) | 24 (92) | 70 (36) | <.001 |
| TEE performed | 72 (12) | 22 (85) | 49 (25) | <.001 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: IE, infective endocarditis; TEE, trans-esophageal echocardiography; TTE, trans-thoracic echocardiography.
| Outcome . | All Cases (N = 339) . | IE Confirmed (n = 26) . | IE Excluded (n = 197) . | P Value, IE Confirmed vs Excluded . |
|---|---|---|---|---|
| Death within 30 d | 48 (14) | 1 (4) | 5 (3) | .5 |
| Death within 6 mo | 97 (29) | 4 (15) | 11 (6) | .08 |
| Days hospitalized, median (range) | 9 (0–139) | 21 (0–45) | 8 (0–139) | <.001 |
| Length of antibiotic treatment, median (range) | 13 (0–150) | 28 (0–95) | 12 (0–150) | <.001 |
| TTE performed | 118 (35) | 24 (92) | 70 (36) | <.001 |
| TEE performed | 72 (12) | 22 (85) | 49 (25) | <.001 |
| Outcome . | All Cases (N = 339) . | IE Confirmed (n = 26) . | IE Excluded (n = 197) . | P Value, IE Confirmed vs Excluded . |
|---|---|---|---|---|
| Death within 30 d | 48 (14) | 1 (4) | 5 (3) | .5 |
| Death within 6 mo | 97 (29) | 4 (15) | 11 (6) | .08 |
| Days hospitalized, median (range) | 9 (0–139) | 21 (0–45) | 8 (0–139) | <.001 |
| Length of antibiotic treatment, median (range) | 13 (0–150) | 28 (0–95) | 12 (0–150) | <.001 |
| TTE performed | 118 (35) | 24 (92) | 70 (36) | <.001 |
| TEE performed | 72 (12) | 22 (85) | 49 (25) | <.001 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: IE, infective endocarditis; TEE, trans-esophageal echocardiography; TTE, trans-thoracic echocardiography.
Risk Factors for IE and the HANDOC Score
Several factors that differed significantly between the IE and non-IE groups were tested for their suitability in a scoring system. Using such variables, the HANDOC risk score (HANDOC = Heart murmur or valve disease; Aetiology with the groups of Streptococcus mutans, Streptococcus bovis, Streptococcus sanguinis, or Streptococcus anginosus; Number of positive blood cultures ≥2; Duration of symptoms of ≥7 days; Only 1 species growing in blood cultures; and Community-acquired infection) was chosen, with parameters that were common and differed significantly between patients with and without IE. The score is presented in Table 4.
| Variable . | Components of Score . | Univariate Association Odds Ratio for Cases With IE vs IE Excluded (95% CI) . | P Value . |
|---|---|---|---|
| Heart murmur or valvular disease (H) 1 point for the presence of a valvular disease or prosthesis or the finding of a heart murmur. | Heart murmur | 8.0 (3–19) | <.001 |
| Heart valve disease | 14 (5–34) | <.001 | |
| Heart murmur or heart valve disease | 20 (6.6–62) | <.001 | |
| Aetiology (A) 1 point if the species is in the S. bovis, S. sanguinis, or S. mutans group. Subtract 1 point if in S. anginosus group. Other streptococcal groups neither give nor subtract points. | S. bovis group | 4.0 (1.3–13) | .02 |
| S. mutans group | 8.8 (2–38) | .003 | |
| S. anginosus group | 0.039 (.002–.7) | .02 | |
| S. sanguinis group | 4.4 (2–11) | <.001 | |
| S. mitis group | 0.4 (.1–1.2) | .1 | |
| S. salivarius group | 0.6 (.1–2.6) | .5 | |
| Number of cultures (N) 1 point if the number of blood cultures containing NBHS is 2 or more. | 45 (6–340) | <.001 | |
| Duration of symptoms (D) 1 point if the duration of symptoms is 7 days or more | 13 (5–33) | <.001 | |
| Only 1 species (O) 1 point if there is only 1 bacterial species in the blood cultures | 42 (5–310) | <.001 | |
| Community acquired (C) 1 point if the infection is community acquired | 3.0 (1–7) | .02 |
| Variable . | Components of Score . | Univariate Association Odds Ratio for Cases With IE vs IE Excluded (95% CI) . | P Value . |
|---|---|---|---|
| Heart murmur or valvular disease (H) 1 point for the presence of a valvular disease or prosthesis or the finding of a heart murmur. | Heart murmur | 8.0 (3–19) | <.001 |
| Heart valve disease | 14 (5–34) | <.001 | |
| Heart murmur or heart valve disease | 20 (6.6–62) | <.001 | |
| Aetiology (A) 1 point if the species is in the S. bovis, S. sanguinis, or S. mutans group. Subtract 1 point if in S. anginosus group. Other streptococcal groups neither give nor subtract points. | S. bovis group | 4.0 (1.3–13) | .02 |
| S. mutans group | 8.8 (2–38) | .003 | |
| S. anginosus group | 0.039 (.002–.7) | .02 | |
| S. sanguinis group | 4.4 (2–11) | <.001 | |
| S. mitis group | 0.4 (.1–1.2) | .1 | |
| S. salivarius group | 0.6 (.1–2.6) | .5 | |
| Number of cultures (N) 1 point if the number of blood cultures containing NBHS is 2 or more. | 45 (6–340) | <.001 | |
| Duration of symptoms (D) 1 point if the duration of symptoms is 7 days or more | 13 (5–33) | <.001 | |
| Only 1 species (O) 1 point if there is only 1 bacterial species in the blood cultures | 42 (5–310) | <.001 | |
| Community acquired (C) 1 point if the infection is community acquired | 3.0 (1–7) | .02 |
Abbreviations: CI, confidence interval; IE, infective endocarditis; NBHS, non-β-hemolytic streptococci.
| Variable . | Components of Score . | Univariate Association Odds Ratio for Cases With IE vs IE Excluded (95% CI) . | P Value . |
|---|---|---|---|
| Heart murmur or valvular disease (H) 1 point for the presence of a valvular disease or prosthesis or the finding of a heart murmur. | Heart murmur | 8.0 (3–19) | <.001 |
| Heart valve disease | 14 (5–34) | <.001 | |
| Heart murmur or heart valve disease | 20 (6.6–62) | <.001 | |
| Aetiology (A) 1 point if the species is in the S. bovis, S. sanguinis, or S. mutans group. Subtract 1 point if in S. anginosus group. Other streptococcal groups neither give nor subtract points. | S. bovis group | 4.0 (1.3–13) | .02 |
| S. mutans group | 8.8 (2–38) | .003 | |
| S. anginosus group | 0.039 (.002–.7) | .02 | |
| S. sanguinis group | 4.4 (2–11) | <.001 | |
| S. mitis group | 0.4 (.1–1.2) | .1 | |
| S. salivarius group | 0.6 (.1–2.6) | .5 | |
| Number of cultures (N) 1 point if the number of blood cultures containing NBHS is 2 or more. | 45 (6–340) | <.001 | |
| Duration of symptoms (D) 1 point if the duration of symptoms is 7 days or more | 13 (5–33) | <.001 | |
| Only 1 species (O) 1 point if there is only 1 bacterial species in the blood cultures | 42 (5–310) | <.001 | |
| Community acquired (C) 1 point if the infection is community acquired | 3.0 (1–7) | .02 |
| Variable . | Components of Score . | Univariate Association Odds Ratio for Cases With IE vs IE Excluded (95% CI) . | P Value . |
|---|---|---|---|
| Heart murmur or valvular disease (H) 1 point for the presence of a valvular disease or prosthesis or the finding of a heart murmur. | Heart murmur | 8.0 (3–19) | <.001 |
| Heart valve disease | 14 (5–34) | <.001 | |
| Heart murmur or heart valve disease | 20 (6.6–62) | <.001 | |
| Aetiology (A) 1 point if the species is in the S. bovis, S. sanguinis, or S. mutans group. Subtract 1 point if in S. anginosus group. Other streptococcal groups neither give nor subtract points. | S. bovis group | 4.0 (1.3–13) | .02 |
| S. mutans group | 8.8 (2–38) | .003 | |
| S. anginosus group | 0.039 (.002–.7) | .02 | |
| S. sanguinis group | 4.4 (2–11) | <.001 | |
| S. mitis group | 0.4 (.1–1.2) | .1 | |
| S. salivarius group | 0.6 (.1–2.6) | .5 | |
| Number of cultures (N) 1 point if the number of blood cultures containing NBHS is 2 or more. | 45 (6–340) | <.001 | |
| Duration of symptoms (D) 1 point if the duration of symptoms is 7 days or more | 13 (5–33) | <.001 | |
| Only 1 species (O) 1 point if there is only 1 bacterial species in the blood cultures | 42 (5–310) | <.001 | |
| Community acquired (C) 1 point if the infection is community acquired | 3.0 (1–7) | .02 |
Abbreviations: CI, confidence interval; IE, infective endocarditis; NBHS, non-β-hemolytic streptococci.
Figure 2 shows a receiver operating characteristic (ROC) curve of the HANDOC score using the patients with and without IE. The area under the curve is 0.96 (95% confidence interval [CI], .93–.98) using a binomial exact CI. With a sensitivity of 100% (95% CI, 88%–100%) and specificity of 73% (95% CI, 67%–80%), the cutoff was set between 2 and 3 points. There was no significant difference in specificity between men (73%) and women (74%). When the HANDOC score was tested against the whole cohort (including also the unknown category), the performance of the score was similar (75% specificity; area under the ROC curve, 0.96) to that when applied only to IE and non-IE cases. The resulting negative predictive value was 100% and the positive predictive value was 23% with the prevalence of 7.6% as in the main cohort.
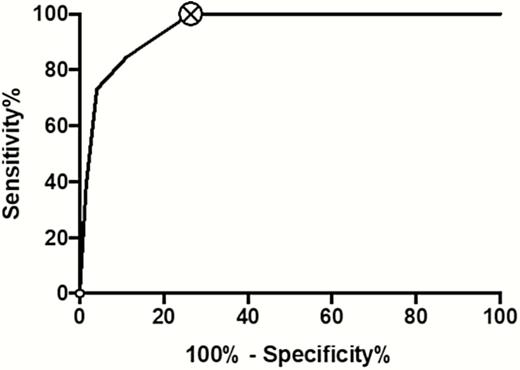
Receiver operating characteristic curve for HANDOC in the first cohort, excluding patients with unknown status.
Validation Cohort
Between 1 July 2013 and 31 December 2014, blood cultures with NBHS from 522 patients were received. The inclusion and exclusion of patients is presented in Supplementary Appendix 3. HANDOC was applied to the patients with (n = 37) and without (n = 264) IE; using a cutoff score of ≥3, the resulting sensitivity was 100% (95% CI, 91%–100%) and the specificity was 76% (95% CI, 71%–81%). When HANDOC was applied to the entire validation cohort, including also the unknown group, 77% of the cases without confirmed IE had a score of ≤2 points.
Consequences of HANDOC on the Need for Echocardiography
Echocardiography was performed on 42% of all patients in the 2 cohorts. Thirty percent of the patients with a HANDOC score of ≤2 and 69% of the patients with a HANDOC score of ≥3 underwent echocardiography. If HANDOC had been used to guide the need for echocardiography, the investigation would have been performed on 31% of the patients.
As a subpopulation analysis, we applied the HANDOC score in patients where echocardiography of any kind was performed; the resulting sensitivity was 100% and the specificity 62%. Including only the cases where TEE was performed, the sensitivity was 100% and the specificity was 47%.
DISCUSSION
In our clinical setting, IE is relatively uncommon (8.5%) in bacteremia with NBHS. However, the suspicion of IE is often raised in this condition and clinicians need tools to determine which patients should undergo echocardiography. We suggest the HANDOC score to guide the use of echocardiography. This score includes parameters that differ significantly between patients with confirmed and excluded IE, are relatively common among patients with IE, and are easily accessible for the clinician. With the established cutoff of 3 points, HANDOC had excellent sensitivity (100%) and good specificity (74%) in the first cohort. Importantly, the specificity was similar for men and women and was also unaffected by inclusion of the group of patients where IE could formally not be ruled out. Thus HANDOC is a well-suited tool for its purpose when applied to the cohort in which it was created. To validate the score, we applied it in the second cohort of patients and found it to be highly sensitive (100%) and specific (76%). Neither the PREDICT score nor the NOVA score was validated in the original publications [22, 25], and it is a major advantage that we could herein confirm the suitability of HANDOC in another cohort of patients. The NOVA score was later validated in a different cohort of patients [26] and the HANDOC score would also benefit from further external validation. The fact that both the score creation and the score validation cohorts consisted of patients from the same geographical area and from the same hospitals is a limitation of the study and makes it difficult to draw definite conclusions about the suitability of the score in other settings.
The “heart murmur or valvular disease” and “aetiology” criteria are similar to those included in the Duke criteria, and the “number of cultures” criterion of HANDOC is included in the Duke criteria. However, “duration of symptoms,” “only 1 species,” and “community acquired” are parameters not part of the Duke criteria. Some features of the Duke criteria were deemed not to be suited for inclusion in our score. Fever was not discriminatory between cases with and without IE, whereas embolization was indicative of IE. Embolization was, however, uncommon, and the retrospective nature of our study made it difficult to reliably determine if embolization was present at the time where HANDOC would have been applied. We thus chose not to include signs of embolization in the score, but the presence of septic emboli should of course alert the clinician to the risk of IE regardless of HANDOC score, and a low HANDOC score should not withhold the use of echocardiography in patients where the clinician has other reasons to suspect IE.
In the univariable analysis, both the presence of heart murmur and underlying heart disease were associated with IE, but we chose to combine these variables as they are strongly interconnected mechanistically and were significantly correlated (P < .0001 using 2-tailed Pearson’s test). A long duration of symptoms is a textbook description of NBHS IE and was found to be highly indicative of IE in our investigation and should clearly be included in a scoring system. Presence of only 1 species is also highly motivated as it is the rule in IE. Community acquisition is a typical feature of IE caused by S. aureus [32] and is part of the PREDICT scoring system [22]. The association of community acquisition also with NBHS IE in our study made it reasonable to include this variable in the score. The retrospective design of the study makes it sensitive to systematic biases. For example, a physician who strongly suspects IE might be more prone to record a long duration of symptoms and more prone to take additional blood cultures. This might increase the likelihood of a recorded long duration of symptoms and of having >2 positive blood cultures. The number of positive cultures and number of cultures taken correlated significantly in our study (P < .0001 with 2-tailed Pearson correlation). We therefore compared the number of positive cultures in a subgroup where 2 cultures had been taken (n = 180). The number of positive blood cultures in this subgroup was significantly higher (P < .0001, Fisher exact test) in the group with IE than in the group where IE had been excluded. The finding of an NBHS in a single flask in a set of blood cultures is by some clinicians regarded as a contamination and no further consideration of the finding is made. We did not find cases of IE with growth of NBHS in only 1 flask but the HANDOC score was ≥3 in only 18 patients with growth in one bottle only, the majority of which had long duration of symptoms, heart murmur on auscultation, or preexisting heart valve disease. In our experience, IE occurs also in patients with a single positive flask and such a finding should not preclude the patient from echocardiography guided by a risk stratification using HANDOC.
A limitation of this study is that the group of patients with IE was too small to allow multivariable analysis. Thus, it may well be possible that the variables associated with IE in our analyses are not truly directly linked to the outcome. Irrespective of causality, however, a model using simple variables, such as HANDOC, might be more robust than sophisticated models using more information [33, 34]. The microbiological variables “aetiology,” “number of cultures,” and “only 1 species” may typically not all be independently associated with IE, but as they are easily accessible and work well in the model, we find it reasonable to include them anyway. After careful consideration, we chose to suggest that an S. anginosus group NBHS should subtract 1 point from the score despite the possibility that this makes the score more complicated to use. We chose to make the analyses on the group of NBHS since the MALDI-TOF MS method is robust for group identification but not necessarily for determination of all species [9]. This conservative approach makes the application of the score easier in other contexts, but it risks missing the possibility that certain species of NBHS might be over- or underrepresented in IE. An interesting finding is the high proportion of IE in cases of bacteremia with S. sanguinis group streptococci, which is in contrast to previous findings by our group showing that S. mitis group streptococci were the most common cause [12]. The reason for this is most probably that the S. sanguinis group previously has been included in the S. mitis group.
We chose to exclude patients with neutropenia due to several lines of argument. NBHS bacteremia in neutropenic patients has been argued to be a very different entity of infection [35], which has led to a widespread notion that such patients are not at risk for IE. This is supported by the fact that IE with NBHS, to our knowledge, has not been reported in patients with neutropenia. Because few of the patients with neutropenia had undergone TEE and most had received long antibiotic treatments, almost all patients with neutropenia would have been classified into the unknown group.
Implementing HANDOC as a guide for when to use echocardiography in NBHS bacteremia would presumably reduce the overall number of investigations and direct the use toward patients with a higher risk of IE. If echocardiography had only been performed in the cases with a HANDOC score of ≥3, the total number of investigations would have been decreased from 307 to 225. In our study, the number needed to screen to find 1 case of IE was 3.6.
In summary, HANDOC is an easy-to-use score to be utilized when a clinician is alerted to a blood culture containing NBHS. Three of the 6 criteria are available directly in the report from the microbiological laboratory, interviewing the patient assesses 2, and the final point is auscultatory or anamnestic. The HANDOC score has an excellent sensitivity and high specificity that should make it useful in clinical practice.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We acknowledge the help of Lena Hyllebusk, Dr Malin Inghammar, Dr Oonagh Shannon, and Dr Susann Ullén for important discussions.
Financial support. This work was supported by the Swedish Government Fund for Clinical Research (ALF); Royal Physiographic Society in Lund; Marianne and Marcus Wallenberg Foundation; Crafoord Foundation; Alfred Österlund Foundation; Anna-Lisa & Sven-Erik Lundgren Foundation; Sigurd & Elsa Golje Foundation; and Längman Foundation.
Potential conflicts of interest. M. R. has received grants from Kungliga Fysiografiska Sällskapet i Lund, Alfred Österlunds Stiftelse, Marianne och Marcus Wallenbergs stiftelse, Crafoordska stiftelsen, and Anna-Lisa och Sven-Erik Lundgrens stiftelse. T. S. has received grants from Stiftelsen Sigurd och Elsa Goljes Minne, Kungliga Fysiografiska Sällskapet i Lund, and Längmanska Kulturfonden. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Meeting for Infectious Diseases Specialists, Karlskrona, Sweden; and 14th International Symposium on Modern Concepts in Endocarditis and Cardiovascular Infections, Dublin, Ireland, June 2017.




