-
PDF
- Split View
-
Views
-
Cite
Cite
Fitsum G Tadesse, Hannah C Slater, Wakweya Chali, Karina Teelen, Kjerstin Lanke, Mulualem Belachew, Temesgen Menberu, Girma Shumie, Getasew Shitaye, Lucy C Okell, Wouter Graumans, Geert-Jan van Gemert, Soriya Kedir, Addisu Tesfaye, Feleke Belachew, Wake Abebe, Hassen Mamo, Robert Sauerwein, Taye Balcha, Abraham Aseffa, Delenasaw Yewhalaw, Endalamaw Gadisa, Chris Drakeley, Teun Bousema, The Relative Contribution of Symptomatic and Asymptomatic Plasmodium vivax and Plasmodium falciparum Infections to the Infectious Reservoir in a Low-Endemic Setting in Ethiopia, Clinical Infectious Diseases, Volume 66, Issue 12, 15 June 2018, Pages 1883–1891, https://doi.org/10.1093/cid/cix1123
Close - Share Icon Share
Abstract
The majority of Plasmodium vivax and Plasmodium falciparum infections in low-endemic settings are asymptomatic. The relative contribution to the infectious reservoir of these infections compared to clinical malaria cases is currently unknown.
We assessed infectivity of passively recruited symptomatic malaria patients (n = 41) and community-recruited asymptomatic individuals with microscopy-detected (n = 41) and polymerase chain reaction (PCR)–detected infections (n = 82) using membrane feeding assays with Anopheles arabiensis mosquitoes in Adama, Ethiopia. Malaria incidence and prevalence data were used to estimate the contributions of these populations to the infectious reservoir.
Overall, 34.9% (29/83) of P. vivax– and 15.1% (8/53) P. falciparum–infected individuals infected ≥1 mosquitoes. Mosquito infection rates were strongly correlated with asexual parasite density for P. vivax (ρ = 0.63; P < .001) but not for P. falciparum (ρ = 0.06; P = .770). Plasmodium vivax symptomatic infections were more infectious to mosquitoes (infecting 46.5% of mosquitoes, 307/660) compared to asymptomatic microscopy-detected (infecting 12.0% of mosquitoes, 80/667; P = .005) and PCR-detected infections (infecting 0.8% of mosquitoes, 6/744; P < .001). Adjusting for population prevalence, symptomatic, asymptomatic microscopy-detected, and PCR-detected infections were responsible for 8.0%, 76.2%, and 15.8% of the infectious reservoir for P. vivax, respectively. For P. falciparum, mosquito infections were sparser and also predominantly from asymptomatic infections.
In this low-endemic setting aiming for malaria elimination, asymptomatic infections were highly prevalent and responsible for the majority of onward mosquito infections. The early identification and treatment of asymptomatic infections might accelerate elimination efforts.
Malaria continues to be a major public health problem, with 212 million cases and 429000 deaths in 2015 [1]. Despite this sobering figure, considerable reductions in incidence occurred over the last decade. In areas with low or declining transmission intensity, infections are commonly present at low parasite densities that may be undetectable by conventional rapid diagnostic tests (RDTs) and microscopy [2]. These infections generally do not elicit symptoms and may persist for several months [3]. Although there is increasing evidence that these asymptomatic infections may have health consequences for the infected host [4], their main importance may lie in sustaining onward malaria transmission.
Malaria transmission depends on the presence of mature gametocytes in the peripheral blood. The production of gametocytes from their asexual progenitors differs between Plasmodium species. In Plasmodium vivax gametocytes, generation begins early during infection, with gametocytes appearing in the bloodstream 2–3 days after the first asexual parasites and typically disappearing within 3 days after asexual infections are cleared [5]. In contrast, mature Plasmodium falciparum gametocytes first appear 10–12 days after asexual parasites and may circulate for several weeks after asexual parasites have been cleared [6]. As a result, gametocyte density is closely associated with asexual parasite density in P. vivax [7], while this association is weaker for P. falciparum [6, 8].
Because of the rapid production of gametocytes and the relatively long period between infection and symptoms [9], many P. vivax infections may be infectious to mosquitoes before clinical presentation at health facilities [10]. There is inconclusive evidence on the infectivity of asymptomatic P. vivax infections [10–12]. By comparison, considerably more data are available on the infectiousness of asymptomatic parasite carriers for P. falciparum. These data almost exclusively come from highly endemic African settings. In those settings, asymptomatic infections, including those undetectable by microscopy or RDT [13], frequently result in onward transmission to mosquitoes, although this has not been directly compared with the transmission from symptomatic infections [14]. Importantly, a study from a low-endemic setting in Asia suggested that symptomatic patients with microscopically detectable gametocytes formed the most important source of P. falciparum mosquito infection [15]. These contrasting findings highlight the necessity to directly assess the relative contribution of symptomatic and asymptomatic infections to onward transmission to mosquitoes. These data are particularly relevant for low-transmission and elimination settings to inform policy on the added value of specifically targeting (low-density) asymptomatic infections [16].
Here, we present the first study to directly quantify the relative contribution to malaria transmission of symptomatic malaria patients and asymptomatic microscopy-detected or polymerase chain reaction (PCR)–detected P. falciparum and P. vivax infections in a low-endemic setting in Ethiopia.
METHODS
Study Area and Population
This study was conducted in Adama district (woreda) in the Oromia Region, approximately 100 kilometers southeast of Addis Ababa. Both P. falciparum and P. vivax are endemic in the district, with transmission peaking following the 2 rainy seasons in September–November (major season) and April–May (short season). Anopheles arabiensis is the dominant vector in Ethiopia [17].
Symptomatic and asymptomatic malaria-infected individuals were recruited simultaneously in October 2016–December 2016 (Figure 1). Self-presenting microscopy-detected P. falciparum and P. vivax malaria patients were passively recruited at the malaria clinic in Adama city. Asymptomatic malaria infections were recruited from the community of the Batu Degaga kebelle, an administrative unit located within Adama woreda (1440–1580 meters). Written informed consent was obtained from all participants and/or parents or guardians. This study received approval from the ethics review boards of Addis Ababa University, Jimma University, Armauer Hansen Research Institute, the National Research Ethics Review Committee, and the London School of Hygiene and Tropical Medicine.
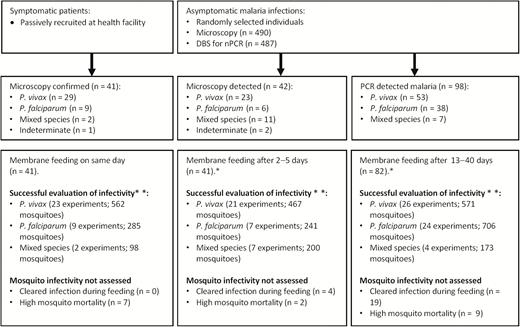
Patient and community recruitment strategies and assessments of infectivity. Mosquito feeding experiments were not done (*) either because participants were not available or did not consent to donate venous blood samples during the moment of feeding (n = 17). Mosquito infectivity experiments were disregarded (**) because of negative quantitative polymerase chain reaction results during feeding (n = 23) or survival of fewer mosquitoes on day 12 post-feeding (n = 18). Abbreviations: DBS, dried blood spot; nPCR, nested polymerase chain reaction; PCR, polymerase chain reaction.
Parasitology
Finger-prick blood samples were collected from clinical patients and during screening of community members. This sample was used for microscopy and to prepare dried blood spots (DBS) [18]. Microscopic investigation was done by 2 expert microscopists, each screening 100 microscopic fields before declaring a slide negative. The18S based nested PCR (nPCR) [19] was done in the field to inform feeding assays (Figure 1). A MagNAPure LC automatic extractor (Roche Applied Science) was used to extract DNA from DBS collected at the moments of screening and membrane feeding for 18S based quantitative PCR (qPCR) and total nucleic acids from 100 µL venous blood samples collected in RNAprotect at the moment of membrane feeding. Quantitative reverse-transcription PCR (qRT-PCR) for gametocytes was performed, targeting Pvs25 and Pfs25 mRNA for female P. vivax and P. falciparum gametocytes, respectively, on DNase-treated material [18] and PfMGET mRNA for male P. falciparum gametocytes without prior DNase treatment [20]. Full details on nucleic acid extraction and molecular assays are provided in the supplemental information (Supplementary Materials 1).
Assessment of Infectivity by Mosquito Membrane Feeding
Symptomatic patients, asymptomatic microscopy-detected, and nPCR-detected community members were invited to participate in feeding assays on the day of diagnosis, within 1–5 and 13–40 days, respectively (Figure 1). Following sampling for feeding experiments and molecular analyses, malaria-infected individuals were treated according to national guidelines [21]. Membrane feeding assays were conducted following an established protocol [22] using 2- to 6-day-old female A. arabiensis mosquitoes that were locally reared at 26 oC–30oC and 60%–80% humidity. The 1- to 2-day-old mosquitoes were transported from the insectary in Sekoru to Adama (approximately 350 km) in humidity- and temperature-maintained containers and allowed to acclimatize for 1 day prior to experiments. Fully fed mosquitoes were provided with glucose for 12 days when they were frozen with desiccant at −80°C. Mosquitoes that fed on qPCR-confirmed parasite-positive blood samples were homogenized by bead-beating and tested for infection using circumsporozoite protein–based enzyme-linked immunosorbent assay (ELISA) followed by confirmation with 18S based qPCR [23].
Analyses
Symptomatic malaria was defined as microscopy-detected malaria (at any density) in the presence of measured fever or reported fever in the last 48 hours. Asymptomatic malaria infections were defined as microscopy- or PCR-detected infections without reported symptoms. These categories were defined at the time of presentation with symptomatic malaria at the clinic or the time point of the community survey when an asymptomatic malaria infection was detected. Parasite densities fluctuated during the time between enrollment and mosquito feeding such that PCR-detected infections became detectable by microscopy at the time of feeding. Since these infections were undetectable by microscopy during the community survey, we nevertheless classified these as asymptomatic PCR-detected infections.
Statistical analyses were performed using STATA 13 (StataCorp., Texas) and Graph Pad Prism 5.0 (Graph Pad Software Inc., California). Mann-Whitney tests and unpaired Student t tests were used for continuous variables. Spearman rank correlation coefficient (ρ) was used for correlations between continuous variables. Proportions were compared using Pearson χ2 test or Fisher exact test. The detectability and contribution to the infectious reservoir of individuals in each of the 3 categories (ie, symptomatic, asymptomatic microscopy-detected, and PCR-detected infections) for both P. falciparum and P. vivax were estimated by calculating the proportion of infected individuals in each infection category that were detectable for a range of diagnostic thresholds [14]. The expected proportion of infected individuals that would be in each category in a cross-section of a population was estimated using incidence data from the district (Supplementary Materials 2) and the prevalence of the asymptomatic categories estimated directly from the data. The proportion of the infectious reservoir attributable to each category and to different parasite densities was calculated as the proportion of the infected population in each category weighted by the relative infectivity to mosquitoes of each category (Supplementary Materials 3) [14]. The detectability of P. vivax infections in relation to copy number (Supplementary Materials 4) and uncertainties in P. vivax clinical incidence estimates (Supplementary Materials 5) were incorporated in these estimates.
RESULTS
During the 3-month study period, 41 individuals reported to the clinic with symptomatic microscopy-confirmed malaria. Of 490 individuals who participated in community surveys, 8.6% (42/490) were malaria parasite positive by microscopy and 98 additional individuals were positive by nPCR (Table 1). The majority of microscopy-detected infections were PCR confirmed (Table 1). Symptomatic patients were on average older than asymptomatically infected individuals detected in community surveys (P < .001; Table 1) and were more likely to be male (P < .001; Table 1).
| Characteristic . | Symptomatic Microscopy-Detected . | Asymptomatic Microscopy-Detected . | Asymptomatic Polymerase Chain Reaction–Detected . | P Value . |
|---|---|---|---|---|
| Female sex, % (n/N) | 12.2 (5/41) | 52.4 (22/42) | 44.9 (44/98) | < .001a |
| Age in years, median (IQR) | 25.5 (20.0–39.0) | 9.5 (5.0–14.0) | 13.0 (7.0–26.0) | < .001b |
| 0–5 years, % (n/N) | 0.0 (0/41) | 28.6 (12/42) | 23.5 (23/98) | |
| 5–15 years, % (n/N) | 7.3 (3/41) | 50.0 (21/42) | 39.8 (39/98) | |
| Above 15 years, % (n/N) | 92.7 (38/41) | 21.4 (9/42) | 36.7 (36/98) | |
| Duration of symptoms, median days (IQR) | 4(3,7) | N/A | N/A | N/A |
| Hemoglobin, g/dL, median (IQR) | 13.4 (8.7–15.0) | 12.8 (12.6–13.5) | 13.5 (12.2–14.3) | .426+ |
| Plasmodium spp. infection screeningc | N/A | |||
| P. vivax, n (%) | 29 | 24 (4.9) | 53 (10.9) | |
| P. falciparum, n (%) | 9 | 8 (1.6) | 38 (7.8) | |
| Mixed species infection, n (%) | 2 | 8 (1.6) | 7 (1.4) | |
| Characteristic . | Symptomatic Microscopy-Detected . | Asymptomatic Microscopy-Detected . | Asymptomatic Polymerase Chain Reaction–Detected . | P Value . |
|---|---|---|---|---|
| Female sex, % (n/N) | 12.2 (5/41) | 52.4 (22/42) | 44.9 (44/98) | < .001a |
| Age in years, median (IQR) | 25.5 (20.0–39.0) | 9.5 (5.0–14.0) | 13.0 (7.0–26.0) | < .001b |
| 0–5 years, % (n/N) | 0.0 (0/41) | 28.6 (12/42) | 23.5 (23/98) | |
| 5–15 years, % (n/N) | 7.3 (3/41) | 50.0 (21/42) | 39.8 (39/98) | |
| Above 15 years, % (n/N) | 92.7 (38/41) | 21.4 (9/42) | 36.7 (36/98) | |
| Duration of symptoms, median days (IQR) | 4(3,7) | N/A | N/A | N/A |
| Hemoglobin, g/dL, median (IQR) | 13.4 (8.7–15.0) | 12.8 (12.6–13.5) | 13.5 (12.2–14.3) | .426+ |
| Plasmodium spp. infection screeningc | N/A | |||
| P. vivax, n (%) | 29 | 24 (4.9) | 53 (10.9) | |
| P. falciparum, n (%) | 9 | 8 (1.6) | 38 (7.8) | |
| Mixed species infection, n (%) | 2 | 8 (1.6) | 7 (1.4) | |
Abbreviations: IQR, interquartile range (25th–75th percentile); N/A, not available.
At the moment of presentation with symptomatic malaria at the clinic and during screening of community-recruited study participants, infection status was determined by microscopy and subsequently confirmed with nested polymerase chain reaction (PCR) and quantitative PCR#; the denominator for the asymptomatic PCR-detected infections was 487 as 3 dried blood spots were missing, while 490 individuals were screened by microscopy; values were compared between microscopy-detected symptomatic patients and community-recruited individuals (asymptomatic microscopy-detected and PCR-detected individuals combined), P values were determined by Fischer exact test* and the Mann-Whitney test+. Species identification by PCR failed in 3 microscopy-positive infections where infections were only confirmed at generic level.
| Characteristic . | Symptomatic Microscopy-Detected . | Asymptomatic Microscopy-Detected . | Asymptomatic Polymerase Chain Reaction–Detected . | P Value . |
|---|---|---|---|---|
| Female sex, % (n/N) | 12.2 (5/41) | 52.4 (22/42) | 44.9 (44/98) | < .001a |
| Age in years, median (IQR) | 25.5 (20.0–39.0) | 9.5 (5.0–14.0) | 13.0 (7.0–26.0) | < .001b |
| 0–5 years, % (n/N) | 0.0 (0/41) | 28.6 (12/42) | 23.5 (23/98) | |
| 5–15 years, % (n/N) | 7.3 (3/41) | 50.0 (21/42) | 39.8 (39/98) | |
| Above 15 years, % (n/N) | 92.7 (38/41) | 21.4 (9/42) | 36.7 (36/98) | |
| Duration of symptoms, median days (IQR) | 4(3,7) | N/A | N/A | N/A |
| Hemoglobin, g/dL, median (IQR) | 13.4 (8.7–15.0) | 12.8 (12.6–13.5) | 13.5 (12.2–14.3) | .426+ |
| Plasmodium spp. infection screeningc | N/A | |||
| P. vivax, n (%) | 29 | 24 (4.9) | 53 (10.9) | |
| P. falciparum, n (%) | 9 | 8 (1.6) | 38 (7.8) | |
| Mixed species infection, n (%) | 2 | 8 (1.6) | 7 (1.4) | |
| Characteristic . | Symptomatic Microscopy-Detected . | Asymptomatic Microscopy-Detected . | Asymptomatic Polymerase Chain Reaction–Detected . | P Value . |
|---|---|---|---|---|
| Female sex, % (n/N) | 12.2 (5/41) | 52.4 (22/42) | 44.9 (44/98) | < .001a |
| Age in years, median (IQR) | 25.5 (20.0–39.0) | 9.5 (5.0–14.0) | 13.0 (7.0–26.0) | < .001b |
| 0–5 years, % (n/N) | 0.0 (0/41) | 28.6 (12/42) | 23.5 (23/98) | |
| 5–15 years, % (n/N) | 7.3 (3/41) | 50.0 (21/42) | 39.8 (39/98) | |
| Above 15 years, % (n/N) | 92.7 (38/41) | 21.4 (9/42) | 36.7 (36/98) | |
| Duration of symptoms, median days (IQR) | 4(3,7) | N/A | N/A | N/A |
| Hemoglobin, g/dL, median (IQR) | 13.4 (8.7–15.0) | 12.8 (12.6–13.5) | 13.5 (12.2–14.3) | .426+ |
| Plasmodium spp. infection screeningc | N/A | |||
| P. vivax, n (%) | 29 | 24 (4.9) | 53 (10.9) | |
| P. falciparum, n (%) | 9 | 8 (1.6) | 38 (7.8) | |
| Mixed species infection, n (%) | 2 | 8 (1.6) | 7 (1.4) | |
Abbreviations: IQR, interquartile range (25th–75th percentile); N/A, not available.
At the moment of presentation with symptomatic malaria at the clinic and during screening of community-recruited study participants, infection status was determined by microscopy and subsequently confirmed with nested polymerase chain reaction (PCR) and quantitative PCR#; the denominator for the asymptomatic PCR-detected infections was 487 as 3 dried blood spots were missing, while 490 individuals were screened by microscopy; values were compared between microscopy-detected symptomatic patients and community-recruited individuals (asymptomatic microscopy-detected and PCR-detected individuals combined), P values were determined by Fischer exact test* and the Mann-Whitney test+. Species identification by PCR failed in 3 microscopy-positive infections where infections were only confirmed at generic level.
Parasite Densities in Clinical and Asymptomatic Infections at Enrollment
Plasmodium vivax 18S copy numbers by qPCR were highest in clinical malaria infections (median, 23139.6 copies/μL; interquartile range [IQR], 12268.9–52479.0; P < .001) followed by microscopy-detected asymptomatic infections (median, 550.1; IQR, 169.5–1821.3) and PCR-detected asymptomatic infections (median, 65.7; IQR, 43.9–184.8; Figure 2A). Similarly, the P. falciparum qPCR parasite density was highest in clinical malaria infections (median, 7190.6 parasites/µL; IQR, 674.5–12721.1) followed by microscopy-detected asymptomatic infections (median, 189.9; IQR, 67.1–380.1; P = .024) and PCR-detected asymptomatic infections (median, 4.0; IQR, 1.7–42.4; P < .001; Figure 2B).
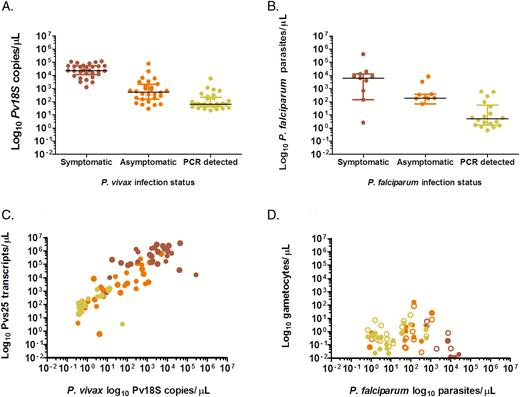
Plasmodium vivax and Plasmodium falciparum parasite and gametocyte densities in symptomatic patients and asymptomatically infected individuals. The y-axes shows the log10 transformed P. vivax 18S copy numbers/µL (A) and P. falciparum parasite densities/µL (B) during the screening surveys at the clinic and in the community with status indicated on the x-axes. Log10 transformed P. vivax Pvs25 transcripts/µL (C) and log10 transformed P. falciparum gametocytes/µL (D) on the y-axes and log10 transformed P. vivax 18S copy numbers/µL (C) and P. falciparum parasite densities/µL (D) during membrane feeding experiments. Data are presented for the 3 defined groups of clinical malaria cases (red circles), asymptomatic microscopy-detected infections (orange circles), and asymptomatic polymerase chain reaction–detected (microscopy negative) infections (yellow circles). In (D) unfilled circles indicate male gametocytes, whereas filled circles indicate female gametocytes. In this figure, each sample provides 2 observations for male and female gametocyte density. In A and B lines refer to the median parasite density and the interquartile ranges. Abbreviation: PCR, polymerase chain reaction.
Gametocyte Carriage and Infectiousness to Mosquitoes
At the time of membrane feeding, female gametocytes were detected by qRT-PCR in 92.8% (77/83) of P. vivax qPCR-positive individuals (Table 2); gametocyte density being positively associated with total parasite density (Figure 2C; ρ = .87; P < .001). Plasmodium falciparum male and/or female gametocytes were detected by qRT-PCR in 56.6% (30/53) of P. falciparum qPCR-positive individuals (Table 2). No association was observed between P. falciparum qPCR parasite density and qRT-PCR gametocyte density at the moment of feeding (Figure 2D; ρ = .02; P = .889).
Relative Infectiousness of Symptomatic and Asymptomatic Plasmodium falciparum-Infected and Plasmodium vivax-Infected Individuals
| Status . | P. vivax infection, % (n/N) . | P. falciparum infection, % (n/N) . | ||||
|---|---|---|---|---|---|---|
| Gametocyte-Positive Individuals . | Infectious Individuals . | Infected Mosquitoes . | Gametocyte-Positive Individuals . | Infectious Individuals . | Infected Mosquitoes . | |
| Symptomatic microscopy detected | 100.0(25/25) | 76.0(19/25) | 46.5(307/660) | 36.4(4/11) | 9.1(1/11) | 0.5(2/383) |
| Asymptomatic microscopy detected | 96.4(27/28) | 32.1(9/28) | 12.0(80/667) | 61.5(8/14) | 28.6(4/14) | 2.3(10/441) |
| Asymptomatic polymerase chain reaction–detected | 83.3(25/30) | 3.3(1/30) | 0.8(6/744) | 64.3(18/28) | 10.7(3/28) | 0.1(1/879) |
| Status . | P. vivax infection, % (n/N) . | P. falciparum infection, % (n/N) . | ||||
|---|---|---|---|---|---|---|
| Gametocyte-Positive Individuals . | Infectious Individuals . | Infected Mosquitoes . | Gametocyte-Positive Individuals . | Infectious Individuals . | Infected Mosquitoes . | |
| Symptomatic microscopy detected | 100.0(25/25) | 76.0(19/25) | 46.5(307/660) | 36.4(4/11) | 9.1(1/11) | 0.5(2/383) |
| Asymptomatic microscopy detected | 96.4(27/28) | 32.1(9/28) | 12.0(80/667) | 61.5(8/14) | 28.6(4/14) | 2.3(10/441) |
| Asymptomatic polymerase chain reaction–detected | 83.3(25/30) | 3.3(1/30) | 0.8(6/744) | 64.3(18/28) | 10.7(3/28) | 0.1(1/879) |
The denominators for the infectious individuals indicate experiments for which samples that were successfully processed for mosquito infection status. Experiments with fewer than 20 surviving mosquitoes on day 12 post-feeding (n = 18) or with quantitive polymerase chain reaction (PCR)–negative participant blood samples at the time of experiments (n = 23) were not processed and do not appear in this table. Infection status was determined using parasitology results at the screening visit (asymptomatic infections) or the time of presentation at the clinic (symptomatic infections). Mixed species infections (2 symptomatic microscopy-detected infections; 7 asymptomatic microscopy-detected infections, and 4 asymptomatic PCR-detected infections) appear in both P. vivax and P. falciparum results. More details are provided in the Supplementary Materials.
Relative Infectiousness of Symptomatic and Asymptomatic Plasmodium falciparum-Infected and Plasmodium vivax-Infected Individuals
| Status . | P. vivax infection, % (n/N) . | P. falciparum infection, % (n/N) . | ||||
|---|---|---|---|---|---|---|
| Gametocyte-Positive Individuals . | Infectious Individuals . | Infected Mosquitoes . | Gametocyte-Positive Individuals . | Infectious Individuals . | Infected Mosquitoes . | |
| Symptomatic microscopy detected | 100.0(25/25) | 76.0(19/25) | 46.5(307/660) | 36.4(4/11) | 9.1(1/11) | 0.5(2/383) |
| Asymptomatic microscopy detected | 96.4(27/28) | 32.1(9/28) | 12.0(80/667) | 61.5(8/14) | 28.6(4/14) | 2.3(10/441) |
| Asymptomatic polymerase chain reaction–detected | 83.3(25/30) | 3.3(1/30) | 0.8(6/744) | 64.3(18/28) | 10.7(3/28) | 0.1(1/879) |
| Status . | P. vivax infection, % (n/N) . | P. falciparum infection, % (n/N) . | ||||
|---|---|---|---|---|---|---|
| Gametocyte-Positive Individuals . | Infectious Individuals . | Infected Mosquitoes . | Gametocyte-Positive Individuals . | Infectious Individuals . | Infected Mosquitoes . | |
| Symptomatic microscopy detected | 100.0(25/25) | 76.0(19/25) | 46.5(307/660) | 36.4(4/11) | 9.1(1/11) | 0.5(2/383) |
| Asymptomatic microscopy detected | 96.4(27/28) | 32.1(9/28) | 12.0(80/667) | 61.5(8/14) | 28.6(4/14) | 2.3(10/441) |
| Asymptomatic polymerase chain reaction–detected | 83.3(25/30) | 3.3(1/30) | 0.8(6/744) | 64.3(18/28) | 10.7(3/28) | 0.1(1/879) |
The denominators for the infectious individuals indicate experiments for which samples that were successfully processed for mosquito infection status. Experiments with fewer than 20 surviving mosquitoes on day 12 post-feeding (n = 18) or with quantitive polymerase chain reaction (PCR)–negative participant blood samples at the time of experiments (n = 23) were not processed and do not appear in this table. Infection status was determined using parasitology results at the screening visit (asymptomatic infections) or the time of presentation at the clinic (symptomatic infections). Mixed species infections (2 symptomatic microscopy-detected infections; 7 asymptomatic microscopy-detected infections, and 4 asymptomatic PCR-detected infections) appear in both P. vivax and P. falciparum results. More details are provided in the Supplementary Materials.
In 164 membrane-feeding experiments, 8936 mosquitoes were successfully fed (median of 56 per experiment; IQR, 39–66). Of the mosquitoes that survived until day 12 post-feeding (median survivorship, 67.4%; IQR, 47.2%–80.5%), a minimum of 20 mosquitoes were examined per experiment on qPCR-positive individuals (123 experiments). The number of successful membrane-feeding experiments (Figure 1) is lower than the original study population in Table 1 because some individuals were qPCR negative at the time of membrane feeding (n = 23), others did not consent to donate venous blood during feeding (n = 17), or there were <20 surviving mosquitoes (n = 18).
Of individuals infected with P. vivax, 34.9% (29/83) infected ≥1 mosquito and 19.0% (393/2071) of all mosquitoes became infected. Infectious individuals (ie, individuals who infected at least 1 mosquito) infected a mean of 49.0% (range, 5%–96%) of mosquitoes (Table 2, Supplementary Table S1 and Table S2). Strong positive associations were observed between the proportion of infected mosquitoes and P. vivax parasite (ρ = .63; P < .001) and gametocyte (ρ = .72; P < .001) densities (Figure 3A and 3B; Supplementary Figure S1). Of P. falciparum–infected individuals, 15.1% (8/53) infected ≥1 mosquitoes, with 0.8% (13/1703) of all mosquitoes becoming infected. Infectious individuals infected a mean of 7.8% (range, 1.7%–29.4%) mosquitoes (Table 2, Supplementary Table S1 and Table S3). While there was no difference in P. falciparum asexual parasite densities between infectious and noninfectious individuals, infectiousness was positively associated with both female (ρ = .42, P = .024) and male (ρ = .42; P = .044) gametocyte densities (Figure 3C and 3D; Supplementary Figure S2). No significant difference was observed in the duration of symptoms and hemoglobin level between infectious and noninfectious groups for both species (Table 1). A large fraction of infectious individuals for both P. vivax (72.4%; 21/29) and P. falciparum (50.0%; 4/8) were aged >15 years.
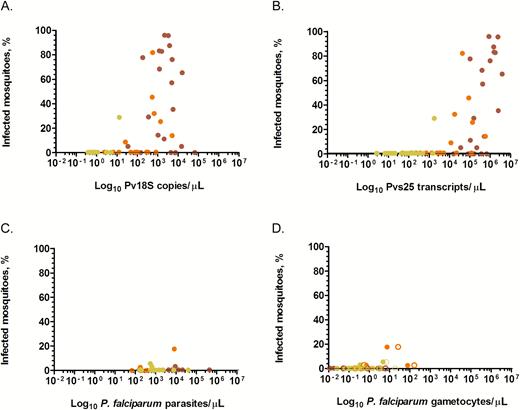
Percentage of infected mosquitoes in relation to parasite (A and C) and gametocyte densities (B and D). Shown on the y-axes are percentages of infected mosquitoes. Presented on the x-axes are log10 transformed copy numbers/µL of the 18S rRNA gene (A) and Pvs25 transcripts/µL (B) of Plasmodium vivax parasites and Plasmodium falciparum parasites/µL (C), P. falciparum female (filled circles) and male (unfilled circles) gametocytes/µL (D) measured on samples collected at the moment of feeding. D, Each sample contributes 2 observations, that is, for male and female gametocyte density. Red circles are microscopy-detected symptomatic infections; orange circles are asymptomatic microscopy-detected infections; and yellow circles are polymerase chain reaction–detected asymptomatic infections.
Relative Contribution to the Infectious Reservoir
Parasite prevalence and density fluctuated in the time period between the initial community screening and membrane feeding (Supplementary Materials 6). Because the initial community screening best reflects the detectability of infections during single-round screening efforts, the classification of infections at screening (Table 1) was maintained in all analyses. These recruitment parasite densities differed among P. vivax clinical malaria cases, asymptomatic microscopy-detected infections, and PCR-detected infections (Figure 4A). The probability of detection by microscopy as a function of the P. vivax copy number (Supplementary Materials 4) indicated that infections with ≥584 copies/μL had 80% probability of detection by microscopy. Three detection thresholds were used in the analysis shown in Figure 4B–4D (97, 584, and 4925 copies/μL, corresponding to 50%, 80%, and 95% probability of detection by microscopy). The estimated number of clinical malaria cases per 1000 person-years was 27.6 for P. vivax and 28.8 for P. falciparum (Supplementary Materials 2 and 5). Accordingly, clinical P. vivax cases and asymptomatic microscopy-detected or PCR-detected infections were estimated to be responsible for 8.0%, 76.2%, and 15.8% of the infectious reservoir, respectively (Figure 4D). A diagnostic with a sensitivity of 584 copies/μL would detect 90.7% of the infectious reservoir (Figure 4D). If we assume the prevalence of asymptomatic microscopy-detected and PCR-detected infections to be at the lower end of the 95% confidence intervals around their estimated prevalence and assume a higher clinical incidence (Supplementary Materials 5), clinical P. vivax cases and asymptomatic microscopy-detected and PCR-detected infections would be responsible for 30.4%, 56.0%, and 13.6% of the infectious reservoir, respectively. For P. falciparum, fewer mosquito infections were observed and estimates were less precise. Based on the available data, clinical cases and asymptomatic microscopy- or PCR-detected infections were estimated to be responsible for 0.8%, 69.5%, and 29.7% of the infectious reservoir for P. falciparum, respectively (Supplementary Figure S3).
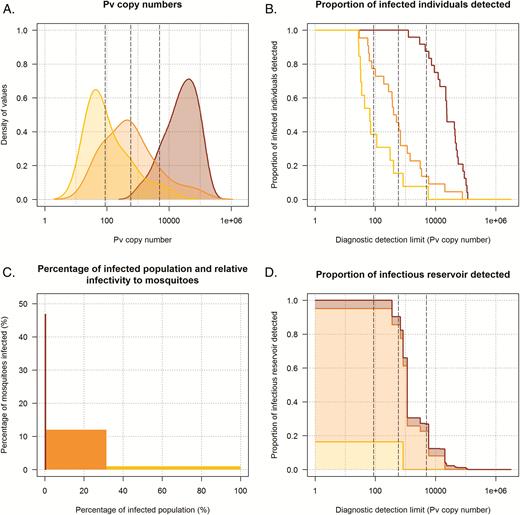
Contribution of symptomatic patients and asymptomatically infected individuals to the infectious reservoir and the detectability of Plasmodium vivax infections. In all panels, dark red, orange, and yellow indicate individuals with clinical malaria cases, asymptomatic microscopy-detected infections, and asymptomatic polymerase chain reaction (PCR)–detected infections respectively. A, Smoothed histograms showing the P. vivax 18S copy numbers/µL of individuals in each category. B, The proportion of individuals in each category that would be detected for diagnostics with different detection limits based on P. vivax 18S copy numbers/µL (x-axis). The 3 vertical dashed gray lines indicate the P. vivax copy number associated with a 50%, 80%, and 95% probability (left to right) of detection by microscopy. These correspond to values of 87, 584, and 4925 copies/µL (left to right) and were obtained using a logistic regression model with detectability by microscopy as the dependent variable and P. vivax 18S copy numbers/µL as the independent variable (this also applies to the 3 vertical lines in 4B and 4D). Details of the regression model are provided in the Supplementary Materials 4. The proportion of each histogram that is to the right of these lines indicates the fraction of individuals in this category that is detected by a diagnostic with this sensitivity. C, The proportion of the infected population in each category (x-axis) and the infectiousness to mosquitoes of each category (y-axis). D, The contribution to the infectious reservoir of individuals in each category in relation to P. vivax 18S copy numbers/µL. Clinical cases, asymptomatic microscopy-detected, and PCR-detected infections are responsible for 8.0%, 76.2%, and 15.8% of the infectious reservoir, respectively. At different diagnostic detection limits, different fractions of the total infectious reservoir are detected; for example, with a limit of detection of 584 copies/µL, 90.7% of the infectious reservoir is detected. Details on the calculations for D are presented in the Supplementary Materials 3.
DISCUSSION
Here, we directly quantified the relative contribution to the infectious reservoir of clinical malaria cases and microscopy-detected and PCR-detected asymptomatic P. vivax and P. falciparum malaria-infected individuals. While symptomatic P. vivax patients were highly infectious, their contribution to the infectious reservoir was limited as a consequence of the much larger population of asymptomatically infected individuals. Compared to P. vivax, a smaller fraction of P. falciparum-infected individuals were infectious to mosquitoes; P. falciparum mosquito infections were predominantly observed from asymptomatically infected individuals.
The majority of malaria infections that are detected across endemic settings are not associated with clinical symptoms that elicit treatment-seeking behavior [2]. The contribution of these asymptomatic infections to transmission is a matter of current debate [24]. In P. vivax, the level of parasitemia was strongly correlated with gametocyte density [7, 25] and, as a consequence, the probability of mosquito infection [10, 26]. Symptomatic P. vivax malaria cases with high parasite densities were highly infectious in our study (46.5% of mosquitoes infected). We observed a sharp increase in the proportion of mosquito infections at microscopically detected parasite densities, which is in agreement with a recent study from Thailand [10]. In line with that study, PCR-detected infections were unlikely to infect mosquitoes; only 1 individual who was microscopy negative but PCR positive during screening was infectious to mosquitoes (3% of mosquitoes infected). That single infection was microscopically detectable at the time of mosquito feeding, reflecting temporal fluctuation in parasite densities that impacts on the likelihood that infections are detected by different diagnostics. Because the infection was missed during the community survey, the individual was nevertheless classified as a PCR-detected asymptomatic infection. Importantly, we observed that mosquito infections were common from asymptomatic microscopy-detected infections (12% of mosquitoes infected). The relative infectiousness of these individuals increases when their relative prevalence in the population is considered. The prevalence of symptomatic infections among the total population was relatively low in the study area based on national and local malaria incidence data [27]. An estimated 0.1%–3.9% of all P. vivax–infected individuals were found to be symptomatic at any given time during the peak transmission season, making the contribution of the symptomatic group to the overall infectious reservoir relatively small (8.0% as best estimate from the study data; 30.4% as upper estimate from a simple sensitivity analysis). In contrast with the study by Kiattibutr et al [10], where all asymptomatic infections had parasite densities below the microscopic threshold for detection and contributed very little to transmission, 8.6% of the population was microscopy parasite positive in our survey and 20.1% by nPCR. These 2 categories of asymptomatically infected individuals were responsible for approximately 76.2% (microscopy detected) and 15.8% (PCR detected) of the total infectious reservoir.
Mosquito infection rates were generally low for P. falciparum and, similar to P. vivax, clinical malaria cases had a modest contribution to mosquito infections. The low infectivity of P. falciparum infections is most likely related to the low parasite and gametocyte densities in our population. Similar to our findings, only 10.1% of qPCR-positive individuals in a high-endemic site in Burkina Faso and 2.9% in a low-endemic site in coastal Kenya were infectious to mosquitoes, and the proportion of infected mosquitoes increased rapidly at densities >10 female gametocytes/µL [28]. A study in Cambodia concluded that most mosquito infections are caused by high-density gametocyte carriers and that asymptomatic low-density infections may be less relevant for transmission [15]. Our findings, although based on a small number of infectious P. falciparum–infected individuals, are in line with other findings from African settings that indicate that a nonnegligible fraction of mosquito infections arise from asymptomatic infections, including asymptomatic PCR-detected infections [29]. Competency of vectors, Anopheles dirus in the Cambodia study and A. arabiensis in our study, might provide an explanation for the observed differences and is highly relevant in estimating the infectivity of low-density infections in different geographical settings [30].
Although our study was conducted at 1 site and during 1 season and few P. falciparum infections resulted in onward transmission to mosquitoes, our findings are of relevance for malaria control and elimination initiatives. Asymptomatic infections formed an important source of mosquito infections in this low-endemic setting. It is conceivable that efforts that identify and target these infections would accelerate malaria elimination efforts [31]. It is unclear whether these asymptomatic infections initially elicited symptoms that would have allowed their detection by enhanced case management. Although this study was not powered to assess temporal dynamics of detectability and parasite densities, our repeated assessments of parasite densities reaffirm that the detectability of infections fluctuates over time. Understanding the dynamics of (asymptomatic) infections in relation to parasite densities, relapses for P. vivax, gametocyte production and infectivity is needed to add a temporal element to the contribution of these individuals to the infectious reservoir. A large proportion of chronic infections that are transmissible over several weeks or months would add weight to suggestions that asymptomatic infections need to be targeted in some transmission settings to achieve malaria elimination, and more sensitive diagnostics are require to do this.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the study participants for their willingness to participate in the study and the Adama woreda health office. The roles of the microscopists at Adama Malaria Clinic (Tsehay Orlando and Tewabech Lemma), mosquito rearing staff of the Tropical and Infectious Diseases Research Center of Jimma University at Sekoru, and community mobilizers at Batu Degaga kebelle were substantial. The following reagent was obtained through BEI Resources, National Institute of Allergy and Infectious Diseases, National Institutes of Health: monoclonal antibody sporozoite ELISA kit, anti-Plasmodium vivax circumsporozoite proteins, MRA-1028K, contributed by Robert A. Wirtz.
Financial support. This work was supported by grants from the Netherlands Organization for Scientific Research (Vidi fellowship NWO 016.158.306) and fellowship from the European Research Council (ERC-2014-StG 639776 to T. B.), the Bill & Melinda Gates Foundation (AFIRM; OPP1034789 to T. B., K. L., and C. D.), the Netherlands Organization for International Cooperation in Higher Education (Nuffic; NFP-PhD.14/150 to F. G. T.), and the Armauer Hansen Research Institute (via its core funding from Norwegian Agency for Development Cooperation and Swedish International Development Cooperation).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




