-
PDF
- Split View
-
Views
-
Cite
Cite
Luke C Kingry, Melissa Anacker, Bobbi Pritt, Jenna Bjork, Laurel Respicio-Kingry, Gongping Liu, Sarah Sheldon, David Boxrud, Anna Strain, Stephanie Oatman, Jon Berry, Lynne Sloan, Paul Mead, David Neitzel, Kiersten J Kugeler, Jeannine M Petersen, Surveillance for and Discovery of Borrelia Species in US Patients Suspected of Tickborne Illness, Clinical Infectious Diseases, Volume 66, Issue 12, 15 June 2018, Pages 1864–1871, https://doi.org/10.1093/cid/cix1107
Close - Share Icon Share
Abstract
Tick-transmitted Borrelia fall into 2 heterogeneous bacterial complexes comprised of multiple species, the relapsing fever (RF) group and the Borrelia burgdorferi sensu lato group, which are the causative agents of Lyme borreliosis (LB), the most common tickborne disease in the Northern Hemisphere. Geographic expansion of LB in the United States and discovery of emerging Borrelia pathogens underscores the importance of surveillance for disease-causing Borrelia.
De-identified clinical specimens, submitted by providers throughout the United States, for patients suspected of LB, anaplasmosis, ehrlichiosis, or babesiosis were screened using a Borrelia genus-level TaqMan polymerase chain reaction (PCR). Borrelia species and sequence types (STs) were characterized by multilocus sequence typing (MLST) utilizing next-generation sequencing.
Among 7292 specimens tested, 5 Borrelia species were identified: 2 causing LB, B. burgdorferi (n = 25) and B. mayonii (n = 9), and 3 RF borreliae, B. hermsii (n = 1), B. miyamotoi (n = 8), and Candidatus B. johnsonii (n = 1), a species previously detected only in the bat tick, Carios kelleyi. ST diversity was greatest for B. burgdorferi–positive specimens, with new STs identified primarily among synovial fluids.
These results demonstrate that broad PCR screening followed by MLST is a powerful surveillance tool for uncovering the spectrum of disease-causing Borrelia species, understanding their geographic distribution, and investigating the correlation between B. burgdorferi STs and joint involvement. Detection of Candidatus B. johnsonii in a patient with suspected tickborne disease suggests this species may be a previously undetected cause of illness in humans exposed to bat ticks.
The Borrelia genus encompasses at least 50 known species of spirochetes, only a subset of which are known to cause human illness [1, 2]. Species are generally divided into 2 major complexes, Borrelia burgdorferi sensu lato (Bbsl) and relapsing fever (RF), comprised of 21 and 29 different species, respectively. The Bbsl complex includes the causative agents of Lyme borreliosis (LB), the most common tickborne illness in the Northern Hemisphere, which are transmitted through the bite of infected Ixodes species hard ticks [3, 4]. The second complex includes the etiologic agents of tickborne and louseborne RF [1, 5]. Tickborne RF is most often associated with transmission by infected soft (argasid) ticks; however, the emerging pathogen, Borrelia miyamotoi, is transmitted by Ixodes ticks.
In the United States, the geographic distribution of human cases of LB and RF is complex and linked to the range of key tick vectors and varied ecological factors [2, 6]. For example, in the Upper Midwest, 3 human disease–causing Borrelia species are transmitted by Ixodes scapularis. These include the LB-causing spirochetes B. burgdorferi sensu stricto (hereafter called B. burgdorferi) and Borrelia mayonii, as well as the RF spirochete B. miyamotoi [7, 8]. In the Northeast and mid-Atlantic, B. burgdorferi and B. miyamotoi, but not B. mayonii, also cause human illness [9]. In mountainous regions of western states, the RF spirochete Borrelia hermsii, transmitted by the bite of the soft tick Ornithodoros hermsi, causes human disease [6]. Adding to this complexity, ticks can carry multiple Borrelia species that have not been associated with human illness [10].
Clinical presentations of LB and RF include a range of overlapping symptoms early in infection, with more distinct symptoms at later stages of disease [3, 6, 8, 11]. Early LB is typically characterized by localized skin infection resulting in an erythema migrans rash, frequently accompanied by fatigue, fever, and headache. Without early treatment, spirochetes can disseminate to secondary sites, causing neurologic effects, cardiac abnormalities, or arthritis. Arthritis is more commonly associated with B. burgdorferi infection, whereas LB caused by B. mayonii has been associated with higher spirochetemia [8, 12]. Manifestations of RF consist of fever, fatigue, headache, chills, myalgia, arthralgia, and nausea, with rash rarely reported. Recurring febrile episodes can occur as untreated illness advances, particularly for B. hermsii, but appears to be less frequent in B. miyamotoi infection [6, 11, 13].
In patients infected with B. burgdorferi, the most common cause of LB in the United States, the number of spirochetes/genomic copies in blood is low, estimated at only 0.1/102–103/mL [14, 15], limiting sensitivity of direct detection methods. Laboratory diagnosis therefore relies primarily on serologic assays to detect the patient’s immune response to the infection [16], which is useful for diagnosis, but precludes surveillance for Bbsl genospecies and strain types causing human illness. In the case of the 2 emerging Borrelia species, B. mayonii and B. miyamotoi, higher numbers of spirochetes and/or genomic copies have been reported in infected patients, at 105–106/mL and 104–105/mL of blood, respectively [8, 9]. Concentrations of B. hermsii spirochetes in patient blood are estimated at >106/mL [6]. As spirochete levels for many Borrelia infections are directly detectable, a broad molecular approach (genus-level polymerase chain reaction [PCR] followed by next-generation multilocus sequence typing [MLST]) was utilized to screen clinical specimens from patients suspected of tickborne illness in order to expand knowledge of Borrelia species and strain types associated with human disease in the United States and the geographic regions where these infections occur.
MATERIALS AND METHODS
Specimen Collection
Residual clinical specimens (whole blood [ethylenediaminetetraacetic acid], synovial fluid, cerebrospinal fluid [CSF], and tissue) submitted to Mayo Clinic from healthcare providers nationwide for patients suspected of having a tickborne illness (ie, testing by either tickborne pathogen PCR panel [Babesia, Ehrlichia/Anaplasma] or Lyme [Borrelia burgdorferi sensu lato] PCR) and the accompanying nucleic acid extract were stored at 4°C or –70°C, de-identified, and shipped to the Minnesota Department of Health (MDH). Synovial fluid, CSF, and tissue specimens were originally submitted for Lyme PCR, whereas blood specimens were submitted for either the tickborne pathogen PCR panel or Lyme PCR. Aliquots of each clinical specimen were prepared, frozen at –70°C, and shipped to the Centers for Disease Control and Prevention (CDC), Fort Collins, Colorado. Associated patient information included specimen type, originating state of the ordering provider, patient age, and sex. As travel history of patients was not available, the state of the ordering provider does not necessarily correlate to the patient’s state of residence or exposure. Analysis of de-identified specimens was approved by the Institutional Review Board at Mayo Clinic (Protocol ID: 14-001148). Review at MDH and CDC determined the protocol to be non–human subjects research.
Polymerase Chain Reaction
DNA was extracted at Mayo Clinic using the MagNA Pure 2.0 Instrument (Roche Diagnostics, Indianapolis, Indiana). Residual DNA (2.5 μL) was tested at MDH using a 16S ribosomal RNA (rRNA) pan-Borrelia TaqMan PCR assay. At CDC, 16S rRNA pan-Borrelia results were confirmed by testing DNA independently extracted from aliquots of the Borrelia-positive specimens using the MagNA Pure 96 (Roche).
The pan-Borrelia TaqMan assay was modified from Parola et al [17]. Degenerate nucleotides were incorporated into the forward primer and probe sequences, based on alignment of 19 Bbsl and RF Borrelia species. Primer and probe sequences are as follows: forward: 5ʹ-AGCYTTTAAAGCTTCGCTTGTAG-3ʹ, reverse: 5ʹ-GCCTCCCGTAGGAGTCTG G-3ʹ, probe: 5ʹ-FAM-CCGGCCTGAGA GGGTGAWCGG-BHQ-3. Optimized final PCR concentrations included 1X PerfeCTa FastMixII or quantitative PCR ToughMix (Quanta Biosciences, Beverly, Massachusetts), 600 nM of each primer, and 200 nM of probe. Real-time PCR (ViiA 7 [Thermo Fisher] or 7500 Fast [Applied Biosystems]) cycling conditions were 95°C for 5 minutes, followed by 40–45 cycles at 95°C for 15 seconds, and 60°C for 30 seconds. Sensitivity and specificity were analyzed as described (Supplementary Materials).
Amplicon Sequencing
Portions of the 16S rRNA, flaB, glpQ, uvrA, rplB, recG, pyrG, pepX, clpX, nifS, and clpA genes were amplified using previously described primers and cycling conditions [18–20] (http://pubmlst.org/borrelia and Premix Ex Taq hot start master mix (Takara Bio USA, Mountain View, California) (Supplementary Materials). DNA concentration of PCR products was measured using the Qubit fluorimeter (ThermoFisher, Grand Island, New York) and normalized to 0.2 ng/µL. Multiplexed libraries were prepared using the Nextera XT DNA library preparation kit (Illumina, San Diego, California) per the manufacturer’s protocol with unique Nextera indexes (Illumina) added to all amplicons from the same specimen. Sequencing was performed on the MiSeq platform (Illumina) using the V2 300 cycle reagent kit (Illumina).
Sequence Analysis
Amplicon sequence reads were de-multiplexed and adapter sequences removed, followed by import into CLC Genomics Workbench 8.0 (Qiagen, Valencia, California). Sequence reads were mapped to 8 reference housekeeping genes, clpA, clpX, nifS, pepX, pyrG, recG, rplB, and uvrA from B. burgdorferi B31, B. mayonii MN14-1420, and B. miyamotoi CT13-2396 using default parameters [21–23]. Sequence reads for 16S ribosomal DNA (rDNA), flaB, and glpQ from sample 15–3581 were de novo assembled.
Consensus sequences for the 8 housekeeping genes were trimmed to lengths present in the Borrelia PubMLST database (https://pubmlst.org/borrelia/), concatenated in frame in the order clpA, clpX, nifS, pepX, pyrG, recG, rplB, and uvrA using Lasergene 12 (DNASTAR, Madison, Wisconsin). Consensus sequences for glpQ, flaB, and 16S rDNA were concatenated. Concatenated sequences were imported into MEGA 6 and aligned (ClustalW), and phylogenetic trees were constructed by maximum likelihood analysis using the generalized time-reversible nucleotide substitution model with gamma distribution (4 categories) followed by bootstrap analysis (1000 replicates). Pairwise genetic distances were calculated using the Kimura-2 model and Bbsl species identified using the threshold (98.3% similarity, genetic distance 0.017). Sequences for Borrelia species included in analyses were obtained from http://pubmlst.org/borrelia/, and National Center for Biotechnology Information iTOL version 3 was used for circularization of phylogenetic trees. Alleles and STs were assigned using http://pubmlst.org/borrelia/.
RESULTS
PCR Detection of Borrelia Species in Specimens From Patients Suspected of Tickborne Illness
Residual extracted DNA and originating specimens for 7292 de-identified clinical specimens from patients suspected of LB, anaplasmosis, ehrlichiosis, or babesiosis were available for testing. Specimens were submitted by providers in 47 states and the District of Columbia (Figure 1; Supplementary Table 1) and were collected between 1 May 2014 and 15 November 2014 and between 1 May 2015 and 11 December 2015. The majority (84%) of specimens were blood, followed by CSF (11.8%), synovial fluid (4.1%), and tissue (0.1%).
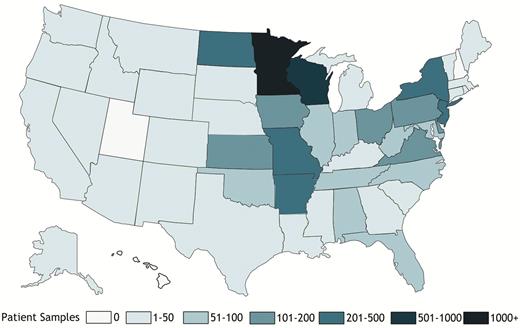
Origin of 7292 clinical specimens from patients suspected of tickborne illness tested in this study. Differential shading indicates the number of patient specimens originating from each state.
Of the 7292 residual DNA specimens tested by Borrelia genus TaqMan PCR at the MDH, 44 were positive (31 bloods, 12 synovial fluids, and 1 CSF). Independent DNA extraction and PCR testing at CDC confirmed all 44 specimens as Borrelia positive.
MLST of Borrelia Species in Clinical Specimens
Eight housekeeping genes (clpA, clpX, nifS, pepX, pyrG, recG, rplB, and uvrA) were amplified from 43 of the 44 Borrelia PCR–positive specimens. For 1 specimen, amplicons were produced for 6 of the 8 housekeeping genes, likely due to a low level of spirochetal DNA. Sequence analysis of the 6 genes amplified confirmed the species as B. burgdorferi. Analysis of 8 concatenated housekeeping gene sequences (4791 nucleotides) for the remaining 43 Borrelia-positive specimens identified 5 species falling into the 2 groups, LB or RF (Figure 2). These included 2 LB pathogens, B. burgdorferi (n = 24; ≥99.2% identity to B. burgdorferi B31) and B. mayonii (n = 9; >99.97% identity to B. mayonii MN14-1420), and 3 different RF Borrelia, B. miyamotoi (n = 8; 100% identity to B. miyamotoi LB-2001), B. hermsii (n = 1; 100% identity to B. hermsii YOR), and a Borrelia species (n = 1) displaying highest sequence identity to Borrelia parkeri (97.5%).
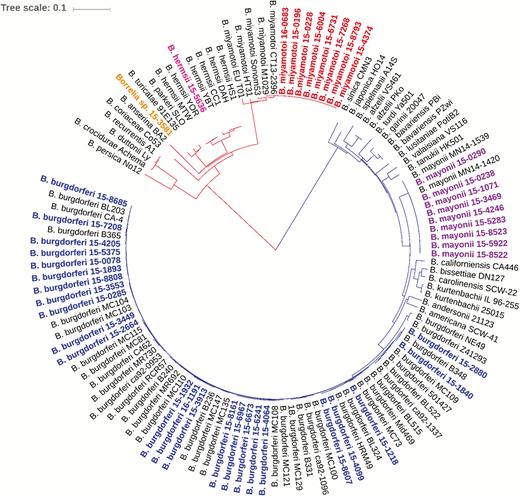
Phylogenetic relationships of identified Borrelia species. The phylogenetic tree is based on alignment of in frame concatenated DNA sequence fragments from 8 housekeeping genes (clpA, clpX, nifS, pepX, pyrG, recG, rplB, and uvrA) (n = 4791 bp). Strains sequenced in this study are highlighted as follows: blue, B. burgdorferi; purple, B. mayonii; red, B. miyamotoi; pink, B. hermsii, and orange, Candidatus B. johnsonii. Publicly available sequences for 18 different Bbsl genospecies and 10 different relapsing fever borreliae (black) were included for comparison. The scale bar corresponds to 0.1 substitutions per nucleotide position.
Of the 25 B. burgdorferi–positive specimens, 1 was CSF and the remainder were comprised equally of blood and synovial fluid. The remaining non–B. burgdorferi positive specimens were all blood (Table 1). The B. burgdorferi–positive specimens originated from 8 states in the Midwest, mid-Atlantic, and Northeast (Iowa, Maryland, Minnesota, Missouri, New Jersey, New York, Pennsylvania, Virginia, and Wisconsin), whereas all 9 B. mayonii positives were submitted only from Minnesota or Wisconsin. The B. burgdorferi positivity rate for synovial fluid samples differed between the Upper Midwest (Minnesota [2/75; 2.7%]) and 4 northeastern and mid-Atlantic states (Maryland, Virginia, Pennsylvania, and New York [9/99; 9%]]). The B. miyamotoi positives came from states in the Upper Midwest and the Northeast (Minnesota, New Jersey, and Wisconsin). The single B. hermsii–positive specimen originated from Montana.
| Sample ID . | Originating State . | Specimen Type . | clpA . | clpX . | nifS . | pepX . | pyrG . | recG . | rplB . | uvrA . | ST . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Borrelia burgdorferi | |||||||||||
| 15–2664 | MN | Blood | 18 | 12 | 1 | 11 | 2 | 15 | 1 | 2 | 29 |
| 15–3449 | MN | Blood | 4 | 12 | 1 | 11 | 2 | 15 | 1 | 2 | 756a |
| 15–4099 | MN | Blood | 8 | 1 | 1 | 1 | 4 | 16 | 1 | 7 | 32 |
| 15–6241 | MN | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–6673 | MN | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–6967 | MN | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–8161 | MN | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–3553 | PA | Blood | 5 | 5 | 4 | 5 | 5 | 5 | 1 | 6 | 8 |
| 15–0285 | WI | Blood | 8 | 1 | 1 | 1 | 4 | 16 | 1 | 7 | 32 |
| 15–3913 | WI | Blood | 128 | 12 | 1 | 8 | 1 | 6 | 1 | 10 | 758a |
| 15–4064 | WI | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–1932 | IA | CSF | 8 | 1 | 1 | 14 | 2 | 6 | 1 | 10 | 48 |
| 15–0078 | MD | Synovial fluid | 4 | 1 | 1 | 1 | 224a | 6 | 1 | 217a | 754a |
| 15–1218 | MN | Synovial fluid | 24 | 14 | 4 | 18 | 11 | 19 | 1 | 12 | 56 |
| 16–1191 | MN | Synovial fluid | 8 | 1 | 1 | 14 | 2 | 6 | 1 | 10 | 48 |
| 15–2880 | MO | Synovial fluid | 4 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 19 |
| 15–5375 | NY | Synovial fluid | 4 | 1 | 1 | 1 | 225a | 6 | 1 | 7 | 759a |
| 15–7208 | NY | Synovial fluid | 6 | 1 | 5 | 1 | 1 | 7 | 1 | 8 | 7 |
| 15–8685 | NY | Synovial fluid | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 15–4205 | PA | Synovial fluid | 14 | 1 | 1 | 2 | 1 | 6 | 200a | 7 | 758a |
| 15–8607 | PA | Synovial fluid | 5 | 5 | 188a | 5 | 5 | 5 | 1 | 6 | 760a |
| 15–1840 | VA | Synovial fluid | 4 | 4 | 3 | 217a | 3 | 3 | 3 | 3 | 755a |
| 15–1893 | VA | Synovial fluid | 4 | 1 | 1 | 1 | 1 | 6 | 1 | 7 | 3 |
| 15–8808 | VA | Synovial fluid | 4 | 1 | 189a | 1 | 1 | 6 | 1 | 7 | 761a |
| Borrelia mayonii | |||||||||||
| 15–0238 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–0290 | MN | Blood | 218 | 182 | 166 | 191 | 202 | 209 | 174 | 193 | 675 |
| 15–1071 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–3469 | WI | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–4246 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–5283 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–5922 | MN | Blood | 218 | 182 | 166 | 218a | 201 | 209 | 174 | 193 | 762a |
| 15–8522 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–8523 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| Borrelia miyamotoi | |||||||||||
| 15–0196 | WI | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–0228 | MN | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–4374 | NJ | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–6004 | MN | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–6731 | NJ | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–7268 | NJ | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–8793 | MN | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 16–0683 | NJ | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| B. hermsii | |||||||||||
| 15–5636 | MT | Blood | 246a | 206a | 190a | 219a | 226a | 236a | 201a | 218a | 763a |
| Candidatus Borrelia johnsonii | |||||||||||
| clpA | clpX | nifS | pepX | pyrG | recG | rplB | uvrA | ST | |||
| 15–3581 | WI | Blood | 247a | 207a | 191a | 220a | 227a | 237a | 202a | 219a | 764a |
| Sample ID . | Originating State . | Specimen Type . | clpA . | clpX . | nifS . | pepX . | pyrG . | recG . | rplB . | uvrA . | ST . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Borrelia burgdorferi | |||||||||||
| 15–2664 | MN | Blood | 18 | 12 | 1 | 11 | 2 | 15 | 1 | 2 | 29 |
| 15–3449 | MN | Blood | 4 | 12 | 1 | 11 | 2 | 15 | 1 | 2 | 756a |
| 15–4099 | MN | Blood | 8 | 1 | 1 | 1 | 4 | 16 | 1 | 7 | 32 |
| 15–6241 | MN | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–6673 | MN | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–6967 | MN | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–8161 | MN | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–3553 | PA | Blood | 5 | 5 | 4 | 5 | 5 | 5 | 1 | 6 | 8 |
| 15–0285 | WI | Blood | 8 | 1 | 1 | 1 | 4 | 16 | 1 | 7 | 32 |
| 15–3913 | WI | Blood | 128 | 12 | 1 | 8 | 1 | 6 | 1 | 10 | 758a |
| 15–4064 | WI | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–1932 | IA | CSF | 8 | 1 | 1 | 14 | 2 | 6 | 1 | 10 | 48 |
| 15–0078 | MD | Synovial fluid | 4 | 1 | 1 | 1 | 224a | 6 | 1 | 217a | 754a |
| 15–1218 | MN | Synovial fluid | 24 | 14 | 4 | 18 | 11 | 19 | 1 | 12 | 56 |
| 16–1191 | MN | Synovial fluid | 8 | 1 | 1 | 14 | 2 | 6 | 1 | 10 | 48 |
| 15–2880 | MO | Synovial fluid | 4 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 19 |
| 15–5375 | NY | Synovial fluid | 4 | 1 | 1 | 1 | 225a | 6 | 1 | 7 | 759a |
| 15–7208 | NY | Synovial fluid | 6 | 1 | 5 | 1 | 1 | 7 | 1 | 8 | 7 |
| 15–8685 | NY | Synovial fluid | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 15–4205 | PA | Synovial fluid | 14 | 1 | 1 | 2 | 1 | 6 | 200a | 7 | 758a |
| 15–8607 | PA | Synovial fluid | 5 | 5 | 188a | 5 | 5 | 5 | 1 | 6 | 760a |
| 15–1840 | VA | Synovial fluid | 4 | 4 | 3 | 217a | 3 | 3 | 3 | 3 | 755a |
| 15–1893 | VA | Synovial fluid | 4 | 1 | 1 | 1 | 1 | 6 | 1 | 7 | 3 |
| 15–8808 | VA | Synovial fluid | 4 | 1 | 189a | 1 | 1 | 6 | 1 | 7 | 761a |
| Borrelia mayonii | |||||||||||
| 15–0238 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–0290 | MN | Blood | 218 | 182 | 166 | 191 | 202 | 209 | 174 | 193 | 675 |
| 15–1071 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–3469 | WI | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–4246 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–5283 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–5922 | MN | Blood | 218 | 182 | 166 | 218a | 201 | 209 | 174 | 193 | 762a |
| 15–8522 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–8523 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| Borrelia miyamotoi | |||||||||||
| 15–0196 | WI | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–0228 | MN | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–4374 | NJ | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–6004 | MN | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–6731 | NJ | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–7268 | NJ | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–8793 | MN | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 16–0683 | NJ | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| B. hermsii | |||||||||||
| 15–5636 | MT | Blood | 246a | 206a | 190a | 219a | 226a | 236a | 201a | 218a | 763a |
| Candidatus Borrelia johnsonii | |||||||||||
| clpA | clpX | nifS | pepX | pyrG | recG | rplB | uvrA | ST | |||
| 15–3581 | WI | Blood | 247a | 207a | 191a | 220a | 227a | 237a | 202a | 219a | 764a |
Abbreviation: CSF, cerebrospinal fluid; IA, Iowa; MD, Maryland; MN, Minnesota; MO, Missouri; MT, Missouri; NJ, New Jersey; NY, New York; PA, Pennsylvania; ST, sequence type; VA, Virginia; WI, Wisconsin.
aDenotes allele or sequence type identified in this study.
| Sample ID . | Originating State . | Specimen Type . | clpA . | clpX . | nifS . | pepX . | pyrG . | recG . | rplB . | uvrA . | ST . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Borrelia burgdorferi | |||||||||||
| 15–2664 | MN | Blood | 18 | 12 | 1 | 11 | 2 | 15 | 1 | 2 | 29 |
| 15–3449 | MN | Blood | 4 | 12 | 1 | 11 | 2 | 15 | 1 | 2 | 756a |
| 15–4099 | MN | Blood | 8 | 1 | 1 | 1 | 4 | 16 | 1 | 7 | 32 |
| 15–6241 | MN | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–6673 | MN | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–6967 | MN | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–8161 | MN | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–3553 | PA | Blood | 5 | 5 | 4 | 5 | 5 | 5 | 1 | 6 | 8 |
| 15–0285 | WI | Blood | 8 | 1 | 1 | 1 | 4 | 16 | 1 | 7 | 32 |
| 15–3913 | WI | Blood | 128 | 12 | 1 | 8 | 1 | 6 | 1 | 10 | 758a |
| 15–4064 | WI | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–1932 | IA | CSF | 8 | 1 | 1 | 14 | 2 | 6 | 1 | 10 | 48 |
| 15–0078 | MD | Synovial fluid | 4 | 1 | 1 | 1 | 224a | 6 | 1 | 217a | 754a |
| 15–1218 | MN | Synovial fluid | 24 | 14 | 4 | 18 | 11 | 19 | 1 | 12 | 56 |
| 16–1191 | MN | Synovial fluid | 8 | 1 | 1 | 14 | 2 | 6 | 1 | 10 | 48 |
| 15–2880 | MO | Synovial fluid | 4 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 19 |
| 15–5375 | NY | Synovial fluid | 4 | 1 | 1 | 1 | 225a | 6 | 1 | 7 | 759a |
| 15–7208 | NY | Synovial fluid | 6 | 1 | 5 | 1 | 1 | 7 | 1 | 8 | 7 |
| 15–8685 | NY | Synovial fluid | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 15–4205 | PA | Synovial fluid | 14 | 1 | 1 | 2 | 1 | 6 | 200a | 7 | 758a |
| 15–8607 | PA | Synovial fluid | 5 | 5 | 188a | 5 | 5 | 5 | 1 | 6 | 760a |
| 15–1840 | VA | Synovial fluid | 4 | 4 | 3 | 217a | 3 | 3 | 3 | 3 | 755a |
| 15–1893 | VA | Synovial fluid | 4 | 1 | 1 | 1 | 1 | 6 | 1 | 7 | 3 |
| 15–8808 | VA | Synovial fluid | 4 | 1 | 189a | 1 | 1 | 6 | 1 | 7 | 761a |
| Borrelia mayonii | |||||||||||
| 15–0238 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–0290 | MN | Blood | 218 | 182 | 166 | 191 | 202 | 209 | 174 | 193 | 675 |
| 15–1071 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–3469 | WI | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–4246 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–5283 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–5922 | MN | Blood | 218 | 182 | 166 | 218a | 201 | 209 | 174 | 193 | 762a |
| 15–8522 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–8523 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| Borrelia miyamotoi | |||||||||||
| 15–0196 | WI | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–0228 | MN | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–4374 | NJ | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–6004 | MN | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–6731 | NJ | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–7268 | NJ | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–8793 | MN | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 16–0683 | NJ | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| B. hermsii | |||||||||||
| 15–5636 | MT | Blood | 246a | 206a | 190a | 219a | 226a | 236a | 201a | 218a | 763a |
| Candidatus Borrelia johnsonii | |||||||||||
| clpA | clpX | nifS | pepX | pyrG | recG | rplB | uvrA | ST | |||
| 15–3581 | WI | Blood | 247a | 207a | 191a | 220a | 227a | 237a | 202a | 219a | 764a |
| Sample ID . | Originating State . | Specimen Type . | clpA . | clpX . | nifS . | pepX . | pyrG . | recG . | rplB . | uvrA . | ST . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Borrelia burgdorferi | |||||||||||
| 15–2664 | MN | Blood | 18 | 12 | 1 | 11 | 2 | 15 | 1 | 2 | 29 |
| 15–3449 | MN | Blood | 4 | 12 | 1 | 11 | 2 | 15 | 1 | 2 | 756a |
| 15–4099 | MN | Blood | 8 | 1 | 1 | 1 | 4 | 16 | 1 | 7 | 32 |
| 15–6241 | MN | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–6673 | MN | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–6967 | MN | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–8161 | MN | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–3553 | PA | Blood | 5 | 5 | 4 | 5 | 5 | 5 | 1 | 6 | 8 |
| 15–0285 | WI | Blood | 8 | 1 | 1 | 1 | 4 | 16 | 1 | 7 | 32 |
| 15–3913 | WI | Blood | 128 | 12 | 1 | 8 | 1 | 6 | 1 | 10 | 758a |
| 15–4064 | WI | Blood | 14 | 1 | 5 | 2 | 2 | 1 | 1 | 10 | 530 |
| 15–1932 | IA | CSF | 8 | 1 | 1 | 14 | 2 | 6 | 1 | 10 | 48 |
| 15–0078 | MD | Synovial fluid | 4 | 1 | 1 | 1 | 224a | 6 | 1 | 217a | 754a |
| 15–1218 | MN | Synovial fluid | 24 | 14 | 4 | 18 | 11 | 19 | 1 | 12 | 56 |
| 16–1191 | MN | Synovial fluid | 8 | 1 | 1 | 14 | 2 | 6 | 1 | 10 | 48 |
| 15–2880 | MO | Synovial fluid | 4 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 19 |
| 15–5375 | NY | Synovial fluid | 4 | 1 | 1 | 1 | 225a | 6 | 1 | 7 | 759a |
| 15–7208 | NY | Synovial fluid | 6 | 1 | 5 | 1 | 1 | 7 | 1 | 8 | 7 |
| 15–8685 | NY | Synovial fluid | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 15–4205 | PA | Synovial fluid | 14 | 1 | 1 | 2 | 1 | 6 | 200a | 7 | 758a |
| 15–8607 | PA | Synovial fluid | 5 | 5 | 188a | 5 | 5 | 5 | 1 | 6 | 760a |
| 15–1840 | VA | Synovial fluid | 4 | 4 | 3 | 217a | 3 | 3 | 3 | 3 | 755a |
| 15–1893 | VA | Synovial fluid | 4 | 1 | 1 | 1 | 1 | 6 | 1 | 7 | 3 |
| 15–8808 | VA | Synovial fluid | 4 | 1 | 189a | 1 | 1 | 6 | 1 | 7 | 761a |
| Borrelia mayonii | |||||||||||
| 15–0238 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–0290 | MN | Blood | 218 | 182 | 166 | 191 | 202 | 209 | 174 | 193 | 675 |
| 15–1071 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–3469 | WI | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–4246 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–5283 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–5922 | MN | Blood | 218 | 182 | 166 | 218a | 201 | 209 | 174 | 193 | 762a |
| 15–8522 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| 15–8523 | MN | Blood | 218 | 182 | 166 | 191 | 201 | 209 | 174 | 193 | 674 |
| Borrelia miyamotoi | |||||||||||
| 15–0196 | WI | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–0228 | MN | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–4374 | NJ | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–6004 | MN | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–6731 | NJ | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–7268 | NJ | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 15–8793 | MN | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| 16–0683 | NJ | Blood | 200 | 117 | 151 | 173 | 184 | 191 | 160 | 70 | 634 |
| B. hermsii | |||||||||||
| 15–5636 | MT | Blood | 246a | 206a | 190a | 219a | 226a | 236a | 201a | 218a | 763a |
| Candidatus Borrelia johnsonii | |||||||||||
| clpA | clpX | nifS | pepX | pyrG | recG | rplB | uvrA | ST | |||
| 15–3581 | WI | Blood | 247a | 207a | 191a | 220a | 227a | 237a | 202a | 219a | 764a |
Abbreviation: CSF, cerebrospinal fluid; IA, Iowa; MD, Maryland; MN, Minnesota; MO, Missouri; MT, Missouri; NJ, New Jersey; NY, New York; PA, Pennsylvania; ST, sequence type; VA, Virginia; WI, Wisconsin.
aDenotes allele or sequence type identified in this study.
Sequence type diversity was greatest among the B. burgdorferi–positive specimens (Figure 2). Eighteen different STs from MLST were identified, including 10 known STs (ST1, ST3, ST7, ST8, ST19, ST29, ST32, ST48, ST56, ST530) and 8 new STs (ST754, ST755, ST756, ST757, ST758, ST759, ST760, ST761) comprised of either known loci in previously unobserved combinations or newly observed loci (Table 1). New B. burgdorferi STs were predominately associated with synovial fluid specimens (6/8; 75%). The 6 synovial fluid specimens assigned new STs were submitted by providers in northeastern and mid-Atlantic states (Table 1).
In contrast to the B. burgdorferi–positive specimens, limited or no sequence diversity across the 8 housekeeping genes was observed among the B. mayonii– or B. miyamotoi–positive specimens, respectively (Table 1). For the 9 B. mayonii–positive specimens, 3 different MLST STs were identified, which included the known ST674 and ST675, and a new ST (ST762), which has a single nucleotide variant in pepX as compared to ST674 (Supplementary Table 3).
Identification of Candidatus Borrelia johnsonii in a Patient Specimen
To further characterize the RF Borrelia species (15–3581) most closely related to B. parkeri, sequencing and phylogenetic analysis of the 16S rRNA, flaB, and glpQ genes was performed. As these genes have historically been utilized for characterization of RF Borrelia, more sequences are publicly available as compared to the housekeeping genes more commonly used for Bbsl species. Analysis of the concatenated glpQ, flaB, and 16S rDNA sequence (3048 nucleotides) demonstrated 100% identity between 15–3581 and Candidatus B. johnsonii strain IA-1, a Borrelia species previously identified only in bat ticks (Carios kelleyi) (Figure 3) [24]. The Candidatus B. johnsonii–positive blood specimen was submitted from a provider in Wisconsin.
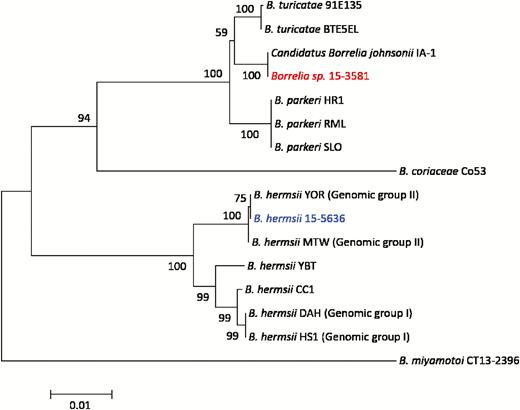
Identification of Candidatus Borrelia johnsonii in patient blood. Phylogenetic relationship of Borrelia species 15–3581 (red) based on analysis of concatenated glpQ, flaB, and 16S rDNA sequences (n = 3048 bp). Publicly available sequences for 6 different relapsing fever borreliae (black) and Borrelia hermsii 15–5636 (blue) from this study were included for comparison. Bootstrap support values >50% are shown. The scale bar corresponds to 0.01 substitutions per nucleotide position.
DISCUSSION
A better understanding of the scope and geographic distribution of Borrelia species infecting humans is essential for improving clinical recognition, laboratory diagnosis, and prevention. Here, we demonstrated a broad molecular surveillance approach that successfully detected both known pathogens and a novel Borrelia species in specimens from US patients suspected of tickborne illness. The 5 Borrelia species identified included the 2 Bbsl genospecies known to cause LB, B. burgdorferi and B. mayonii, the 2 known RF pathogens, B. hermsii and B. miyamotoi, and a third RF species, Candidatus B. johnsonii, not previously associated with human illness.
Prior description of Candidatus B. johnsonii is limited to molecular and microscopic detection in bat ticks, C. kelleyi, collected from an Iowa farmhouse [24, 25]. Carios kelleyi ticks are widely distributed in the Americas and can be found in houses and buildings infested with bats. Although this tick prefers to feed on bats, it will also feed on humans, and persons presumably bitten by C. kelleyi ticks have reported expanding, erythematous skin lesions, lymphadenopathy, fever, weight loss, malaise, and fatigue [26]. Whether these symptoms were due to infection with Candidatus B. johnsonii is unknown. The clinical presentation for the Candidatus B. johnsonii–infected patient identified here is also unknown. Nonetheless, we can infer that the physician suspected a tickborne illness, given that the blood specimen was originally submitted for either Lyme or tickborne pathogen PCR panel.
Notably, 10 infections due to 3 RF Borrelia species, B. miyamotoi (n = 8), B. hermsii (n = 1) and Candidatus B. johnsonii (n = 1), associated with 3 different tick species (I. scapularis, O. hermsi, and C. kelleyi, respectively), were detected in patient samples submitted for tickborne diseases other than RF. As B. miyamotoi is transmitted by I. scapularis, the same tick that transmits B. burgdorferi, B. mayonii, Anaplasma phagocytophilum, Ehrlichia muris subspecies eauclairensis, and Babesia microti, its detection in this sample set is not unexpected and has been reported previously [9, 27, 28]. All 8 B. miyamotoi–positive samples originated from patients in the Northeast and Upper Midwest where human cases or B. miyamotoi–infected I. scapularis have been previously described [7, 9, 27]. Detection of B. hermsii in these samples was unexpected. The specimen originated from Montana where cases of RF are reported due to the bite of infected O. hermsi, and locally acquired cases of LB, anaplasmosis, ehrlichiosis, and babesiosis are not known to occur.
Of the 11 Bbsl genospecies identified in ticks or animals in the United States [2, 5, 29], only B. burgdorferi and B. mayonii were detected in samples from >7000 US patients. Consistent with the initial description of B. mayonii as a cause of LB in the Upper Midwest [8], all 9 B. mayonii–positive specimens described here were submitted by providers in either Minnesota or Wisconsin. Given that 3 of the identified B. mayonii positives were from 2014, the same time period in which the initial 6 B. mayonii–infected patients were detected by Lyme PCR [9], it appears likely the providers suspected these patients of having a tickborne illness other than LB. All 9 B. mayonii–positive specimens were blood, as compared to B. burgdorferi–positive specimens, which were an equal distribution of synovial fluid and blood. The B. burgdorferi–positive specimens originated from Lyme-endemic regions in the Northeast, mid-Atlantic, and Upper Midwest, with the exception of a synovial fluid specimen from Missouri. Due to lack of patient travel information, exposure to ticks in a Lyme-endemic region for this patient cannot be excluded and appears likely, given the ST of the infecting strain, ST19, has only been observed previously in the Northeast. No Bbsl positives were identified among specimens originating from 10 southeast or south-central states (Florida, Georgia, South Carolina, Alabama, Tennessee, North Carolina, Louisiana, Arkansas, Oklahoma, and Texas), where I. scapularis is also present, and which accounted for 10% (739/7292) of the samples tested.
ST diversity among B. burgdorferi–positive specimens contrasted with what was observed for B. mayonii– and B. miyamotoi–positive specimens. Only a single ST from MLST, ST634, was observed among B. miyamotoi–positive specimens from the upper midwestern and northeastern United States, in agreement with previous studies demonstrating low genetic variability [30]. ST diversity among B. mayonii–positive specimens was also limited, with 7 of the 9 identified as ST674. The 3 identified B. mayonii STs differ by only 1 or 2 loci, and thus appear to belong to the same clonal complex. In contrast, among the B. burgdorferi–positive samples, 18 STs were identified. The most common B. burgdorferi ST in this study, ST530, appears to have a localized geographic distribution in the Upper Midwest (Minnesota, Wisconsin). Consistent with this, the only previous reported detection of this ST is from I. scapularis collected from Manitoba, Canada (http://pubmlst.org/borrelia/).
Newly observed B. burgdorferi MLST STs were most abundant in synovial fluids (71%), a specimen type for which 8 housekeeping MLST data are publicly lacking [18, 19, 31–33]. Four of the STs identified in synovial fluid specimens submitted by providers in the Northeast and mid-Atlantic regions, grouped with MLST ST3 (node support 0.89), an ST previously associated with disseminated LB [32]. Whether MLST STs may be predictive of arthritic outcome requires further study.
It is important to clarify that the 0.6% (44/7292) Borrelia positivity rate in this sample set does not reflect disease incidence or PCR positivity rates for a given Borrelia species. To enhance the likelihood of uncovering the spectrum of Borrelia species causing human disease in US patients, specimens from persons suspected of a number of tickborne diseases (LB, anaplasmosis, ehrlichiosis, or babesiosis) were tested. The percentage of samples submitted specifically for Lyme as opposed to tickborne pathogen PCR panel (Babesia, Ehrlichia/Anaplasma) was unknown. Second, the ability to detect Borrelia DNA by PCR is dependent on a number of factors, including when the specimen is collected in relation to illness onset, whether the sample is taken pretreatment, and the level of spirochetemia resulting from the infecting Borrelia species. These factors could lead to over- and underrepresentation of specific Borrelia species or even MLST STs. This is particularly relevant to B. burgdorferi, where the number of spirochetes/genomic copies in blood is low, often below the limit of PCR detection [14, 15].
Our results demonstrate that broad PCR followed by MLST utilizing next-generation sequencing is a powerful surveillance tool for uncovering Borrelia species causing human disease, understanding the geographic distribution of disease-causing Borrelia, and improving understanding of the pathogenic properties of B. burgdorferi STs. Ongoing surveillance for Borrelia pathogens in US patients suspected of tickborne illness is in progress. In ill patients with potential exposure to bat ticks, Candidatus B. johnsonii may be considered.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We express our thanks to Katherine Caflisch and the Mayo Clinic Microbiology Initial Processing Laboratory for their work collecting, aliquoting, recording, and shipping of specimens; John Young for sample receiving and processing; Alison Hinckley and Sarah Hook for study coordination; and Kirk Smith for critical review of the manuscript.
Disclaimer. The views and opinions expressed herein are those of the authors alone and do not represent the official position of the Centers for Disease Control and Prevention (CDC).
Financial support. This work was supported by the CDC’s Office of Advanced Molecular Detection (project ID AMD90).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




