-
PDF
- Split View
-
Views
-
Cite
Cite
Ane B Fisker, Eric Nebie, Anja Schoeps, Cesario Martins, Amabelia Rodrigues, Alphonse Zakane, Moubassira Kagone, Stine Byberg, Sanne M Thysen, Justin Tiendrebeogo, Boubacar Coulibaly, Osman Sankoh, Heiko Becher, Hilton C Whittle, Fiona R M van der Klis, Christine S Benn, Ali Sie, Olaf Müller, Peter Aaby, A Two-Center Randomized Trial of an Additional Early Dose of Measles Vaccine: Effects on Mortality and Measles Antibody Levels, Clinical Infectious Diseases, Volume 66, Issue 10, 15 May 2018, Pages 1573–1580, https://doi.org/10.1093/cid/cix1033
Close - Share Icon Share
Abstract
In addition to protecting against measles, measles vaccine (MV) may have beneficial nonspecific effects. We tested the effect of an additional early MV on mortality and measles antibody levels.
Children aged 4–7 months at rural health and demographic surveillance sites in Burkina Faso and Guinea-Bissau were randomized 1:1 to an extra early standard dose of MV (Edmonston-Zagreb strain) or no extra MV 4 weeks after the third diphtheria-tetanus-pertussis-hepatitis B-Haemophilus influenzae type b vaccine. All children received routine MV at 9 months. We assessed mortality through home visits and compared mortality from enrollment to age 3 years using Cox proportional hazards models, censoring for subsequent nontrial MV. Subgroups of participants had blood sampled to assess measles antibody levels.
Among 8309 children enrolled from 18 July 2012 to 3 December 2015, we registered 145 deaths (mortality rate: 16/1000 person-years). The mortality was lower than anticipated and did not differ by randomization group (hazard ratio, 1.05; 95% confidence interval, 0.75–1.46).
At enrollment, 4% (16/447) of children in Burkina Faso and 21% (90/422) in Guinea-Bissau had protective measles antibody levels. By age 9 months, no measles-unvaccinated/-unexposed child had protective levels, while 92% (306/333) of early MV recipients had protective levels. At final follow-up, 98% (186/189) in the early MV group and 97% (196/202) in the control group had protective levels.
Early MV did not reduce all-cause mortality. Most children were susceptible to measles infection at age 4–7 months and responded with high antibody levels to early MV.
NCT01644721.
The World Health Organization recommends the first dose of measles vaccine (MV) be administered at age 9 months in areas with measles transmission [1]. The decision to vaccinate at age 9 months was made in the late 1970s and was a compromise. It was reasoned that vaccinating earlier would lead to poor seroconversion rates and high rates of subsequent “vaccine failures,” while vaccinating later would lead to many children catching measles infection early in life. However, data on different ages at the time of measles vaccination and the impact on child survival were not collected [2].
In the 1970s, most mothers had experienced measles infection. Mothers with a history of natural measles infection transmit higher levels of maternal antibodies to their children [3]. Today, mothers are more likely to have received MV in childhood [4, 5]. They therefore transmit fewer measles antibodies to their children who become susceptible to measles infection at age 3–4 months [4, 6].
In addition to protecting against measles, observational studies [7–9] and randomized trials [10, 11] indicate that MV may have beneficial nonspecific effects (NSEs), lowering all-cause mortality due to prevention of nonmeasles infections. Such beneficial NSEs have also been shown for other live vaccines such as BCG [12–14] and oral polio vaccine (OPV) [15].
In a previous trial from Guinea-Bissau, provision of an early standard dose of Edmonston-Zagreb MV vs no early MV at age 4.5 months was associated with a hazard ratio (HR) of 0.67 (95% confidence interval [CI], 0.38–1.19) between enrollment and 9-month vaccination. All children received MV at age 9 months, but the early MV group also had a survival advantage from age 9–36 months (HR, 0.71; 95% CI, 0.50–1.01). Observational studies from the introduction of MV in Guinea-Bissau [16] and MV campaigns [17, 18] also indicate an additional benefit of receiving several doses of MV.
In this trial, we tested the hypothesis that an extra early dose of MV reduces all-cause mortality.
METHODS
Setting, Study Design, and Enrollment of Participants
In this individually randomized, open-label, 2-center trial, we assessed the effect of an early dose of MV on mortality between enrollment after age 4 months and age 36 months. The trial was conducted by the Centre de Recherche en Santé de Nouna in Burkina Faso and by the Bandim Health Project in Guinea-Bissau. Both research centers run health and demographic surveillance systems (HDSSs).
The routine vaccination program includes 3 doses of pentavalent vaccine (Penta: diphtheria-tetanus-pertussis-hepatitis B-Haemophilus influenzae type B) at 4-week intervals starting at age 6 weeks in Guinea-Bissau and at age 2 months in Burkina Faso. At age 9 months, children receive MV and yellow fever vaccine. During the trial, Burkina Faso changed to measles and rubella vaccine at age 9 months and started providing a second dose at age15 months.
Enrollment was initiated in July 2012 in the Bandim HDSS and in May 2013 in the Nouna HDSS. Potentially eligible children were visited at home. Children aged 121–215 days were eligible for enrollment 4 weeks after the third dose of Penta provided that they were registered as HDSS residents. After informed consent, children were randomized 1:1 to early Edmonston-Zagreb MV at a standard dose (Supplementary Materials) or no early MV in blocks stratified by sex and enrollment team. Following randomization, the child received either a standard dose of MV or no vaccine according to group. Since we were interested in testing the NSEs of MV, we did not use a placebo vaccine that could also have NSEs [19] (Supplementary Materials).
Assessment of Outcomes
All children were followed through the HDSS routines and, in Burkina Faso, also through the health facilities. At the first visit after age 9 months, the child was invited back to the vaccination post to receive the routine MV. The primary outcome was all-cause mortality between randomization and age 36 months or “end of study.” Since the purpose of the trial was to examine the effect of 1 vs 2 doses of MV, it was prespecified to censor children who received nonstudy MVs. In October 2014, Burkina Faso introduced a second dose of MV at age 15 months (which was also provided to children up to age 18 months). This shortened the follow-up period considerably in Burkina Faso. MV campaigns that targeted all children aged 9–59 months occurred in December 2012 and December 2015 in Guinea-Bissau and in November 2014 in Burkina Faso, further shortening the follow-up time. The “end of study” depended on the timeline for funding and was 4 December 2015 in Guinea-Bissau when the MV campaign occurred and 31 January 2016 in Burkina Faso.
For all registered deaths, a specially trained field assistant conducted a standard verbal autopsy [20], and a physician assigned the probable cause of death (Supplementary Materials).
As a secondary outcome, we assessed levels of measles antibody. In a subgroup of children, we collected blood samples at enrollment and at 9 months (after 1 vs no MV); a third and final blood sample was obtained at 15 months in Burkina Faso and at 24 months in Guinea-Bissau (after 2 vs 1 MV). Blood samples from the mothers were collected at enrollment.
A subgroup of children were visited 14 days after enrollment to register adverse events (Supplementary Materials).
Statistical Analyses
The sample size was based on a significance level of 5% and 80% power and a hypothesized reduction of 32% in mortality between age 4 months and age 36 months. We assumed an average follow-up of 1.4 years. With an expected mortality rate (MR) of 30/1000 person-years in Guinea-Bissau and 25/1000 in Burkina Faso, we arrived at minimum samples of 3750 in Guinea-Bissau and 4050 in Burkina Faso (Supplementary Materials). The analysis plan was reviewed and approved by the Data Safety and Ethics Monitoring Board prior to data lock.
Mortality was compared using Cox proportional hazards models, with time since enrollment as the underlying timescale and stratified by site and sex. The primary analysis was based on the per-protocol population. In the per-protocol analysis, follow-up was censored at age 3 years, migration, the end of study, or deviations from the planned vaccination schedule (18 months if the scheduled 9-month MV had not been received by then, registration of reception of nontrial MV, eligibility to MV campaigns, or routine second dose of MV).
Secondary analyses assessed the effect of early MV on mortality in 2 intervals: before the 9-month vaccination and between the 9-month vaccination and end of study (Supplementary Materials). In a secondary intention-to-treat analysis, follow-up was censored at age 3 years, migration, or end of study. Deaths due to accidents were censored in a sensitivity analysis.
Antibody concentrations were measured using multiplex immunoassay [21] and described by geometric mean concentration and the log-transformed concentrations compared by group using Student t test. As in prior publications [5, 22, 23], we compared the proportion of samples with concentrations of protective measles antibody levels using the internationally accepted cutoff of ≥125 mIU/mL.
The proportions of children for whom adverse events were reported at the home visit 14 days post-enrollment were compared by calculating relative risks for the intervention relative to the control group stratified by site.
The trial protocol was approved by the relevant ethical committees in Guinea-Bissau (Comité Nacional de Ética na Saúde), Burkina Faso (Le Comité d’Ethique pour la Recherche en Santé, Comité technique d’autorisation d’essais cliniques and le Comité Institutionnel d’Éthique de Nouna), Germany (the Ethical Committee of the Medical School at University of Heidelberg), and Denmark (the Danish Central Ethical Committee [consultative approval]). Due to changes in national vaccination policies during the course of the study, protocol modifications with respect to timing of blood sample collection and sample size were submitted and approved by the ethical committees. The data safety and ethics monitoring board approved the suggested changes before submission.
RESULTS
Between 18 July 2012 and 3 December 2015, when the planned sample size was reached in Guinea-Bissau, we enrolled 8309 children, 4559 in Burkina Faso (12% more than the originally planned sample of 4050 to compensate for censoring) and 3750 in Guinea-Bissau. Among these children, 4153 were randomized to the intervention group and received early measles vaccination and 4156 were randomized to the control group (Figure 1). A total of 104 children were excluded due to protocol violations (Figure 1), retaining 8205 children in the analyses. Only 6% of children were followed until completing age 3 years of age in the per-protocol analysis (Figure 1).
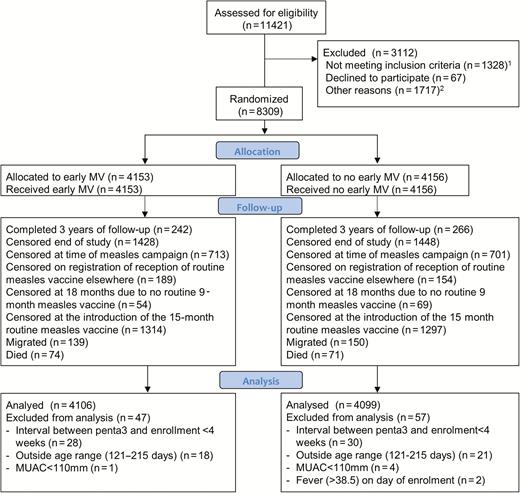
Flowchart of children in the early measles vaccination trial in Burkina Faso and Guinea-Bissau. 1Not meeting inclusion criteria (n = 1328): migrated (108) or died (54) before 28 days after Penta3; no Penta3 before too old (516); interval <28 days since Penta3 (102); too old when seen (500); mid upper-arm circumference <110 (34); ill (12); malformation (2). 2Other reasons (n = 1717): absent/travelling (1385); vaccination card not seen (64); no guardian present (10); already received measles vaccine elsewhere (7); residential status not confirmed (251). Abbreviations: MV, measles vaccine; MUAC, mid upper-arm circumference.
The randomization resulted in balanced groups with regard to baseline demographic and health characteristics (Table 1). Eighty percent of children had been exposed to OPV campaigns before enrollment (Table 1, Supplementary Table S1).
By the end of the study, we had registered 145 deaths in the per-protocol analysis. There was no difference in survival between the 2 randomized groups; the early MV group had a MR of 16.8/1000 person-years and the control group 15.9/1000 person-years, the HR being 1.05 (0.75–1.46). There was no indication of a site- or sex-differential effect (Figure 2, Table 2).
| Characteristic . | Early Measles Vaccine . | Control . |
|---|---|---|
| Number | 4106 | 4099 |
| Male, n (%) | 2056 (50) | 2048 (50) |
| Age/days, median (IQR) | 170 (158–188) | 170 (157–187) |
| Enrolled in the rainy season,a n (%) | 2485 (61) | 2455 (60) |
| Site | ||
| Burkina Faso, n (%) | 2258 (55) | 2238 (55) |
| Guinea-Bissau, n (%) | 1848 (45) | 1861 (45) |
| Markers of child health | ||
| Mid-upper-arm circumference, median (IQR) | 138 (130–146) | 138 (130–146) |
| Admitted to hospital prior to enrollment, n (%) | 43 (1) | 38 (1) |
| Socioeconomic status | ||
| Number of persons sleeping in the bed of the child, median (IQR) | 3 (2–3) | 3 (2–3) |
| Prior OPV campaign | ||
| Eligible for campaign OPV prior to enrollment, n (%) | 3290 (80) | 3247 (79) |
| Characteristic . | Early Measles Vaccine . | Control . |
|---|---|---|
| Number | 4106 | 4099 |
| Male, n (%) | 2056 (50) | 2048 (50) |
| Age/days, median (IQR) | 170 (158–188) | 170 (157–187) |
| Enrolled in the rainy season,a n (%) | 2485 (61) | 2455 (60) |
| Site | ||
| Burkina Faso, n (%) | 2258 (55) | 2238 (55) |
| Guinea-Bissau, n (%) | 1848 (45) | 1861 (45) |
| Markers of child health | ||
| Mid-upper-arm circumference, median (IQR) | 138 (130–146) | 138 (130–146) |
| Admitted to hospital prior to enrollment, n (%) | 43 (1) | 38 (1) |
| Socioeconomic status | ||
| Number of persons sleeping in the bed of the child, median (IQR) | 3 (2–3) | 3 (2–3) |
| Prior OPV campaign | ||
| Eligible for campaign OPV prior to enrollment, n (%) | 3290 (80) | 3247 (79) |
Abbreviations: IQR, interquartile range; OPV, oral polio vaccine.
Rainy season defined as in prior studies: Guinea-Bissau, June-November; Burkina Faso, June-October.
| Characteristic . | Early Measles Vaccine . | Control . |
|---|---|---|
| Number | 4106 | 4099 |
| Male, n (%) | 2056 (50) | 2048 (50) |
| Age/days, median (IQR) | 170 (158–188) | 170 (157–187) |
| Enrolled in the rainy season,a n (%) | 2485 (61) | 2455 (60) |
| Site | ||
| Burkina Faso, n (%) | 2258 (55) | 2238 (55) |
| Guinea-Bissau, n (%) | 1848 (45) | 1861 (45) |
| Markers of child health | ||
| Mid-upper-arm circumference, median (IQR) | 138 (130–146) | 138 (130–146) |
| Admitted to hospital prior to enrollment, n (%) | 43 (1) | 38 (1) |
| Socioeconomic status | ||
| Number of persons sleeping in the bed of the child, median (IQR) | 3 (2–3) | 3 (2–3) |
| Prior OPV campaign | ||
| Eligible for campaign OPV prior to enrollment, n (%) | 3290 (80) | 3247 (79) |
| Characteristic . | Early Measles Vaccine . | Control . |
|---|---|---|
| Number | 4106 | 4099 |
| Male, n (%) | 2056 (50) | 2048 (50) |
| Age/days, median (IQR) | 170 (158–188) | 170 (157–187) |
| Enrolled in the rainy season,a n (%) | 2485 (61) | 2455 (60) |
| Site | ||
| Burkina Faso, n (%) | 2258 (55) | 2238 (55) |
| Guinea-Bissau, n (%) | 1848 (45) | 1861 (45) |
| Markers of child health | ||
| Mid-upper-arm circumference, median (IQR) | 138 (130–146) | 138 (130–146) |
| Admitted to hospital prior to enrollment, n (%) | 43 (1) | 38 (1) |
| Socioeconomic status | ||
| Number of persons sleeping in the bed of the child, median (IQR) | 3 (2–3) | 3 (2–3) |
| Prior OPV campaign | ||
| Eligible for campaign OPV prior to enrollment, n (%) | 3290 (80) | 3247 (79) |
Abbreviations: IQR, interquartile range; OPV, oral polio vaccine.
Rainy season defined as in prior studies: Guinea-Bissau, June-November; Burkina Faso, June-October.
Mortality by Group Allocation in the Early Measles Vaccine Trial in Burkina Faso and Guinea-Bissau
| . | Mortality Rate/1000 Person-Years (Deaths/Person-Years) . | Hazard Ratio (95% Confidence Interval) . | |
|---|---|---|---|
| Early Measles Vaccine . | Control . | ||
| Per-protocol analysisa | |||
| All | 16.8 (74/4398) | 15.9 (71/4453) | 1.05 (0.75–1.46) |
| Boys | 16.3 (36/2214) | 16.1 (36/2238) | 1.01 (0.63–1.62) |
| Girls | 17.4 (38/2184) | 15.8 (35/2215) | 1.09 (0.69–1.72) |
| Intention-to-treat analysisb | |||
| All | 18.4 (129/6999) | 16.4 (114/6960) | 1.12 (0.87–1.44) |
| Boys | 19.1 (67/3505) | 17.2 (60/3491) | 1.11 (0.78–1.58) |
| Girls | 17.7 (62/3493) | 15.6 (54/3469) | 1.13 (0.79–1.63) |
| . | Mortality Rate/1000 Person-Years (Deaths/Person-Years) . | Hazard Ratio (95% Confidence Interval) . | |
|---|---|---|---|
| Early Measles Vaccine . | Control . | ||
| Per-protocol analysisa | |||
| All | 16.8 (74/4398) | 15.9 (71/4453) | 1.05 (0.75–1.46) |
| Boys | 16.3 (36/2214) | 16.1 (36/2238) | 1.01 (0.63–1.62) |
| Girls | 17.4 (38/2184) | 15.8 (35/2215) | 1.09 (0.69–1.72) |
| Intention-to-treat analysisb | |||
| All | 18.4 (129/6999) | 16.4 (114/6960) | 1.12 (0.87–1.44) |
| Boys | 19.1 (67/3505) | 17.2 (60/3491) | 1.11 (0.78–1.58) |
| Girls | 17.7 (62/3493) | 15.6 (54/3469) | 1.13 (0.79–1.63) |
Follow-up time as follows: Early measles vaccine (MV): mean, 1.07 years; median, 0.87 years (interquartile range [IQR], 0.61–1.37). Control: mean, 1.09 years; median, 0.87 years (IQR, 0.61–1.37).
Follow-up time as follows: Early MV: mean, 1.70 years; median, 1.79 years (IQR, 1.29–2.32). Control: mean, 1.70 years; median, 1.81 years (IQR, 1.29–2.33).
Mortality by Group Allocation in the Early Measles Vaccine Trial in Burkina Faso and Guinea-Bissau
| . | Mortality Rate/1000 Person-Years (Deaths/Person-Years) . | Hazard Ratio (95% Confidence Interval) . | |
|---|---|---|---|
| Early Measles Vaccine . | Control . | ||
| Per-protocol analysisa | |||
| All | 16.8 (74/4398) | 15.9 (71/4453) | 1.05 (0.75–1.46) |
| Boys | 16.3 (36/2214) | 16.1 (36/2238) | 1.01 (0.63–1.62) |
| Girls | 17.4 (38/2184) | 15.8 (35/2215) | 1.09 (0.69–1.72) |
| Intention-to-treat analysisb | |||
| All | 18.4 (129/6999) | 16.4 (114/6960) | 1.12 (0.87–1.44) |
| Boys | 19.1 (67/3505) | 17.2 (60/3491) | 1.11 (0.78–1.58) |
| Girls | 17.7 (62/3493) | 15.6 (54/3469) | 1.13 (0.79–1.63) |
| . | Mortality Rate/1000 Person-Years (Deaths/Person-Years) . | Hazard Ratio (95% Confidence Interval) . | |
|---|---|---|---|
| Early Measles Vaccine . | Control . | ||
| Per-protocol analysisa | |||
| All | 16.8 (74/4398) | 15.9 (71/4453) | 1.05 (0.75–1.46) |
| Boys | 16.3 (36/2214) | 16.1 (36/2238) | 1.01 (0.63–1.62) |
| Girls | 17.4 (38/2184) | 15.8 (35/2215) | 1.09 (0.69–1.72) |
| Intention-to-treat analysisb | |||
| All | 18.4 (129/6999) | 16.4 (114/6960) | 1.12 (0.87–1.44) |
| Boys | 19.1 (67/3505) | 17.2 (60/3491) | 1.11 (0.78–1.58) |
| Girls | 17.7 (62/3493) | 15.6 (54/3469) | 1.13 (0.79–1.63) |
Follow-up time as follows: Early measles vaccine (MV): mean, 1.07 years; median, 0.87 years (interquartile range [IQR], 0.61–1.37). Control: mean, 1.09 years; median, 0.87 years (IQR, 0.61–1.37).
Follow-up time as follows: Early MV: mean, 1.70 years; median, 1.79 years (IQR, 1.29–2.32). Control: mean, 1.70 years; median, 1.81 years (IQR, 1.29–2.33).

Kaplan-Meier survival graphs for children in the early measles vaccination trial in Burkina Faso and Guinea-Bissau. Based on per-protocol population. Abbreviation: MV, measles vaccine.
Splitting the observation time at the 9-month MV, the mortality did not differ by group assignment between enrollment and 9 months (comparing measles vaccinated vs unvaccinated children; HR, 1.10; 95% CI, 0.66–1.83) or between 9 months and end of study (2 doses of MV vs 1 dose; HR, 1.01; 95% CI, 0.66–1.56; Supplementary Table S2). Due to restrictions on opening the 10-dose yellow fever vaccine vials and national stock-outs, 42% of children in Guinea-Bissau received no yellow fever vaccine on the date of the 9-month MV but were offered the vaccine at a subsequent visit if they had not been vaccinated through the national program. We found no indication that the effect of early MV varied by whether or not yellow fever vaccine was received with the 9-month MV (Supplementary Table S3).
The intention-to-treat analysis, which extended the follow-up beyond the nontrial MVs, included 243 deaths, 129 in the early MV group (MR, 18.4/1000 person-years) and 114 in the control group (MR, 16.4/1000 person-years). The HR was 1.12 (95% CI, 0.87–1.44; Table 2).
Half of the deaths occurred at home, and the proportion of deaths that occurred in the hospital did not differ by group (Supplementary Table S4). Deaths were mainly classified as being caused by infectious diseases (Supplementary Materials). Two deaths in the per-protocol analysis were classified as due to accidents (1 early MV, 1 control; Supplementary Table S4). Censoring these deaths, the HR remained unchanged. In the intention-to-treat analysis, when 4 additional deaths due to accidents were censored from the early MV arm, the HR was 1.09 (0.84–1.40; Supplementary Table S5).
We found no indication of acute adverse events among the 1543 children who were visited 14 days after enrollment (Table 3, Supplementary Materials).
Reported Adverse Events Within the First 14 Days After Enrollment in the Early Measles Vaccine Trial in Guinea-Bissau and Burkina Faso
| Adverse Events Information . | Early Measles Vaccine, n (%) . | Control, n (%) . | Relative Risk (95% Confidence Interval) . |
|---|---|---|---|
| Guinea-Bissau | |||
| Number visited | 278 | 284 | |
| Number with information | 217 (78) | 228 (80) | |
| Any symptom since enrollment | 71 (33) | 82 (36) | 0.91 (0.70–1.18) |
| Symptoms reported | |||
| Fever | 62 (29) | 63 (28) | 1.03 (0.77–1.39) |
| Convulsions | 0 (0) | 0 (0) | NA |
| Othera | 71 (33) | 82 (36) | 0.91 (0.70–1.18) |
| Sought health centers | 20 (9) | 21 (9) | 1.00 (0.56–1.79) |
| Burkina Faso | |||
| Number visited | 480 | 501 | |
| Number with information | 473 (99) | 498 (99) | |
| Any symptom since enrollment | 98 (21) | 94 (19) | 1.10 (0.85–1.41) |
| Symptoms reported | |||
| Fever | 70 (15) | 74 (15) | 1.00 (0.74–1.35) |
| Convulsions | 0 (0) | 0 (0) | NA |
| Otherb | 68 (14) | 59 (12) | 1.21 (0.88–1.68) |
| Sought health centers | 47 (10) | 54 (11) | 0.92 (0.63–1.33) |
| Adverse Events Information . | Early Measles Vaccine, n (%) . | Control, n (%) . | Relative Risk (95% Confidence Interval) . |
|---|---|---|---|
| Guinea-Bissau | |||
| Number visited | 278 | 284 | |
| Number with information | 217 (78) | 228 (80) | |
| Any symptom since enrollment | 71 (33) | 82 (36) | 0.91 (0.70–1.18) |
| Symptoms reported | |||
| Fever | 62 (29) | 63 (28) | 1.03 (0.77–1.39) |
| Convulsions | 0 (0) | 0 (0) | NA |
| Othera | 71 (33) | 82 (36) | 0.91 (0.70–1.18) |
| Sought health centers | 20 (9) | 21 (9) | 1.00 (0.56–1.79) |
| Burkina Faso | |||
| Number visited | 480 | 501 | |
| Number with information | 473 (99) | 498 (99) | |
| Any symptom since enrollment | 98 (21) | 94 (19) | 1.10 (0.85–1.41) |
| Symptoms reported | |||
| Fever | 70 (15) | 74 (15) | 1.00 (0.74–1.35) |
| Convulsions | 0 (0) | 0 (0) | NA |
| Otherb | 68 (14) | 59 (12) | 1.21 (0.88–1.68) |
| Sought health centers | 47 (10) | 54 (11) | 0.92 (0.63–1.33) |
Abbreviation: NA, not applicable.
Respiratory symptoms, 99 (early measles vaccine [MV]/control, 42/57); gastrointestinal symptoms, 50 (25/25); other, 11 (8/3) (overlapping symptoms).
Respiratory symptoms, 75 (early MV/control 40/35); gastrointestinal symptoms, 50 (20/30); other, 24 (13/11) (overlapping symptoms).
Reported Adverse Events Within the First 14 Days After Enrollment in the Early Measles Vaccine Trial in Guinea-Bissau and Burkina Faso
| Adverse Events Information . | Early Measles Vaccine, n (%) . | Control, n (%) . | Relative Risk (95% Confidence Interval) . |
|---|---|---|---|
| Guinea-Bissau | |||
| Number visited | 278 | 284 | |
| Number with information | 217 (78) | 228 (80) | |
| Any symptom since enrollment | 71 (33) | 82 (36) | 0.91 (0.70–1.18) |
| Symptoms reported | |||
| Fever | 62 (29) | 63 (28) | 1.03 (0.77–1.39) |
| Convulsions | 0 (0) | 0 (0) | NA |
| Othera | 71 (33) | 82 (36) | 0.91 (0.70–1.18) |
| Sought health centers | 20 (9) | 21 (9) | 1.00 (0.56–1.79) |
| Burkina Faso | |||
| Number visited | 480 | 501 | |
| Number with information | 473 (99) | 498 (99) | |
| Any symptom since enrollment | 98 (21) | 94 (19) | 1.10 (0.85–1.41) |
| Symptoms reported | |||
| Fever | 70 (15) | 74 (15) | 1.00 (0.74–1.35) |
| Convulsions | 0 (0) | 0 (0) | NA |
| Otherb | 68 (14) | 59 (12) | 1.21 (0.88–1.68) |
| Sought health centers | 47 (10) | 54 (11) | 0.92 (0.63–1.33) |
| Adverse Events Information . | Early Measles Vaccine, n (%) . | Control, n (%) . | Relative Risk (95% Confidence Interval) . |
|---|---|---|---|
| Guinea-Bissau | |||
| Number visited | 278 | 284 | |
| Number with information | 217 (78) | 228 (80) | |
| Any symptom since enrollment | 71 (33) | 82 (36) | 0.91 (0.70–1.18) |
| Symptoms reported | |||
| Fever | 62 (29) | 63 (28) | 1.03 (0.77–1.39) |
| Convulsions | 0 (0) | 0 (0) | NA |
| Othera | 71 (33) | 82 (36) | 0.91 (0.70–1.18) |
| Sought health centers | 20 (9) | 21 (9) | 1.00 (0.56–1.79) |
| Burkina Faso | |||
| Number visited | 480 | 501 | |
| Number with information | 473 (99) | 498 (99) | |
| Any symptom since enrollment | 98 (21) | 94 (19) | 1.10 (0.85–1.41) |
| Symptoms reported | |||
| Fever | 70 (15) | 74 (15) | 1.00 (0.74–1.35) |
| Convulsions | 0 (0) | 0 (0) | NA |
| Otherb | 68 (14) | 59 (12) | 1.21 (0.88–1.68) |
| Sought health centers | 47 (10) | 54 (11) | 0.92 (0.63–1.33) |
Abbreviation: NA, not applicable.
Respiratory symptoms, 99 (early measles vaccine [MV]/control, 42/57); gastrointestinal symptoms, 50 (25/25); other, 11 (8/3) (overlapping symptoms).
Respiratory symptoms, 75 (early MV/control 40/35); gastrointestinal symptoms, 50 (20/30); other, 24 (13/11) (overlapping symptoms).
Antibody levels in children were low at enrollment, only 21% of children in Guinea-Bissau and 4% of children in Burkina Faso had protective antibody levels (Table 4). At age 9 months, all but 4% of children in the control group (who had presumably been measles vaccinated/exposed [Supplementary Materials]) had lost protective maternal antibody levels.
Measles Antibody: Proportion Protected and Levels of Antibodies by Randomization Group and Age at Sampling, Stratified by Site
| . | Protective Antibody Level,a % (n/N) . | Test of No Difference . | Geometric Mean Concentration, mIU/mL (95%CI) . | Geometric Mean Ratio (95% CI) . | ||
|---|---|---|---|---|---|---|
| Early MV . | Control . | Early MV . | Control . | |||
| Guinea-Bissau | ||||||
| Child | ||||||
| Enrollment | 21 (41/196) | 22 (49/226) | 0.85 | 57 (49–66) | 59 (52–68) | 0.97 (0.79–1.18) |
| 9 months | 90 (128/142) | 3 (5/176) | <0.001 | 401 (342–471) | 11 (9–13) | 36.5 (29.1–45.7) |
| 24 months | 97 (96/99) | 97 (102/105) | 0.94 | 600 (512–705) | 699 (598–816) | 0.86 (0.69–1.07) |
| Mother | ||||||
| Enrollment | 95 (184/194) | 95 (207/218) | 0.96 | 685 (586–801) | 853 (727–999) | 0.80 (0.64–1.00) |
| Burkina Faso | ||||||
| Child | ||||||
| Enrollment | 3 (7/226) | 4 (9/221) | 0.62 | 19 (17–21) | 18 (16–21) | 1.06 (0.88–1.26) |
| 9 months | 93 (178/191) | 5 (9/187) | <0.0001 | 457 (392–533) | 10 (8–12) | 46.2 (36.5–58.6) |
| 15 months | 100 (90/90) | 97 (94/97) | 0.25 | 800 (681–940) | 614 (513–735) | 1.30 (1.02–1.66) |
| Mother | ||||||
| Enrollment | 93 (211/226) | 93 (201/216) | 0.90 | 634 (550–732) | 596 (504–706) | 1.06 (0.85–1.32) |
| . | Protective Antibody Level,a % (n/N) . | Test of No Difference . | Geometric Mean Concentration, mIU/mL (95%CI) . | Geometric Mean Ratio (95% CI) . | ||
|---|---|---|---|---|---|---|
| Early MV . | Control . | Early MV . | Control . | |||
| Guinea-Bissau | ||||||
| Child | ||||||
| Enrollment | 21 (41/196) | 22 (49/226) | 0.85 | 57 (49–66) | 59 (52–68) | 0.97 (0.79–1.18) |
| 9 months | 90 (128/142) | 3 (5/176) | <0.001 | 401 (342–471) | 11 (9–13) | 36.5 (29.1–45.7) |
| 24 months | 97 (96/99) | 97 (102/105) | 0.94 | 600 (512–705) | 699 (598–816) | 0.86 (0.69–1.07) |
| Mother | ||||||
| Enrollment | 95 (184/194) | 95 (207/218) | 0.96 | 685 (586–801) | 853 (727–999) | 0.80 (0.64–1.00) |
| Burkina Faso | ||||||
| Child | ||||||
| Enrollment | 3 (7/226) | 4 (9/221) | 0.62 | 19 (17–21) | 18 (16–21) | 1.06 (0.88–1.26) |
| 9 months | 93 (178/191) | 5 (9/187) | <0.0001 | 457 (392–533) | 10 (8–12) | 46.2 (36.5–58.6) |
| 15 months | 100 (90/90) | 97 (94/97) | 0.25 | 800 (681–940) | 614 (513–735) | 1.30 (1.02–1.66) |
| Mother | ||||||
| Enrollment | 93 (211/226) | 93 (201/216) | 0.90 | 634 (550–732) | 596 (504–706) | 1.06 (0.85–1.32) |
Abbreviations: CI, confidence interval; MV, measles vaccine.
Protective antibody level is ≥125 mIU/mL.
Measles Antibody: Proportion Protected and Levels of Antibodies by Randomization Group and Age at Sampling, Stratified by Site
| . | Protective Antibody Level,a % (n/N) . | Test of No Difference . | Geometric Mean Concentration, mIU/mL (95%CI) . | Geometric Mean Ratio (95% CI) . | ||
|---|---|---|---|---|---|---|
| Early MV . | Control . | Early MV . | Control . | |||
| Guinea-Bissau | ||||||
| Child | ||||||
| Enrollment | 21 (41/196) | 22 (49/226) | 0.85 | 57 (49–66) | 59 (52–68) | 0.97 (0.79–1.18) |
| 9 months | 90 (128/142) | 3 (5/176) | <0.001 | 401 (342–471) | 11 (9–13) | 36.5 (29.1–45.7) |
| 24 months | 97 (96/99) | 97 (102/105) | 0.94 | 600 (512–705) | 699 (598–816) | 0.86 (0.69–1.07) |
| Mother | ||||||
| Enrollment | 95 (184/194) | 95 (207/218) | 0.96 | 685 (586–801) | 853 (727–999) | 0.80 (0.64–1.00) |
| Burkina Faso | ||||||
| Child | ||||||
| Enrollment | 3 (7/226) | 4 (9/221) | 0.62 | 19 (17–21) | 18 (16–21) | 1.06 (0.88–1.26) |
| 9 months | 93 (178/191) | 5 (9/187) | <0.0001 | 457 (392–533) | 10 (8–12) | 46.2 (36.5–58.6) |
| 15 months | 100 (90/90) | 97 (94/97) | 0.25 | 800 (681–940) | 614 (513–735) | 1.30 (1.02–1.66) |
| Mother | ||||||
| Enrollment | 93 (211/226) | 93 (201/216) | 0.90 | 634 (550–732) | 596 (504–706) | 1.06 (0.85–1.32) |
| . | Protective Antibody Level,a % (n/N) . | Test of No Difference . | Geometric Mean Concentration, mIU/mL (95%CI) . | Geometric Mean Ratio (95% CI) . | ||
|---|---|---|---|---|---|---|
| Early MV . | Control . | Early MV . | Control . | |||
| Guinea-Bissau | ||||||
| Child | ||||||
| Enrollment | 21 (41/196) | 22 (49/226) | 0.85 | 57 (49–66) | 59 (52–68) | 0.97 (0.79–1.18) |
| 9 months | 90 (128/142) | 3 (5/176) | <0.001 | 401 (342–471) | 11 (9–13) | 36.5 (29.1–45.7) |
| 24 months | 97 (96/99) | 97 (102/105) | 0.94 | 600 (512–705) | 699 (598–816) | 0.86 (0.69–1.07) |
| Mother | ||||||
| Enrollment | 95 (184/194) | 95 (207/218) | 0.96 | 685 (586–801) | 853 (727–999) | 0.80 (0.64–1.00) |
| Burkina Faso | ||||||
| Child | ||||||
| Enrollment | 3 (7/226) | 4 (9/221) | 0.62 | 19 (17–21) | 18 (16–21) | 1.06 (0.88–1.26) |
| 9 months | 93 (178/191) | 5 (9/187) | <0.0001 | 457 (392–533) | 10 (8–12) | 46.2 (36.5–58.6) |
| 15 months | 100 (90/90) | 97 (94/97) | 0.25 | 800 (681–940) | 614 (513–735) | 1.30 (1.02–1.66) |
| Mother | ||||||
| Enrollment | 93 (211/226) | 93 (201/216) | 0.90 | 634 (550–732) | 596 (504–706) | 1.06 (0.85–1.32) |
Abbreviations: CI, confidence interval; MV, measles vaccine.
Protective antibody level is ≥125 mIU/mL.
Vaccinated children responded well to early MV, and ≥90% had protective antibody levels at age 9 months. In the final sample at 15/24 months, 97%–100% were protected (Table 4, Figure 3). Previous studies of 2-dose MV strategies have suggested that early MV is associated with lower final antibody levels [23]. A similar tendency was seen in Guinea-Bissau. However, in Burkina Faso where the maternal antibody level was particularly low (Supplementary Materials), an early 2-dose strategy was associated with a significantly higher final measles antibody level (geometric mean ratio, 1.30 [95% CI, 1.02–1.66]) than among the children who had followed the current recommendation of 1 dose at age 9 months (Table 4).
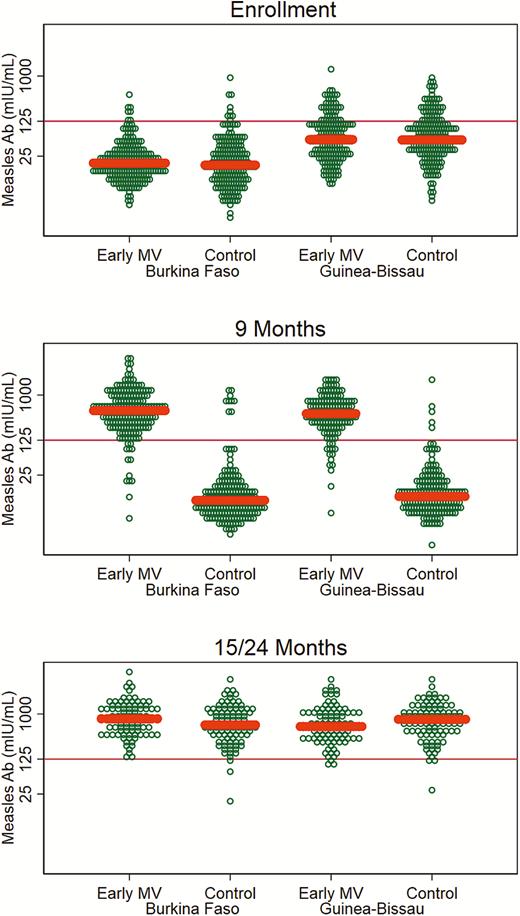
Measles antibody levels at enrollment, 9 months, and 15/24 months for children in the early measles vaccination trial in Burkina Faso and Guinea-Bissau. Median concentration by group indicated by thick line. Protective level: ≥125 mIU/mL. Abbreviations: Ab, antibody; MV, measles vaccine.
DISCUSSION
We found no effect of early MV on all-cause mortality in this trial. The vast majority of children were susceptible to measles infection at age 4–6 months, and practically all measles-unvaccinated children were susceptible by age 9 months. An early 2-dose vaccination schedule gave 97%–100% of the children protective antibodies.
Though the study was large, the power was lower than expected due to censoring of follow-up for children who received MV outside of the trial. This shortened the mean follow-up time in Burkina Faso considerably. Furthermore, in spite of censoring that reduced the follow-up time selectively more among the oldest children, the MR during the trial was 16/1000 person-years and thus much lower than the expected rate in both Guinea-Bissau (30/1000) and Burkina Faso (25/1000). The mortality has fallen markedly at both sites [24, 25]. Though this is certainly a celebratory finding, it meant that we had only half the deaths we anticipated in the study. However, there is no indication that had we had the planned power, we might have found a difference in child mortality. By nesting the trial in the HDSS sites, we were able to follow the enrolled children, and the comparison between the 2 groups was not affected by a loss to follow-up.
Participants were not blind to group allocation, and we used no placebo vaccine. However, with mortality as the outcome, the risk of a differential reporting by group allocation seems unlikely. It could be speculated that mothers who thought that their children were healthier because they had been allocated to the intervention group could have a different threshold for seeking healthcare. We found no indication that this was the case; the number of deaths that occurred in health facilities was similar in the 2 groups.
We assigned causes of death based on verbal autopsies and available health facility data. The diagnostic tools are limited in both settings and the classifications uncertain. Nevertheless, we consider the verbal autopsy data sufficiently accurate to be able to censor deaths due to accidents.
We tested a previous finding that early MV has the potential to lower nonmeasles mortality, but found no effect. Infectious disease is the main cause of death in the studied age group, and the decline in mortality is mainly due to fewer deaths from infectious diseases [26]. Hence, the much lower mortality level may have meant that the remaining causes of death were not influenced by a beneficial NSE of MV.
Other interventions may also have neutralized differences between the 2 randomized groups. Due to previous findings that receiving diphtheria-tetanus-pertussis vaccine (DTP) after high-titer MV was associated with increased female mortality [27] and that receiving neonatal vitamin A supplementation (NVAS) removed the beneficial effect of early MV [10], we enrolled per-protocol-only children who had completed all 3 doses of DTP-containing vaccine and had not received NVAS in our 2-dose trial of standard-titer MV. Moreover, when we planned the trial, we were not aware of any interaction with OPV. Recently, we reanalyzed data from the previous positive early MV trial and found that campaign OPV provided before trial enrollment reduced mortality, especially among children in the control group, and there was no benefit of early MV in this group [28]. Furthermore, campaign OPV before enrollment was associated with a significant reduction in the load of pneumococcus in the control group compared with the early MV group [29]. OPV campaigns became more frequent during the present trial; 80% of our trial children were exposed to OPV campaigns prior to enrollment, whereas only 21% had been exposed in the previous trial [28]. This is another possible explanation as to why early MV had no detectable effect on child survival in the present trial.
It could also be speculated that the use of Penta rather than DTP and the introduction of pneumococcal and rota virus vaccines in the routine vaccination program could have had an impact by reducing infections with pathogens that could otherwise have been prevented nonspecifically by early MV. Yellow fever vaccines were also not part of the routine vaccination program in the prior trial. Prior studies suggest that addition of yellow fever vaccine does not alter the effect of the 9-month MV [30, 31], and we found no indication that this explains why there was no effect in the present trial. However, data are scarce.
In rural Guinea-Bissau, the proportion of mothers with protective measles antibodies was comparable to the level found in the prior trial in urban Guinea-Bissau [23], but the median concentration was lower. The proportion of infants with protective antibodies at the time of enrollment (21%) in Guinea-Bissau was also similar to that in the prior trial, but the proportion in Burkina Faso (4%) was much lower. None of the measles-unvaccinated/-unexposed children had protective measles antibodies at age 9 months. Consistent with the very low proportion of children with protective antibody levels at age 4 months in Burkina Faso, their mothers also had much lower antibody levels than the mothers of Guinean children. Concerns about early waning of levels of measles antibodies have been raised [3, 5, 32]. The present data indicate that this may happen much earlier than age 9 months, leaving many infants susceptible to measles infection.
In the previous trial, the effect of early MV was particularly pronounced among children who had maternal measles antibodies at the time of vaccination [33]. The lack of an effect in the present trial is unlikely to be due merely to the lower levels of maternal antibodies in the present trial since maternal antibodies were still present in Guinea-Bissau.
While the present trial does not confirm that an early measles vaccination strategy would lower mortality (with likely explanations given above), it supports the belief that early measles vaccination may be important for improved measles control. In contrast to the assumed negative effect on long-term measles immunity [34], we did not find any indication that earlier vaccination using a 2-dose strategy hampered long-term immunity. Instead, with the low level of prevaccination antibodies observed in Burkina Faso and the higher attained final antibody level in the early MV group, earlier vaccination may improve measles control. We also found no indication that there are any severe adverse reactions connected with early vaccination. Since early vaccination with Edmonston-Zagreb MV is both safe and effective in controlling measles infection, it should be considered in future vaccination strategies.
In conclusion, early MV did not reduce all-cause mortality in this 2-center trial. This could possibly be explained by interference from the NSEs of the frequent OPV campaigns [28, 35]. If that is the case, with the current phase-out of OPV campaigns [36, 37] and with the worldwide cessation of OPV, it would still be worthwhile to examine whether early MV has beneficial NSEs in addition to its specific protective effects against measles infection.
Nearly all enrolled children in both Burkina Faso and Guinea-Bissau were susceptible to measles infection well before the currently recommended age for MV. Providing the first dose of MV at age 9 months may therefore not be ideal to control measles infection, and a 2-dose MV strategy with an early MV followed by MV at 9 months would provide better protection against measles infection.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. A. B. F., E. N., A. Si., C. M., A. R., H. B., H. C. W., O. M., C. S. B., and P.A. designed the trial. A. B. F., E. N., A. Si., C. M., A. R., A. Z., M. K., S. B., S. M. T., J. T., B. C., and F. K. obtained the data. A. B. F., A. Sc., H. B., and P.A. conducted or supervised the analysis. A. B. F., E. N., A. Sc., H. B., H. C. W., O. M., C. S. B., and P. A. interpreted the data. A. B. F., E. N., A. Sc., H. C. W., F. K., O. M., C. S. B., and P. A. were the core writing team for the manuscript.
Acknowledgments. We are indebted to the Data Safety and Ethics Monitoring Board, which included Professor Kim Mulholland, Dr Rana Hajjeh, and Dr Jukka Jokinen who oversaw the trial and provided advice on implementation. We thank Dr Henrik Ravn for assistance in trial design and valuable input to the analysis plan, the dedicated field teams at the Bandim and Nouna health and demographic surveillance systems and the personnel who implemented and supervised data collection, entry, and cleaning (Marie Pedersen, Line Storgaard, Bibi Uhre Nielsen, Katarina Funch, Jesper Sloth Hansen, Mette Møller Jensen, Gauthier Tougri, Mamadou Bountogo, Cheick Bagagnan, Maurice Yé, and the Nouna Health District team). We thank the Burkina Faso and Guinea-Bissau Expanded Programme on Immunization for collaboration and all the study participants and their families.
Financial support. This work was supported by the European Union FP7 support for OPTIMUNISE (Health-F3-2011-261375). Grants from the Danish Council for Independent Research (DFF-1333-00192), Fonden af 17-12-1981, The Danish National Research Foundation via CVIVA (DNRF108), and The Novo Nordisk Foundation supported the work of some of the authors.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
A. B. F. and E. N. contributed equally to the article.




