-
PDF
- Split View
-
Views
-
Cite
Cite
Yarden Yavne, Eyal Leshem, Yael Paran, Eyal Nadir, Miriam Weinberger, Michal Stein, Neta Petersiel, Dafna Yahav, Tamar Grossman, Eli Schwartz, Plasmodium malariae in Israeli Travelers: A Nationwide Study, Clinical Infectious Diseases, Volume 65, Issue 9, 1 November 2017, Pages 1516–1522, https://doi.org/10.1093/cid/cix560
Close - Share Icon Share
Abstract
Little is known about Plasmodium malariae, a relatively rare cause of malaria in returned travelers. Recently, polymerase chain reaction (PCR) use for malaria diagnosis has enhanced specificity of P. malariae detection. The study objective was to describe the unique aspects of P. malariae diagnosis and clinical course in travelers.
Malaria is a reportable disease in Israel. All PCR-proven P. malariae monoinfections in Israeli travelers between January 2008 and January 2017 were retrieved from the Ministry of Health Reference Parasitology Laboratory. Data regarding method and timing of diagnosis, clinical characteristics, and laboratory testing were collected from patient charts.
Eighteen patients with P. malariae were included. All cases were acquired in Africa. During the study period, the relative proportion of P. malariae increased (2%–10% of all malaria cases). Malaria was identified by blood smear in 10 of 18 patients (56%) on admission, and by rapid antigen test in 5 of 18 (29%) patients only, while P. malariae speciation was correctly identified by smear in 2 of 18 (11%) patients. Though all patients reported fever, only 4 of 18 (22%) described a quartan fever course. In 7 of 18 (39%) patients, malaria was contracted despite prophylactic treatment. Five patients had prolonged prepatent periods (median, 55 days), all of whom received prior prophylaxis.
The relative proportion of P. malariae is on the rise. Diagnosis in routine clinical settings is inadequate due to the low sensitivity and specificity of blood smears. PCR should be considered when clinical suspicion is high. Prophylaxis failure, which caused delayed clinical presentation, was documented.
Plasmodium malariae is 1 of the 5 Plasmodium species that infect humans and is known for the quartan (every 72 hours) fever pattern in patients [1]. Plasmodium malariae is endemic throughout tropical Africa, Southeast Asia, and Central and South America [1–3]. Plasmodium malariae is notable for its ability to recrudesce and generate an infection, sometimes years after the initial exposure, a characteristic attributed to the presence of dormant forms that have yet to be found [1, 4–6].
Diagnosis of P. malariae is customarily reached when the characteristic ring stages and band forms can be seen within the normal-sized erythrocyte on a thin blood smear [1]. Lately, several studies have suggested that this method is less sensitive for detection of P. malariae infections, resulting in a high percentage of false-negative results and occasional misdiagnoses [4, 7–12]. Similarly, there is mounting evidence that rapid antigen tests often produce false-negative results in P. malariae cases [10, 12–17]. Polymerase chain reaction (PCR), which is considered to be highly sensitive and specific for P. malariae diagnosis, has subsequently uncovered a higher than suspected relative proportion of P. malariae among malaria patients [1, 18], reaching a prevalence of 23%–39% in symptomatic populations of sub-Saharan Africa and the Southwest Pacific [18].
Plasmodium malariae is not as common as other Plasmodium species, yet it has several unique features as to its recrudescent abilities and diagnosis. However, information regarding the diagnosis, clinical course, and laboratory findings of P. malariae in returned travelers is scarce. The objective of the current study is to report PCR-proven P. malariae monoinfections diagnosed in Israel and the unique clinical and diagnostic characteristics of these patients.
MATERIALS AND METHODS
Plasmodium malariae is a reportable disease in Israel, and all patients suspected of malarial infection are hospitalized. Thin and thick blood smears and rapid malaria diagnostic tests (RDTs) are routinely performed. If a smear or RDT result is positive, or if clinical suspicion for malaria is high, blood samples are sent out for confirmatory real-time PCR analysis at the national Reference Parasitology Laboratory. In this study, we included Israeli patients who contracted a PCR-proven P. malariae monoinfection following a visit in an endemic area during January 2008 and January 2017. PCR diagnoses of P. malariae were retrieved from the Israeli Ministry of Health Reference Parasitology Laboratory registry. The study was approved by Sheba Medical Center’s ethics committee.
Blood samples of patients were tested at the Reference Parasitology Laboratory using an implemented real-time PCR. This test utilizes a general pan-species assay, as well as species-specific assays [12]. In brief, DNA is extracted from 200 μL of blood with ethylenediaminetetraacetic acid, either manually, using the QIAGEN blood and tissue kit (catalog number 69506), or via NUCLISENS easyMAG platform (bioMérieux), according to the manufacturer’s protocol. Elution volume is 200 μL with QIAGEN or 110 μL and adjusted to 200 μL when using easyMAG. Real-time PCR is performed essentially as described by Grossman et al [12]. Sensitivity reports for this assay are high, detecting 1–10 copies of extracted DNA/5 μL of diluted positive controls [19, 20]. The assay is run on an Applied Biosystems 7500 Real-Time PCR machine, using 5 μL of eluted DNA in a total reaction volume of 20 μL.
We contacted infectious diseases units at all hospitals in which P. malariae patients were admitted, and data regarding patient demographics, travel history, diagnosis, clinical, laboratory analyses, treatment, and outcomes were extracted retrospectively from patient charts and laboratory records. The rapid antigen test kits used in this study were VIKIA, BinaxNow, and Carestart, all of which contain antibodies to pan-species antigens. The standard of number of fields scanned during microscopic evaluation of thick blood smears varied among laboratories (range, 10–500 fields/20 µL of blood). Treatment regimens were chosen according to the suspected instigating Plasmodium species. Time to defervescence was defined as a temperature <37.5°C that was stable for at least 72 hours after treatment commencement.
Continuous variables were expressed as the median and interquartile range (IQR) and categorical variables as a percentage. Fisher exact test (2-tailed) was used to compute P values in the prevalence assessment. A P value <.05 was considered significant.
RESULTS
During January 2008–January 2017, 20 of 358 (5.5%) of PCR-diagnosed malaria cases at the Reference Parasitology Laboratory of the Israeli Ministry of Health were P. malariae. Two patients for whom P. malariae was identified as part of a mixed infection were excluded from clinical analyses. Thus, 18 cases were included in the analyses, all of which were contracted during a visit to a malaria-endemic country. The proportion of P. malariae infections among all malaria cases has significantly increased in the past 3 years, from 2.1% at the beginning of the study to 10.1% (P = .02; Figure 1).
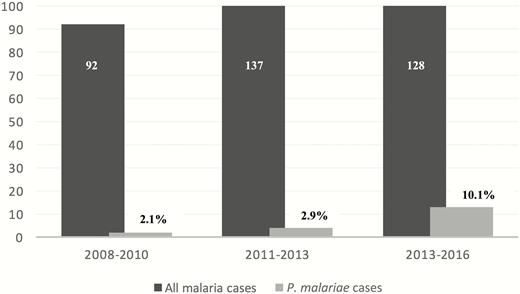
Proportion of Plasmodium malariae infection among all malaria cases, Israel, 2008–2016.
Demographics and Travel History
The median age of patients was 43 years (range 21–66 years) and 17 of 18 (94%) were males. Most of the patients were expatriates residing in endemic regions or traveling for business purposes (13/18 [72%]), and the rest were tourists. All cases were acquired in Africa, of which 6 (33%) were acquired in Angola (Table 1). Seven patients (39%) contracted malaria despite reported use of antimalarial prophylaxis, of whom 5 received atovaquone/proguanil (AP) and 2 mefloquine. The interval in days between date of departure from the endemic country and symptom onset varied considerably, ranging from 0 (the presence of fever before return to Israel) to 210 days. The median interval of the 7 patients who received prophylaxis was substantially longer than that of the other 11 patients (47 vs 7 days, respectively; Figure 2).
Characteristics and Travel Details of Patients With Plasmodium malariae, Israel, January 2008–January 2017 (N = 18)
| Patient . | Sex/Age, y . | Type of Trip . | Country of Probable Infection . | Year of Diagnosis . | Prophylaxis, Yes/No (Type) . | Days From Symptoms Onset to PCR Diagnosis . | rt-PCR Ct Value . | RDT Kit Used for Diagnosis . |
|---|---|---|---|---|---|---|---|---|
| 1 | M/51 | Expatriate | Angola | 2008 | Yes (mefloquine) | 207 | 29.3 | … |
| 2 | M/54 | Expatriate | Equatorial Guinea | 2011 | No | 11 | 18.5 | CarestartMalaria Pan/Pf |
| 3 | M/49 | Business | Sierra Leone | 2012 | Yes (AP) | 9 | 28 | CarestartMalaria Pan/Pf |
| 4 | M/66 | Expatriate | Angola | 2014 | Yes (AP) | 11 | 30.4 | CarestartMalaria Pan/Pf |
| 5 | M/53 | Business | Angola | 2015 | No | 8 | 31.5 | CarestartMalaria Pan/Pf |
| 6 | M/24 | Business | Angola | 2016 | Yes (AP) | 30 | 33 | CarestartMalaria Pan/Pf |
| 7 | M/63 | Expatriate | Nigeria | 2015 | No | … | 28.6 | Vikia Malaria Ag Pf/Pan |
| 8 | M/51 | Business | Angola | 2009 | No | 31 | 26.5 | CarestartMalaria Pan/Pf |
| 9 | M/29 | Tourism | South Africa, Namibia, Botswana, Zimbabwe, Mozambique | 2013 | No | 51 | 27.5 | CarestartMalaria Pan/Pf |
| 10 | M/21 | Tourism | Sierra Leone | 2014 | Yes (AP) | 12 | 26.6 | CarestartMalaria Pan/Pf |
| 11 | F/27 | Tourism | Ethiopia | 2014 | No | 22 | 22 | CarestartMalaria Pan/Pf |
| 12 | M/47 | Business | Gabon | 2014 | Yes (mefloquine) | 24 | 27.5 | Unknown |
| 13 | M/56 | Expatriate | Angola | 2016 | No | 29 | 28 | CarestartMalaria Pan/Pf |
| 14 | M/33 | Business | Ghana | 2011 | No | … | 31 | Unknown |
| 15 | M/26 | Tourism | Malawi, Ethiopia, Tanzania, Kenya, Uganda | 2016 | No | 88 | 28 | BinaxNow Malaria |
| 16 | M/23 | Business | Equatorial Guinea | 2016 | Yes (AP) | 17 | 26 | CarestartMalaria Pan/Pf |
| 17 | M/39 | Tourism | Mozambique | 2016 | No | 15 | 22 | CarestartMalaria Pan/Pf |
| 18 | M/22 | Business | Equatorial Guinea | 2017 | No | 15 | 32 | CarestartMalaria Pan/Pf |
| Patient . | Sex/Age, y . | Type of Trip . | Country of Probable Infection . | Year of Diagnosis . | Prophylaxis, Yes/No (Type) . | Days From Symptoms Onset to PCR Diagnosis . | rt-PCR Ct Value . | RDT Kit Used for Diagnosis . |
|---|---|---|---|---|---|---|---|---|
| 1 | M/51 | Expatriate | Angola | 2008 | Yes (mefloquine) | 207 | 29.3 | … |
| 2 | M/54 | Expatriate | Equatorial Guinea | 2011 | No | 11 | 18.5 | CarestartMalaria Pan/Pf |
| 3 | M/49 | Business | Sierra Leone | 2012 | Yes (AP) | 9 | 28 | CarestartMalaria Pan/Pf |
| 4 | M/66 | Expatriate | Angola | 2014 | Yes (AP) | 11 | 30.4 | CarestartMalaria Pan/Pf |
| 5 | M/53 | Business | Angola | 2015 | No | 8 | 31.5 | CarestartMalaria Pan/Pf |
| 6 | M/24 | Business | Angola | 2016 | Yes (AP) | 30 | 33 | CarestartMalaria Pan/Pf |
| 7 | M/63 | Expatriate | Nigeria | 2015 | No | … | 28.6 | Vikia Malaria Ag Pf/Pan |
| 8 | M/51 | Business | Angola | 2009 | No | 31 | 26.5 | CarestartMalaria Pan/Pf |
| 9 | M/29 | Tourism | South Africa, Namibia, Botswana, Zimbabwe, Mozambique | 2013 | No | 51 | 27.5 | CarestartMalaria Pan/Pf |
| 10 | M/21 | Tourism | Sierra Leone | 2014 | Yes (AP) | 12 | 26.6 | CarestartMalaria Pan/Pf |
| 11 | F/27 | Tourism | Ethiopia | 2014 | No | 22 | 22 | CarestartMalaria Pan/Pf |
| 12 | M/47 | Business | Gabon | 2014 | Yes (mefloquine) | 24 | 27.5 | Unknown |
| 13 | M/56 | Expatriate | Angola | 2016 | No | 29 | 28 | CarestartMalaria Pan/Pf |
| 14 | M/33 | Business | Ghana | 2011 | No | … | 31 | Unknown |
| 15 | M/26 | Tourism | Malawi, Ethiopia, Tanzania, Kenya, Uganda | 2016 | No | 88 | 28 | BinaxNow Malaria |
| 16 | M/23 | Business | Equatorial Guinea | 2016 | Yes (AP) | 17 | 26 | CarestartMalaria Pan/Pf |
| 17 | M/39 | Tourism | Mozambique | 2016 | No | 15 | 22 | CarestartMalaria Pan/Pf |
| 18 | M/22 | Business | Equatorial Guinea | 2017 | No | 15 | 32 | CarestartMalaria Pan/Pf |
Abbreviations: AP, atovaquone/proguanil; Ct, cycle threshold; F, female; M, male; RDT, rapid diagnostic test; rt-PCR Ct, real-time polymerase chain reaction cycle threshold.
Characteristics and Travel Details of Patients With Plasmodium malariae, Israel, January 2008–January 2017 (N = 18)
| Patient . | Sex/Age, y . | Type of Trip . | Country of Probable Infection . | Year of Diagnosis . | Prophylaxis, Yes/No (Type) . | Days From Symptoms Onset to PCR Diagnosis . | rt-PCR Ct Value . | RDT Kit Used for Diagnosis . |
|---|---|---|---|---|---|---|---|---|
| 1 | M/51 | Expatriate | Angola | 2008 | Yes (mefloquine) | 207 | 29.3 | … |
| 2 | M/54 | Expatriate | Equatorial Guinea | 2011 | No | 11 | 18.5 | CarestartMalaria Pan/Pf |
| 3 | M/49 | Business | Sierra Leone | 2012 | Yes (AP) | 9 | 28 | CarestartMalaria Pan/Pf |
| 4 | M/66 | Expatriate | Angola | 2014 | Yes (AP) | 11 | 30.4 | CarestartMalaria Pan/Pf |
| 5 | M/53 | Business | Angola | 2015 | No | 8 | 31.5 | CarestartMalaria Pan/Pf |
| 6 | M/24 | Business | Angola | 2016 | Yes (AP) | 30 | 33 | CarestartMalaria Pan/Pf |
| 7 | M/63 | Expatriate | Nigeria | 2015 | No | … | 28.6 | Vikia Malaria Ag Pf/Pan |
| 8 | M/51 | Business | Angola | 2009 | No | 31 | 26.5 | CarestartMalaria Pan/Pf |
| 9 | M/29 | Tourism | South Africa, Namibia, Botswana, Zimbabwe, Mozambique | 2013 | No | 51 | 27.5 | CarestartMalaria Pan/Pf |
| 10 | M/21 | Tourism | Sierra Leone | 2014 | Yes (AP) | 12 | 26.6 | CarestartMalaria Pan/Pf |
| 11 | F/27 | Tourism | Ethiopia | 2014 | No | 22 | 22 | CarestartMalaria Pan/Pf |
| 12 | M/47 | Business | Gabon | 2014 | Yes (mefloquine) | 24 | 27.5 | Unknown |
| 13 | M/56 | Expatriate | Angola | 2016 | No | 29 | 28 | CarestartMalaria Pan/Pf |
| 14 | M/33 | Business | Ghana | 2011 | No | … | 31 | Unknown |
| 15 | M/26 | Tourism | Malawi, Ethiopia, Tanzania, Kenya, Uganda | 2016 | No | 88 | 28 | BinaxNow Malaria |
| 16 | M/23 | Business | Equatorial Guinea | 2016 | Yes (AP) | 17 | 26 | CarestartMalaria Pan/Pf |
| 17 | M/39 | Tourism | Mozambique | 2016 | No | 15 | 22 | CarestartMalaria Pan/Pf |
| 18 | M/22 | Business | Equatorial Guinea | 2017 | No | 15 | 32 | CarestartMalaria Pan/Pf |
| Patient . | Sex/Age, y . | Type of Trip . | Country of Probable Infection . | Year of Diagnosis . | Prophylaxis, Yes/No (Type) . | Days From Symptoms Onset to PCR Diagnosis . | rt-PCR Ct Value . | RDT Kit Used for Diagnosis . |
|---|---|---|---|---|---|---|---|---|
| 1 | M/51 | Expatriate | Angola | 2008 | Yes (mefloquine) | 207 | 29.3 | … |
| 2 | M/54 | Expatriate | Equatorial Guinea | 2011 | No | 11 | 18.5 | CarestartMalaria Pan/Pf |
| 3 | M/49 | Business | Sierra Leone | 2012 | Yes (AP) | 9 | 28 | CarestartMalaria Pan/Pf |
| 4 | M/66 | Expatriate | Angola | 2014 | Yes (AP) | 11 | 30.4 | CarestartMalaria Pan/Pf |
| 5 | M/53 | Business | Angola | 2015 | No | 8 | 31.5 | CarestartMalaria Pan/Pf |
| 6 | M/24 | Business | Angola | 2016 | Yes (AP) | 30 | 33 | CarestartMalaria Pan/Pf |
| 7 | M/63 | Expatriate | Nigeria | 2015 | No | … | 28.6 | Vikia Malaria Ag Pf/Pan |
| 8 | M/51 | Business | Angola | 2009 | No | 31 | 26.5 | CarestartMalaria Pan/Pf |
| 9 | M/29 | Tourism | South Africa, Namibia, Botswana, Zimbabwe, Mozambique | 2013 | No | 51 | 27.5 | CarestartMalaria Pan/Pf |
| 10 | M/21 | Tourism | Sierra Leone | 2014 | Yes (AP) | 12 | 26.6 | CarestartMalaria Pan/Pf |
| 11 | F/27 | Tourism | Ethiopia | 2014 | No | 22 | 22 | CarestartMalaria Pan/Pf |
| 12 | M/47 | Business | Gabon | 2014 | Yes (mefloquine) | 24 | 27.5 | Unknown |
| 13 | M/56 | Expatriate | Angola | 2016 | No | 29 | 28 | CarestartMalaria Pan/Pf |
| 14 | M/33 | Business | Ghana | 2011 | No | … | 31 | Unknown |
| 15 | M/26 | Tourism | Malawi, Ethiopia, Tanzania, Kenya, Uganda | 2016 | No | 88 | 28 | BinaxNow Malaria |
| 16 | M/23 | Business | Equatorial Guinea | 2016 | Yes (AP) | 17 | 26 | CarestartMalaria Pan/Pf |
| 17 | M/39 | Tourism | Mozambique | 2016 | No | 15 | 22 | CarestartMalaria Pan/Pf |
| 18 | M/22 | Business | Equatorial Guinea | 2017 | No | 15 | 32 | CarestartMalaria Pan/Pf |
Abbreviations: AP, atovaquone/proguanil; Ct, cycle threshold; F, female; M, male; RDT, rapid diagnostic test; rt-PCR Ct, real-time polymerase chain reaction cycle threshold.
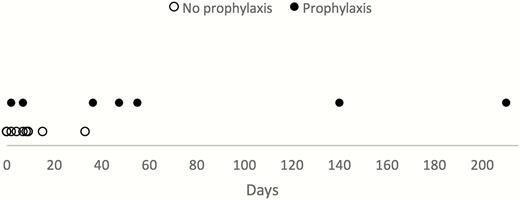
Comparison of Plasmodium malariae interval in patients with and without prior antimalarial prophylaxis, Israel, January 2008–January 2017 (N = 18).
Diagnosis
Diagnosis may be defined as a general diagnosis of Plasmodium infection without species identification or as the detection of the specific Plasmodium species (in this case, malariae). With regard to general malaria diagnosis, initial blood smears at admission were recognized as positive for any type of Plasmodium parasites in 10 of 18 (56%) patients. Rapid antigen tests, which were performed in 17 patients during hospitalization, yielded a diagnosis of non-falciparum malaria in 5 of 17 patients (29%). When combining the results of both diagnostic methods, 10 of 18 (56%) patients were identified as having malaria on admission. Plasmodium was eventually identified by smear or rapid antigen test during hospitalization for 17 of 18 (94%) patients (within a median of 2 days). One patient was diagnosed only by PCR, 88 days after symptoms onset.
The species P. malariae was not diagnosed on initial blood smear of any of the patients and was mistaken for a different species in 3 cases (once as Plasmodium falciparum and twice as Plasmodium vivax). Plasmodium malariae was identified on follow-up blood smears for 2 of 18 patients (11%); thus, correct speciation of P. malariae was added by PCR in 16 of 18 patients (89%) (Figure 3). Therefore, although most patients were diagnosed with malaria shortly after admission, the median time to identification of P. malariae as the causative species after hospitalization was 38 days (Table 2).
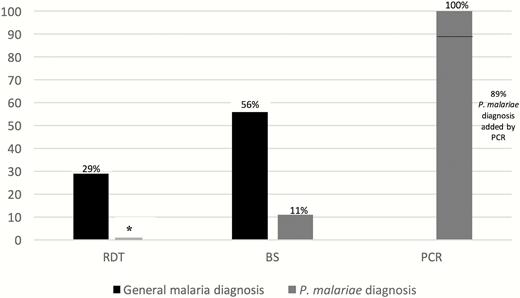
Comparing the detection rate of any Plasmodium on admission and of Plasmodium malariae by different diagnostic methods (N = 18). *Rapid diagnostic test cannot provide a diagnosis of P. malariae. Abbreviations: BS, blood smear; PCR, polymerase chain reaction; RDT, rapid diagnostic test.
Timeline of Plasmodium malariae Diagnosis, Israel, January 2008–January 2017 (N = 18)
| Median No. of Days (IQR) From Departure From Endemic Country Until: . | |||
|---|---|---|---|
| Onset of Symptoms . | Hospital Admission . | Diagnosis of Malaria . | Diagnosis of P. malariae . |
| 8.5 (32.75) | 14 (43) | 26 (45) | 52 (56.25) |
| Median No. of Days (IQR) From Departure From Endemic Country Until: . | |||
|---|---|---|---|
| Onset of Symptoms . | Hospital Admission . | Diagnosis of Malaria . | Diagnosis of P. malariae . |
| 8.5 (32.75) | 14 (43) | 26 (45) | 52 (56.25) |
Abbreviation: IQR, interquartile range.
Timeline of Plasmodium malariae Diagnosis, Israel, January 2008–January 2017 (N = 18)
| Median No. of Days (IQR) From Departure From Endemic Country Until: . | |||
|---|---|---|---|
| Onset of Symptoms . | Hospital Admission . | Diagnosis of Malaria . | Diagnosis of P. malariae . |
| 8.5 (32.75) | 14 (43) | 26 (45) | 52 (56.25) |
| Median No. of Days (IQR) From Departure From Endemic Country Until: . | |||
|---|---|---|---|
| Onset of Symptoms . | Hospital Admission . | Diagnosis of Malaria . | Diagnosis of P. malariae . |
| 8.5 (32.75) | 14 (43) | 26 (45) | 52 (56.25) |
Abbreviation: IQR, interquartile range.
PCR detected P. malariae at cycle threshold (Ct) ranges of 18.5–33 (Table 1). The median Ct required for P. malariae diagnosis was significantly higher than that required for P. falciparum or P. vivax and Plasmodium ovale diagnosis (28 vs 24 and 24, respectively), indicating lower parasitemia in P. malariae. For several patients in whom parasitemia was assessed, parasite densities ranged from 100/μL to 390/µL.
Clinical Features
Although only 8 of 16 (50%) patients were febrile on admission, all patients reported a history of fever. The median maximal temperature reported was 39.9°C (range 38.5–41°C).The nature of the fever course differed between patients, with only 4 of 18 (22%) reporting a quartan course and 5 of 18 (27%) reporting a tertiary course. Other common symptoms were chills (13/18 [72%]), headache (8/18 [44%]), and significant weakness (6/18 [33%]) (Table 3). Diagnosis of general malaria was reached within a median of 12 days from hospitalization.
Laboratory Investigations
Overall, 12 of 18 patients (66%) were anemic: 7 of 18 (39%) presented with anemia and an additional 5 of 18 (28%) developed anemia during hospitalization. The most common laboratory abnormality was thrombocytopenia (16/18 [89%]), and the majority presented with thrombocytopenia on admission (13/18 [72%]) (Table 3).
Clinical and Laboratory Characteristics of Plasmodium malariae Patients, Israel, January 2008–January 2017 (N = 18)
| Characteristic . | Subjects With Information Available, No. . | Symptoms, No. (%) . | |
|---|---|---|---|
| Symptom | |||
| Fever history reported | 18 | 18 (100) | |
| Chills | 18 | 13 (72) | |
| Headache | 18 | 8 (44) | |
| Weakness | 18 | 6 (33) | |
| Diarrhea | 18 | 4 (22) | |
| Myalgia | 18 | 4 (22) | |
| Neck ache | 18 | 4 (22) | |
| Nausea | 18 | 3 (17) | |
| Cough | 18 | 2 (11) | |
| Vomiting | 18 | 2 (11) | |
| Night sweats | 18 | 2 (11) | |
| Dyspnea | 18 | 1 (6) | |
| Dysuria | 18 | 1 (6) | |
| Sign | |||
| Tachycardia (>95 bpm) | 16 | 8 (50) | |
| Fever at presentation | 16 | 8 (50) | |
| Pallor | 18 | 4 (22) | |
| Jaundice | 18 | 2 (11) | |
| Splenomegaly on PE or AUS | 18 | 6 (33) | |
| Median (IQR) | % of Patients With Abnormal Results | ||
| Laboratory parameter | |||
| Hemoglobin, g/dL (nadir) | 18 | 12.54 (2.37) | 67 |
| WBC count, K/μL (nadir) | 17 | 4.2 (1.99) | 39 |
| Absolute lymphocyte count, K/μL (nadir) | 17 | 0.6 (0.98) | 59 |
| Platelets, K/μL (nadir) | 18 | 99.5 (44.75) | 89 |
| Total bilirubin, mg/dL (max) | 17 | 1.18 (0.48) | 65 |
| LDH, IU/L (max) | 15 | 357 (153) | 73 |
| AST, IU/L (max) | 16 | 37.5 (26.25) | 44 |
| ALT, IU/L (max) | 17 | 50 (24) | 53 |
| Creatinine, mg/dL (max) | 17 | 1.03 (0.23) | 71 |
| INR (max) | 14 | 1.17 (0.12) | 11 |
| Characteristic . | Subjects With Information Available, No. . | Symptoms, No. (%) . | |
|---|---|---|---|
| Symptom | |||
| Fever history reported | 18 | 18 (100) | |
| Chills | 18 | 13 (72) | |
| Headache | 18 | 8 (44) | |
| Weakness | 18 | 6 (33) | |
| Diarrhea | 18 | 4 (22) | |
| Myalgia | 18 | 4 (22) | |
| Neck ache | 18 | 4 (22) | |
| Nausea | 18 | 3 (17) | |
| Cough | 18 | 2 (11) | |
| Vomiting | 18 | 2 (11) | |
| Night sweats | 18 | 2 (11) | |
| Dyspnea | 18 | 1 (6) | |
| Dysuria | 18 | 1 (6) | |
| Sign | |||
| Tachycardia (>95 bpm) | 16 | 8 (50) | |
| Fever at presentation | 16 | 8 (50) | |
| Pallor | 18 | 4 (22) | |
| Jaundice | 18 | 2 (11) | |
| Splenomegaly on PE or AUS | 18 | 6 (33) | |
| Median (IQR) | % of Patients With Abnormal Results | ||
| Laboratory parameter | |||
| Hemoglobin, g/dL (nadir) | 18 | 12.54 (2.37) | 67 |
| WBC count, K/μL (nadir) | 17 | 4.2 (1.99) | 39 |
| Absolute lymphocyte count, K/μL (nadir) | 17 | 0.6 (0.98) | 59 |
| Platelets, K/μL (nadir) | 18 | 99.5 (44.75) | 89 |
| Total bilirubin, mg/dL (max) | 17 | 1.18 (0.48) | 65 |
| LDH, IU/L (max) | 15 | 357 (153) | 73 |
| AST, IU/L (max) | 16 | 37.5 (26.25) | 44 |
| ALT, IU/L (max) | 17 | 50 (24) | 53 |
| Creatinine, mg/dL (max) | 17 | 1.03 (0.23) | 71 |
| INR (max) | 14 | 1.17 (0.12) | 11 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUS, abdominal ultrasound; bpm, beats per minute; INR, international normalized ratio; IQR, interquartile range; LDH, lactate dehydrogenase; PE, physical examination; WBC, white blood cell.
Clinical and Laboratory Characteristics of Plasmodium malariae Patients, Israel, January 2008–January 2017 (N = 18)
| Characteristic . | Subjects With Information Available, No. . | Symptoms, No. (%) . | |
|---|---|---|---|
| Symptom | |||
| Fever history reported | 18 | 18 (100) | |
| Chills | 18 | 13 (72) | |
| Headache | 18 | 8 (44) | |
| Weakness | 18 | 6 (33) | |
| Diarrhea | 18 | 4 (22) | |
| Myalgia | 18 | 4 (22) | |
| Neck ache | 18 | 4 (22) | |
| Nausea | 18 | 3 (17) | |
| Cough | 18 | 2 (11) | |
| Vomiting | 18 | 2 (11) | |
| Night sweats | 18 | 2 (11) | |
| Dyspnea | 18 | 1 (6) | |
| Dysuria | 18 | 1 (6) | |
| Sign | |||
| Tachycardia (>95 bpm) | 16 | 8 (50) | |
| Fever at presentation | 16 | 8 (50) | |
| Pallor | 18 | 4 (22) | |
| Jaundice | 18 | 2 (11) | |
| Splenomegaly on PE or AUS | 18 | 6 (33) | |
| Median (IQR) | % of Patients With Abnormal Results | ||
| Laboratory parameter | |||
| Hemoglobin, g/dL (nadir) | 18 | 12.54 (2.37) | 67 |
| WBC count, K/μL (nadir) | 17 | 4.2 (1.99) | 39 |
| Absolute lymphocyte count, K/μL (nadir) | 17 | 0.6 (0.98) | 59 |
| Platelets, K/μL (nadir) | 18 | 99.5 (44.75) | 89 |
| Total bilirubin, mg/dL (max) | 17 | 1.18 (0.48) | 65 |
| LDH, IU/L (max) | 15 | 357 (153) | 73 |
| AST, IU/L (max) | 16 | 37.5 (26.25) | 44 |
| ALT, IU/L (max) | 17 | 50 (24) | 53 |
| Creatinine, mg/dL (max) | 17 | 1.03 (0.23) | 71 |
| INR (max) | 14 | 1.17 (0.12) | 11 |
| Characteristic . | Subjects With Information Available, No. . | Symptoms, No. (%) . | |
|---|---|---|---|
| Symptom | |||
| Fever history reported | 18 | 18 (100) | |
| Chills | 18 | 13 (72) | |
| Headache | 18 | 8 (44) | |
| Weakness | 18 | 6 (33) | |
| Diarrhea | 18 | 4 (22) | |
| Myalgia | 18 | 4 (22) | |
| Neck ache | 18 | 4 (22) | |
| Nausea | 18 | 3 (17) | |
| Cough | 18 | 2 (11) | |
| Vomiting | 18 | 2 (11) | |
| Night sweats | 18 | 2 (11) | |
| Dyspnea | 18 | 1 (6) | |
| Dysuria | 18 | 1 (6) | |
| Sign | |||
| Tachycardia (>95 bpm) | 16 | 8 (50) | |
| Fever at presentation | 16 | 8 (50) | |
| Pallor | 18 | 4 (22) | |
| Jaundice | 18 | 2 (11) | |
| Splenomegaly on PE or AUS | 18 | 6 (33) | |
| Median (IQR) | % of Patients With Abnormal Results | ||
| Laboratory parameter | |||
| Hemoglobin, g/dL (nadir) | 18 | 12.54 (2.37) | 67 |
| WBC count, K/μL (nadir) | 17 | 4.2 (1.99) | 39 |
| Absolute lymphocyte count, K/μL (nadir) | 17 | 0.6 (0.98) | 59 |
| Platelets, K/μL (nadir) | 18 | 99.5 (44.75) | 89 |
| Total bilirubin, mg/dL (max) | 17 | 1.18 (0.48) | 65 |
| LDH, IU/L (max) | 15 | 357 (153) | 73 |
| AST, IU/L (max) | 16 | 37.5 (26.25) | 44 |
| ALT, IU/L (max) | 17 | 50 (24) | 53 |
| Creatinine, mg/dL (max) | 17 | 1.03 (0.23) | 71 |
| INR (max) | 14 | 1.17 (0.12) | 11 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUS, abdominal ultrasound; bpm, beats per minute; INR, international normalized ratio; IQR, interquartile range; LDH, lactate dehydrogenase; PE, physical examination; WBC, white blood cell.
Treatment and Outcomes
Almost all patients (17/18 [94%]) had a benign pattern of disease with a mean hospitalization length of 4.9 days. One patient, who had a history of chronic lymphocytic leukemia, developed acute renal failure and was hospitalized for 2 weeks.
Treatment regimens was varied among hospitals; however, the majority of patients (76%) received 3-day treatment with chloroquine, 2 of whom received follow-up treatment with primaquine. Two patients (12%) were treated for 3 days with AP and 2 (12%) with artemether/lumefantrine. All patients responded promptly to treatment with defervescence occurring within 48 hours.
DISCUSSION
In this series, we analyzed 18 cases of PCR-diagnosed P. malariae monoinfection in returned Israeli travelers. Although all presented with febrile illness, 44% had a false-negative result for any type of malaria on admission. PCR was required for species recognition in 89%, whereas RDTs, which cannot provide a species diagnosis of P. malariae, diagnosed non-falciparum malaria in only 29% (5/17 cases).
PCR usage uncovered a recent significant increase in the proportion of P. malariae infections in Israel, at odds with recent prevalence findings [21]. However, due to the small number of cases included in this study, it is difficult to ascertain whether this finding reflects a true recent increase in P. malariae infections, or whether it has been impacted by other factors.
Although microscopic analysis of thick and thin blood smears has long been the gold standard of malaria diagnosis, as well as species discrimination [1, 22, 23], several reports in the literature disclose accounts of false-negative results for P. malariae diagnosis [4, 8–11]. Many of the current RDT kits, including the 3 kits used in this study (VIKIA, BinaxNOW, and Carestart) target pan-species-specific antigens, such as Plasmodium lactate dehydrogenase and aldolase, in an effort to encompass non-falciparum infections. However, the number of P. malariae infections assessed in RDT sensitivity testing is extremely low and sensitivity reports for this species ranged between 18.8% and 47.6% [15, 17, 24–28]. Plasmodium malariae’s tendency to cause low parasitemia may hinder detection in both methods [15, 24], a finding supported by the correlation between negative RDT and blood smear results and lower parasitemia (as indicated by a high number of Ct cycles on PCR) demonstrated in our cases (Figure 4). Our data confirm the unreliability of both methods for P. malariae diagnosis in a real-life, nonendemic setting and emphasizes the need for prompt PCR utilization on admission for all malaria-suspect cases [1, 12, 18, 24].
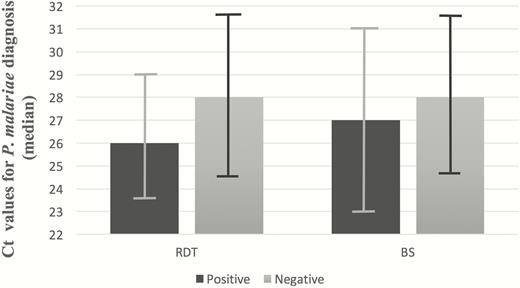
Correlation of rapid diagnostic test (RDT) and blood smear (BS) results with cycle threshold (Ct) value for Plasmodium malariae diagnosis using polymerase chain reaction (N = 18).
Little information exists in the literature regarding the clinical manifestations and laboratory abnormalities of P. malariae infection in a nonimmune population. Fever, the clinical hallmark of malaria, was reported by all of the patients; however, only half were febrile at presentation. The quartan fever pattern caused by P. malariae’s 72-hour replication cycle [1] was reported by only 22%, whereas the fever trend varied considerably for the remainder of cases. Symptomatology was comprised of high fever peaks often accompanied by chills, weakness, headache, and gastrointestinal disturbance, in accord with the characteristic clinical manifestations of malaria [29, 30]. For several patients, diagnosis was delayed due to negative blood smears (in 1 case, up to 82 days), indicating P. malariae as a potential cause for fever of unknown origin.
Thrombocytopenia, as in the case of general malaria [23], was the most common laboratory abnormality (89%); however, the median nadir (99.5 K/μL) was slightly higher than similar evaluations for non-falciparum malaria (63–70 K/μL) [29, 31]. The remainder of the laboratory anomalies were analogous to those previously reported in non-falciparum malaria [1, 23, 31]. Only 1 case answered a defining criterion of severe malaria (creatinine serum level >3 mg/dL); however, this was probably due to comorbidity with chronic lymphocytic leukemia. Nephrotic syndrome, reported to be a rare complication of P. malariae infection [32–34], was not observed in any of these cases that presented with acute disease.
The majority of the cases in our study received treatment with chloroquine, and all patients responded promptly within 48 hours. One report of chloroquine resistance in P. malariae in Indonesia exists in the literature; however, these findings were not replicated in a subsequent study [7, 35]. The results of this series concur with respect to P. malariae’s continued susceptibility to a standard regimen of chloroquine.
In our series, 39% of cases reported receiving prior chemoprophylaxis; however, proper adherence was impossible to assess. Nevertheless, the high proportion of prophylactic failure corroborates similar findings in the literature [7, 14, 15]. Furthermore, almost all of the patients in this series who had a significant delay in symptom development (>30 days) reported using prophylaxis, 1 with mefloquine and 4 with AP, implying an antimalarial suppressive effect. Mefloquine is considered to be mostly effective against the erythrocytic stage, whereas AP is a potent antimalarial for both pre- and erythrocytic stages [13, 36]; however, both therapies apparently have a lengthening effect on P. malariae interval latency [36, 37] (Figure 2). This may be due to a masking of early clinical manifestations of P. malariae infection that emerge later, behavior which is reminiscent of relapses due to prophylaxis-resistant hypnozoites in P. vivax/ovale infections [36–38].
Various hypotheses exist with regard to prophylaxis inefficiency for P. malariae. Schwartz et al [36] observed that the high percentages of failed prophylactic treatment in P. malariae patients with delayed symptom onset (77%) mirrors those found in P. vivax and P. ovale patients (64% and 88%), in contrast to their findings for P. falciparum patients (34%). Intrinsic drug resistance is thought to be highly unlikely as different types of prophylaxis failure have been reported [13, 39, 40]. Despite multiple case reports that describe recrudescence of P. malariae months after treatment with an antimalarial regimen (summarized in Table 4) [3, 13, 39, 41–43], recurrences often remain susceptible to a repeated course of the initial failed treatment [13, 39, 41]. Recrudescence, the recurrence of P. malariae after long periods of dormancy, is generally believed to result from the presence of subclinical levels of parasitemia [1, 5, 43], a hypothesis supported by cases of transfusion-transmitted P. malariae infections [4, 10]. A quiescent intererythrocytic reservoir of P. malariae may explain clinical outbreaks long after prophylaxis administration [13, 43], but fails to clarify the mechanism of P. malariae recrudescence after treatment with blood-stage schizonticides [13, 41]. Notwithstanding the controversy in the literature with regard to the location of the latent P. malariae forms, it seems that the nature of P. malariae’s response to prophylaxis, comparable to that of P. vivax, may indicate the presence of similar hypnozoite-like forms in the liver, the blood system, or perhaps another tissue altogether, which interfere occasionally with response to prophylaxis and treatment.
| Case Report . | Country of Diagnosis . | Country of Infection . | Failed Treatment Regimena . | Interval Between Treatment and P. malariae Symptoms, days . | Successful Regimen . |
|---|---|---|---|---|---|
| Müller-Stöver et al [13] | Germany | Nigeria | AP | 98 | AP |
| Hess et al [41] | Germany | Kenya | Mefloquine, halofantrine | 78, 106 | Halofantrine |
| Kugasia et al [3] | United States | Sierra Leone | Quinine + clindamycin | 730 | Chloroquine |
| Calleri et al [42] | Italy | Uganda | AL | 38 | Chloroquine |
| Visser et al [39] | Holland | Uganda | Chloroquine | 60 | Chloroquine |
| Franken et al [43] | Germany | Kenya | Quinine + doxycycline + AL | 120 | Chloroquine |
| Case Report . | Country of Diagnosis . | Country of Infection . | Failed Treatment Regimena . | Interval Between Treatment and P. malariae Symptoms, days . | Successful Regimen . |
|---|---|---|---|---|---|
| Müller-Stöver et al [13] | Germany | Nigeria | AP | 98 | AP |
| Hess et al [41] | Germany | Kenya | Mefloquine, halofantrine | 78, 106 | Halofantrine |
| Kugasia et al [3] | United States | Sierra Leone | Quinine + clindamycin | 730 | Chloroquine |
| Calleri et al [42] | Italy | Uganda | AL | 38 | Chloroquine |
| Visser et al [39] | Holland | Uganda | Chloroquine | 60 | Chloroquine |
| Franken et al [43] | Germany | Kenya | Quinine + doxycycline + AL | 120 | Chloroquine |
Abbreviations: AL, artemether/lumefantrine; AP, atovaquone/proguanil.
aNo patients were further exposed to malaria-endemic countries.
| Case Report . | Country of Diagnosis . | Country of Infection . | Failed Treatment Regimena . | Interval Between Treatment and P. malariae Symptoms, days . | Successful Regimen . |
|---|---|---|---|---|---|
| Müller-Stöver et al [13] | Germany | Nigeria | AP | 98 | AP |
| Hess et al [41] | Germany | Kenya | Mefloquine, halofantrine | 78, 106 | Halofantrine |
| Kugasia et al [3] | United States | Sierra Leone | Quinine + clindamycin | 730 | Chloroquine |
| Calleri et al [42] | Italy | Uganda | AL | 38 | Chloroquine |
| Visser et al [39] | Holland | Uganda | Chloroquine | 60 | Chloroquine |
| Franken et al [43] | Germany | Kenya | Quinine + doxycycline + AL | 120 | Chloroquine |
| Case Report . | Country of Diagnosis . | Country of Infection . | Failed Treatment Regimena . | Interval Between Treatment and P. malariae Symptoms, days . | Successful Regimen . |
|---|---|---|---|---|---|
| Müller-Stöver et al [13] | Germany | Nigeria | AP | 98 | AP |
| Hess et al [41] | Germany | Kenya | Mefloquine, halofantrine | 78, 106 | Halofantrine |
| Kugasia et al [3] | United States | Sierra Leone | Quinine + clindamycin | 730 | Chloroquine |
| Calleri et al [42] | Italy | Uganda | AL | 38 | Chloroquine |
| Visser et al [39] | Holland | Uganda | Chloroquine | 60 | Chloroquine |
| Franken et al [43] | Germany | Kenya | Quinine + doxycycline + AL | 120 | Chloroquine |
Abbreviations: AL, artemether/lumefantrine; AP, atovaquone/proguanil.
aNo patients were further exposed to malaria-endemic countries.
Our study has several limitations. First, despite uncovering a significant recent increase in the proportion of P. malariae infections, we do not know whether our data may have been impacted by other relevant factors, such as a recent change in Israeli travel patterns or a recent rise in smear-negative cases sent for PCR analysis. Second, there was an underestimation of the pre–patent period length, as date of departure from the endemic country was used in lieu of the date of exposure (difficult to determine in travelers). Third, microscopic assessments and RDTs were performed by various laboratories and kits, which may have contributed to the heterogeneity of the results. However, many of our results coincide with the literature and represent data collected in a real-life setting. Last, the modest span of the series limits the generalizability of its findings. Be that as it may, ours is the only series describing the clinical course and laboratory assessment of imported P. malariae infections so far.
In conclusion, we found a recent increase in P. malariae proportion among the annual malarial case load. Furthermore, it is evident that diagnosis of P. malariae via the standard malarial diagnostic methods (blood smears and RDTs) is unreliable, and it seems that antimalarial prophylactic treatment may have a lengthening effect on the dormancy period. Hence, P. malariae infection should remain in the differential diagnosis of febrile patients returning from endemic countries whose malaria workup is negative, and PCR should be utilized early on. Finally, recent evidence, which has come to light in this study, demonstrates the need for further investigation into the life cycle of this enigmatic parasite.
Note
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




