-
PDF
- Split View
-
Views
-
Cite
Cite
Vimalanand S Prabhu, Oliver A Cornely, Yoav Golan, Erik R Dubberke, Sebastian M Heimann, Mary E Hanson, Jane Liao, Alison Pedley, Mary Beth Dorr, Stephen Marcella, Thirty-Day Readmissions in Hospitalized Patients Who Received Bezlotoxumab With Antibacterial Drug Treatment for Clostridium difficile Infection, Clinical Infectious Diseases, Volume 65, Issue 7, 1 October 2017, Pages 1218–1221, https://doi.org/10.1093/cid/cix523
Close - Share Icon Share
Abstract
We estimated 30-day all-cause and Clostridium difficile infection (CDI)–associated hospital readmissions in participants at high risk of recurrent CDI enrolled in MODIFY I/II. Bezlotoxumab-treated inpatients experienced fewer CDI-associated readmissions compared with placebo-treated inpatients, notably in participants aged ≥65 years and with severe CDI.
Clinical Trials Registration. NCT01241552 (MODIFY I) and NCT01513239 (MODIFY II).
Although antibiotic treatment of primary Clostridium difficile infection (CDI) is often successful, approximately 25% of patients experience recurrent CDI (rCDI) after completing initial antibiotic therapy [1, 2]. After a first recurrence of CDI, the probability of a second recurrence is approximately 38% [3]. Known risk factors for rCDI include concomitant systemic antibiotic use [4], advanced age [5, 6], inadequate immune response to antitoxins [7, 8], severe underlying disease [9], and infection with the BI/NAP1/027 strain [6, 10–12].
Recent model-based estimates place the 2014 economic cost of CDI at $5.4 billion in the United States, mostly attributable to hospitalization [13]. In Europe, extra per-patient costs for treatment of CDI were reported to reach €4396 to €14023, with the majority of costs due to hospitalization [14, 15]. In France, 12.5% of the €163.1 million extra cost of CDI in public acute-care hospitals was attributable to rCDI [15]. Episodes of rCDI are associated with excessive costs, mostly attributable to significantly longer hospital stays, especially in intensive care units in tertiary care settings [16–18]. Hospital readmissions are more common among patients with a CDI discharge diagnosis than among those without one [19] and may contribute to the disease burden. Patients with rCDI are also significantly more likely than patients with nonrecurrent CDI to experience a readmission [20].
MODIFY I and MODIFY II were global trials that investigated the efficacy and safety of bezlotoxumab, a human monoclonal antibody against C. difficile toxin B, for the prevention of rCDI in adults receiving antibacterial drug treatment [21]. In the MODIFY trials, bezlotoxumab significantly reduced rCDI (P < .001, both studies) and had a favorable safety profile [21]. The objective of the current analysis was to estimate 30-day CDI-associated hospital readmission rates and all-cause hospital readmission rates using pooled data from the MODIFY I/MODIFY II trials in the subgroup of participants who were inpatients at the time of study randomization and for participants who had high-risk prognostic factors for rCDI.
METHODS
MODIFY I (NCT01241552) and MODIFY II (NCT01513239) were randomized, double-blind, placebo-controlled, multicenter, global phase 3 trials conducted from 1 November 2011 through 22 May 2015 at 322 sites in 30 countries. The protocols and all amendments were approved by the institutional review board or independent ethics committee at each study center. Each study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Written informed consent was obtained before study procedures were performed.
The eligibility criteria for the MODIFY trials have been described elsewhere [21]. Briefly, adults with primary CDI or rCDI receiving antibiotic treatment for CDI (determined by the treating physician) were enrolled. CDI was defined as diarrhea (≥3 unformed bowel movements in 24 hours) associated with a positive stool test for toxigenic C. difficile. The number of unformed bowel movements was recorded by participants daily for 80–90 days, and new episodes of diarrhea were monitored via scheduled phone contacts between visits.
Participants included in the MODIFY trials received 1 dose of bezlotoxumab (10 mg/kg) or placebo (0.9% saline). Randomization was stratified by oral antibacterial drug treatment for CDI and hospitalization status (inpatient or outpatient).
Thirty-Day readmissions is an emerging policy-relevant quality metric in the United States [22]. All-cause 30-day readmission was defined as the proportion of participants admitted to a healthcare facility at randomization who had any readmission within 30 days of discharge. CDI-associated 30-day readmission was defined as a 30-day readmission that satisfied ≥1 of the following criteria: occurrence within 5 days after onset of a new episode of CDI, onset of a new CDI episode during the readmission, or the discharge diagnosis including terms synonymous with CDI, rCDI, or pseudomembranous colitis, as recorded on the trial case report form.
For this post hoc analysis, we pooled data from MODIFY I and MODIFY II, which were independent trials but nearly identical in design [21]. The analysis population was the subset of modified intent-to-treat participants (defined elsewhere [21]) who were hospitalized at the time of randomization. Subsets of participants at high risk for rCDI were included in the subgroup analysis. The proportion of subjects meeting the end point definitions was estimated, along with the absolute difference in the proportion between the bezlotoxumab and placebo groups (with 95% confidence intervals [CIs]) [23]. Risk factors included age ≥65 years, severe CDI (severity based on Zar score [24]), a history of ≥1 episodes of CDI in the previous 6 months, infection due to 027 strain, and compromised immunity, defined on the basis of medical history or immunosuppressive therapy.
RESULTS
Across the 2 MODIFY trials, 781 bezlotoxumab-treated participants and 773 placebo-treated participants were included in the modified intent-to-treat population. Of these, 530 participants (67.9%) in the bezlotoxumab group and 520 (67.3%) in the placebo group were hospitalized at the time of randomization and were included in this post hoc analysis. Baseline characteristics, including high-risk prognostic factors, were generally similar between the bezlotoxumab and placebo groups (Supplementary Table S1).
In the 30 days after hospital discharge, participants treated with bezlotoxumab had fewer CDI-associated hospital readmissions (absolute difference, −6.1%; 95% CI, −9.5 to −2.8; relative difference, −53.4%). Participants treated with bezlotoxumab also had fewer all-cause readmissions (absolute difference, −3.7%; 95% CI, −9.0 to 1.5; relative difference, −12.1%) than inpatients randomized to placebo (Figure 1A), although the difference did not reach statistical significance. Bezlotoxumab reduced CDI-associated hospital readmissions in participants at high risk for rCDI (Figure 1B), including those aged ≥65 years or with severe CDI. Participants with ≥1 CDI episode in the previous 6 months, compromised immunity, or infection with the 027 strain showed fewer rCDIs with bezlotoxumab treatment than with placebo treatment; however, the 95% CIs for the difference included 0 (Figure 1A).
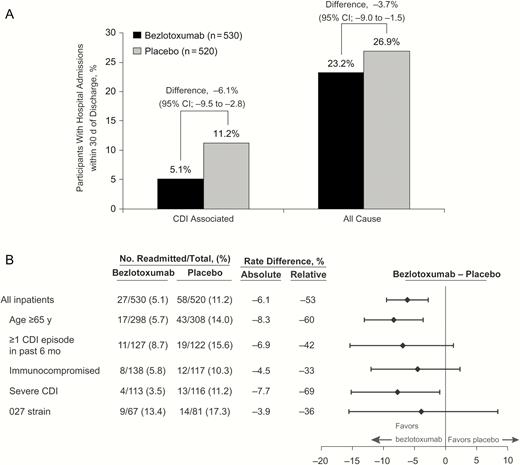
A, Proportion of inpatients with Clostridium difficile (CDI)–associated and all-cause hospital readmissions within 30 days of discharge. CI, confidence interval. B, Summary of CDI-associated readmissions within 30 days of discharge in hospitalized participants with high-risk prognostic factors for recurrent CDI. Participants were defined as immunocompromised based on medical history or use of immunosuppressive therapy. Severe CDI was defined as a Zar score ≥2 based on the following scoring system: (1) age >60 years (1 point); (2) body temperature >38.3°C (>100°F) (1 point); (3) albumin level <2.5 g/dL (1 point); (4) peripheral white blood cell count >15 000/μL within 48 hours (1 point); (5) endoscopic evidence of pseudomembranous colitis (2 points); and (6) treatment in an intensive care unit (2 points).
DISCUSSION
In participants with primary or rCDI treated with antibiotics for CDI, bezlotoxumab reduced CDI-associated 30-day hospital readmissions compared with placebo. Treatment with bezlotoxumab was also more effective at reducing CDI-associated hospital readmissions in participants at high risk for rCDI, including those aged ≥65 years and those with severe CDI.
Prevention of rCDI remains a serious unmet medical need, especially in patients with high-risk prognostic factors, such as the elderly and patients with multiple prior episodes of CDI [25]. Several nonantimicrobial experimental approaches are being studied to address rCDI [25], such as fecal microbiota transplantation [26] and nontoxigenic C. difficile [27]. Bezlotoxumab prevents recurrence by a different mechanism. It binds and neutralizes C. difficile toxin B [21], the primary virulence factor in causing CDI symptoms [28]. This novel approach is designed to passively provide antibody-mediated immune defense, which has been associated with protection against rCDI [29]. Taken together, the results of the current analysis, which demonstrate a reduction in 30-day CDI-associated hospital readmissions, and previously reported findings, demonstrating protective effects of bezlotoxumab against rCDI, provide support for using bezlotoxumab as a valuable treatment option for patients with CDI.
Lost opportunity costs are unaccounted for in many studies that focus on the cost of rCDI in acute-care facilities [30]. Lessa et al [6] noted that C. difficile was responsible for almost half a million infections and was associated with approximately 29000 deaths in 2011. Based on economic modeling, high-risk susceptible individuals represent 5% of the total hospital population and account for 23% of hospitalized patients with CDI [13]. Moreover, the model estimated the economic cost of CDI at $5.4 billion in 2014, with most costs due to hospitalization [13]. rCDI contributes substantially to the cost and burden of CDI, mostly attributable to significantly longer hospital stays [16, 19]. Shah et al [31] reported that the cost of rCDI doubled or tripled that of a first episode of CDI. Despite antibiotic treatment a quarter of patients experience rCDI, with up to 38% experiencing multiple recurrences [3] and with a significantly higher likelihood of hospital readmission [20].
In the current analysis, however, bezlotoxumab treatment was shown to reduce the number of 30-day CDI-associated rehospitalizations by approximately 6% overall. Furthermore, CDI-associated hospital readmissions were reduced by 8% in subpopulations known to be at higher risk for rCDI or CDI-related adverse outcomes (participants ≥65 years old and those with severe CDI). These results suggest that treatment with bezlotoxumab may help reduce some of the costs associated with rCDI by reducing CDI-associated hospital readmissions. Of note, by preventing 1 recurrence, additional future recurrences may also be prevented. It would be of interest to analyze the economic impact of bezlotoxumab through further health economic evaluations, such as cost-effectiveness analyses.
There were some limitations to these post hoc analyses. Although the clinical trial included a broad population with few exclusion criteria, a healthier population (compared with real-world patients with CDI) may have been enrolled, and overall readmissions may be underestimated compared with other reports in the literature. In addition, the proportion of participants with a severe baseline CDI episode may have been underestimated owing to delay in assessment of CDI severity until the antibiotics for CDI had been given for >2 days in the majority of participants (>90%). In addition, these post hoc analyses were not powered for hypothesis testing.
In conclusion, the results of the current analysis demonstrated that treatment with bezlotoxumab, given with C. difficile active antibacterials was shown to reduce CDI-associated rehospitalizations, especially in participants with high-risk prognostic factors.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgment. Carol Zecca, BS, Merck & Co., Inc., Kenilworth, NJ, provided editorial and submission assistance.
Financial support. This work was supported by Merck & Co., Inc., Kenilworth, NJ.
Potential conflicts of interest. O. A. C. has received research grants from Actelion, Aranis, Astellas, AstraZeneca, Basilea, Bayer, Cidara, Duke University (National Institutes of Health grant UM1AI104681), F2G, Gilead, GlaxoSmithKline, Leeds University, MedPace, Melinta Therapeutics, Merck/Merck Sharp & Dohme (MSD), Miltenyi, Pfizer, Rempex, Roche, Sanofi Pasteur, Scynexis, Seres Therapeutics, and The Medicines Company; is a consultant to Anacor, Amplyx, Actelion, Astellas, Basilea, Cidara, Da Volterra, F2G, Gilead, Janssen Pharmaceuticals, Matinas, Menarini Ricerche, Merck/MSD, Paratek Pharmaceuticals, Scynexis, Seres, Summit, Vical, Tetraphase, and Achaogen/Parexel; and has received lecture honoraria from Astellas, Basilea, Gilead, and Merck/MSD. Y. G. is a grant investigator for Merck & Co, and Allergan; a scientific advisor (review panel or advisory committee) for Merck & Co, Achaogen, Allergan, and Cempra; and a member of the speaker’s bureau for Merck & Co, Pfizer, The Medicines Company, and Allergan. E. R. D. is an investigator on behalf of Merck & Co and Rebiotix; has received grants from Sanofi Pasteur; and is a consultant for Merck & Co, GlaxoSmithKline, Velneva, Rebiotix, and Sanofi Pasteur. S. M. H. has received research and travel grants from Astellas and Merck; research grants from Basilea, Gilead, and 3M; travel grants from Pfizer; and lecture honoraria from Astellas and Merck. V. S. P, M. E. H., J. L., A. P., M. B. D., and S. M. are employees of Merck & Co., Inc., Kenilworth, NJ, USA and may own stock or stock options in the company. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




