-
PDF
- Split View
-
Views
-
Cite
Cite
William Vindrios, Nicolas Argy, Solène Le Gal, François-Xavier Lescure, Laurent Massias, Minh Patrick Le, Michel Wolff, Yazdan Yazdanpanah, Gilles Nevez, Sandrine Houze, Richard Dorent, Jean-Christophe Lucet, Outbreak of Pneumocystis jirovecii Infection Among Heart Transplant Recipients: Molecular Investigation and Management of an Interhuman Transmission, Clinical Infectious Diseases, Volume 65, Issue 7, 1 October 2017, Pages 1120–1126, https://doi.org/10.1093/cid/cix495
Close - Share Icon Share
Abstract
An outbreak of Pneumocystis jirovecii pneumonia (PCP) occurred among heart transplant recipients (HTR) at the outpatient clinic of a university hospital, from March to September 2015. Clinical, therapeutic, biological, and molecular data were analyzed to determine its origin and control the outbreak.
Clinical and biological data regarding all HTR followed in the outpatient clinic were collected. PCP diagnosis was based on microscopy and real-time polymerase chain reaction (PCR). Investigations were performed by building a transmission map, completed by genotyping Pneumocystis isolates and by a control of chemoprophylaxis observance. Asymptomatic exposed patients were screened for colonization using real-time PCR.
Among 124 HTR, 7 PCP cases were confirmed. Screening identified 3 additional patients colonized by P. jirovecii. All patients were cured, and no further cases were identified after trimethoprim-sulfamethoxazole prophylaxis was introduced in the entire cohort. Genotyping demonstrated the same strain in all PCP cases and colonized patients. All cases were linked with possible transmission chains from 2 possible index patients. Interhuman transmission was significantly associated with more frequent visits in the outpatient clinic. Six cases were receiving atovaquone as a prophylaxis. The occurrence of PCP was significantly associated with atovaquone prophylaxis.
This is the first outbreak with detailed molecular analysis in HTR so far. Genotyping and transmission chain confirmed interhuman transmission in all colonized/infected PCP cases. Outpatient clinic layout and high encounters probably caused this PCP cluster, which was controlled after systematic trimethoprim-sulfamethoxazole prophylaxis in exposed patients.
Pneumocystis jirovecii is an opportunistic fungus that causes severe pneumonia in immunocompromised patients. Pneumocystis pneumonia (PCP) is still one of the most common initial AIDS manifestation but is also described in other immunocompromised patients, including transplant recipients [1]. Incidence of PCP is variable according to the type of solid organ transplantation. In the absence of prophylaxis, PCP can occur in about 2%–10% of heart transplant recipients (HTR) [2]; however, an attack rate as high as 41% has been reported [3]. Preventive measures are critical to prevent PCP occurrence in solid organ transplant recipients [2].
Recent advances in the epidemiology and pathogenesis of PCP have shown that the reservoir of P. jirovecii is probably limited to human beings. Animal and human studies support an airborne transmission of PCP [4, 5]. Thereby, person-to-person spread is the most likely mode of acquisition of P. jirovecii [5, 6]. Over the recent years, owing to molecular identification, interhuman transmission between immunocompromised patients has been reported [7–9]. Studies of PCP clusters among renal transplant recipients combining patient encounters analysis and P. jirovecii genotyping support the hypothesis of nosocomial acquisition. Main outbreaks of PCP have been reported in renal transplant recipients [7–12], but to the best of our knowledge none in HTR.
We report an outbreak of PCP in HTR attending the outpatient clinic of a university hospital from March to September 2015, in which molecular investigation and transmission map were performed to determine cluster origin; preventive measures were undertaken to control the outbreak.
METHODS
Overview of the Heart Transplantation Activity
The 850-bed Bichat-Claude Bernard University Hospital performs about 40 heart transplantations per year. The long-term follow-up of HTR is managed in the outpatient clinic of the cardiac surgery unit where the patients typically stay 1 day every week for surveillance during the early stage of transplantation, then at broader intervals. In this outpatient clinic, HTR stay in a common waiting room, without physical separation between patients. First-line PCP prophylaxis recommended was trimethoprim-sulfamethoxazole (TMP-SMX) for 1 year after heart transplantation and atovaquone as a second-line PCP prophylaxis. Prophylaxis could be prolonged in high-risk patients.
During the years 2012–2014, only 2 PCP cases were observed in HTR among 53 PCP cases identified in the hospital, 35 of whom were human immunodeficiency virus (HIV)–infected patients. In 2015, a sudden increase of PCP cases was detected in HTR patients in the cardiology outpatient clinic, with 7 cases between 15 March and 8 September 2015. Because of possible contacts between patients, an outbreak with interhuman transmission was suspected. Six of the 7 PCP cases were under atovaquone prophylaxis during this period. Clinical, therapeutic, and molecular data were collected retrospectively to analyse the outbreak.
Patient Data
Data regarding all HTR followed from 15 March (date of the first identified PCP case) through 18 November 2015 in the outpatient clinic were included. Because case patients only attended the outpatient clinic without inpatients, the investigation was limited to this unit. Information concerning the underlying disease, immunosuppressive medications, use and type of PCP prophylaxis, dates of hospital visits, comorbidities, and demographic data as well as the clinical presentation of PCP were recorded.
The diagnosis of PCP was performed in the parasitology and pathology department by direct microscopy (Wright-Giemsa staining, Toluidine Blue O, Gram-Weigert, and/or Grocott-Gomori) on bronchoalveolar lavage (BAL) and by real-time polymerase chain reaction (PCR) targeting the P. jirovecii mitochondrial large subunit ribosomal RNA gene (mtLSUrRNA) (Bioevolution, Bry-sur-Marne, France) on BAL or induced sputum (IS) after automatized DNA extraction by EZ1 DNA tissue kit (Qiagen, Courtaboeuf, France).
The diagnosis of PCP was retained in the presence of a positive direct examination on BAL samples and/or a positive PCR on BAL or IS samples, and if (i) at least 2 of the 4 following items were present: cough, fever (temperature ≥38°C), dyspnea or Sp02 <96%, and radiological signs of PCP (interstitial syndrome) on the chest radiograph or computed tomography scan, and (ii) a favorable outcome was obtained under therapy with TMP-SMX [1, 13].
Colonized patients were defined by isolated positive PCR without clinical and radiological signs of PCP or with another diagnosis than PCP explaining clinical or radiological symptoms. These patients did not develop PCP, despite the absence of specific treatment [9].
Control Measures
Trimethoprim-sulfamethoxazole prophylaxis was systematically prescribed (400 mg sulfamethoxazole/80 mg trimethoprim per day, if estimated glomerular filtration rate [eGFR] >30 mL/minute, half dose if eGFR <30 mL/minute), regardless of the date of heart transplantation, to the entire HTR cohort, within 15 days after outbreak identification. Symptoms and temperature were checked at admission to the outpatient clinic, and patients with respiratory symptoms were separated and placed in a single room for further investigation. In addition, it was requested that all HTR patients wore a surgical mask during their stay in the outpatient clinic.
Screening in Exposed Heart Transplant Recipients
From the beginning of September to mid-November, screening of all contact patients with a PCP case in the outpatient clinic were performed to detect infection or colonization by P. jirovecii. In each encounter, Pneumocystis presence was detected on nasopharyngeal swab by real-time PCR (Bioevolution) after DNA extraction.
Pneumocystis Genotyping
Pneumocystis genotyping was performed using a multilocus sequence typing (MLST) method in all infected HTR patients, whatever their clinical presentation, from extracted DNA of positive respiratory or nasopharyngeal samples. Three loci, mtLSUrRNA, superoxide dismutase (SOD), and cytochrome b (CYB) genes, were analyzed. A 310-bp fragment of the mtLSUrRNA gene was amplified as described by Chabé et al [14]. A 638-bp fragment of CYB and a 652-bp fragment of SOD were amplified using primers described by Esteves et al [15]. PCR products were sequenced from the 2 strands with the dideoxy chain termination method on the 3130XL Genetic Analyzer (BigDye terminator version 1.1 cycle sequencing kit, Applied Biosystems, Foster City, California). Chromatograms were analyzed using Pregap and Gap software (Staden package version 2003.0-beta, Staden Group). Consensus sequences were aligned with reference sequences (GenBank accession numbers M58605 [mtLSUrRNA], AF074871 [CYB], and AF146753 [SOD]) [16–18] using the BioEdit software with the Clustal W program. MtLSUrRNA alleles were named using the nomenclature described previously by Beard et al [19]. CYB and SOD alleles were named using the nomenclature previously described [15, 20]. In parallel, 11 unrelated immunosuppressed patients with PCP admitted in other hospital units in 2015 and without contacts with PCP cases were also genotyped: 4 patients receiving immunosuppressive therapy, 2 HIV-positive patients, 2 liver transplant recipients, and 3 patients with neoplasia (1 squamous cell cancer, 1 pancreatic, and 1 adrenal carcinoma).
Transmission Map
A transmission map was built to detect contacts between HTR during their stay in the outpatient clinic. All infected or colonized patients were regarded as a possible index patient. A possible index patient was defined as a patient for whom a diagnosis of Pneumocystis infection or colonization was confirmed by a positive result of P. jirovecii detection (BAL, IS samples, or nasopharyngeal swab) and who could be at the origin of secondary case(s) before PCP diagnosis. The number of visits between secondary cases and noncases were compared since the first possible transmission from the suspected index patient.
Serum Atovaquone Determination
For HTR patients presenting with PCP, self-reported compliance with PCP prophylaxis in the few previous days, including drug intakes habits, have been collected. Because we suspected that atovaquone prophylaxis was not properly taken in case patients, atovaquone serum concentration was performed the day of PCP diagnosis according to a validated method [21]. Atovaquone serum concentration was also performed in 5 non-PCP-infected HTR receiving atovaquone prophylaxis and representing a control chemoprophylaxis group of exposed noninfected HTR patients to PCP cases. It was admitted that an effective steady-state concentration of atovaquone chemoprophylaxis should be at least 15 µg/mL [22].
Statistical Analysis
Nonparametric variables were represented as median value (range). PCP cases and control patients groups were compared using Wilcoxon and Fisher exact tests. Differences were considered significant at P < .05. Statistical analyses were performed using R software, version 3.
RESULTS
Patient Characteristics and Outcome
Among the 124 HTR regularly followed at the outpatient clinic, 7 confirmed PCP cases were identified, using PCR (7/7) and observation of trophozoites and cysts forms by microscopy on BAL (2/7) or in pulmonary biopsy (1/7) (Table 1). Six cases were receiving atovaquone as prophylaxis (750 mg twice a day) and 1 case had no prophylaxis. All cases had renal failure, 1 was receiving hemodialysis, 4 had eGFR <30 mL/minute/1.73 m2, and 2 had eGFR <50 mL/minute/1.73 m2. The median time to PCP onset after heart transplantation was 260 (range, 103–1383) days. Three patients were admitted to the intensive care unit for severe PCP, but none needed to be mechanically ventilated. All patients were treated by TMP-SMX and had a favorable outcome (Table 1).
Characteristics of the 7 Heart Transplant Recipients During an Outbreak of Pneumocystis jirovecii Pneumonia
| Characteristic . | Case 1 . | Case 2 . | Case 3 . | Case 4 . | Case 5 . | Case 6 . | Case 7 . |
|---|---|---|---|---|---|---|---|
| Sex | M | F | M | M | M | F | M |
| Age, y | 43 | 34 | 68 | 64 | 49 | 68 | 37 |
| Comorbidities | HIV/HBV (CD4 = 20), RTR, hypertension, CRD (eGFR <30 mL/min) | Hodgkin lymphoma, atrial flutter, CRD (eGFR<30) | Diabetes, hypertension, COPD, CRD (eGFR <30 mL/min) | Hypertension, CRD (eGFR <50 mL/min) | Hypertension, CRD (eGFR <50 mL/min) | Legionella pneumonia (July 2015), CRD, (eGFR <30 mL/ min) | Diabetes, nephrectomy, hemodialysis |
| Date of transplantation | 1 Nov 2014 | 7 Jan 2015 | 28 Jan 2013 | 1 Oct 2011 | 19 Nov 2014 | 13 May 2015 | 5 Sept 2012 |
| PCP prophylaxis | ATQ | ATQ | ATQ | None | ATQ | ATQ | ATQ |
| Immunosuppressive therapy | CCS 350 mg/d; MMF 3 g/d; CS 15 mg/d | TCL 2.5 mg/d; MMF 1 g/d; CS 20 mg/d | MMF 1 g/d; CS 10 mg/d | MMF 2 g/d; CS 10 mg/d | MMF 2 g/d; CS 15 mg/d | TCL 6 mg/d; AZA 500 mg/d; CS 20 mg/d | CCS 200 mg/d; CS 10 mg/d |
| Date of PCP | 16 March 2015 | 11 May 2015 | 15 July 2015 | 28 July 2015 | 6 Aug 2015 | 24 Aug 2015 | 8 Sept 2015 |
| Posttransplant period, da | 135 | 124 | 898 | 1383 | 260 | 103 | 1098 |
| Symptoms | Fever, cough, asthenia | Dyspnea, cough, asthenia | Fever, cough | Dyspnea, cough | No clinical symptoms; interstitial syndrome (CXR) | Chest pain, fever, dyspnea | Respiratory distress syndrome |
| Direct microscopic examination on BAL | Negative | Positive | Negative | Negative | Positive | Negative | Negative |
| PCR on IS (gene copies/mL), ×103 | 1.2 | 68.000 | … | … | … | … | … |
| PCR on BAL (gene copies/mL), ×103 | 6.7 | 125.000 | 30.000 | 180 | 1.430 | 1.700 | 2.100 |
| Characteristic . | Case 1 . | Case 2 . | Case 3 . | Case 4 . | Case 5 . | Case 6 . | Case 7 . |
|---|---|---|---|---|---|---|---|
| Sex | M | F | M | M | M | F | M |
| Age, y | 43 | 34 | 68 | 64 | 49 | 68 | 37 |
| Comorbidities | HIV/HBV (CD4 = 20), RTR, hypertension, CRD (eGFR <30 mL/min) | Hodgkin lymphoma, atrial flutter, CRD (eGFR<30) | Diabetes, hypertension, COPD, CRD (eGFR <30 mL/min) | Hypertension, CRD (eGFR <50 mL/min) | Hypertension, CRD (eGFR <50 mL/min) | Legionella pneumonia (July 2015), CRD, (eGFR <30 mL/ min) | Diabetes, nephrectomy, hemodialysis |
| Date of transplantation | 1 Nov 2014 | 7 Jan 2015 | 28 Jan 2013 | 1 Oct 2011 | 19 Nov 2014 | 13 May 2015 | 5 Sept 2012 |
| PCP prophylaxis | ATQ | ATQ | ATQ | None | ATQ | ATQ | ATQ |
| Immunosuppressive therapy | CCS 350 mg/d; MMF 3 g/d; CS 15 mg/d | TCL 2.5 mg/d; MMF 1 g/d; CS 20 mg/d | MMF 1 g/d; CS 10 mg/d | MMF 2 g/d; CS 10 mg/d | MMF 2 g/d; CS 15 mg/d | TCL 6 mg/d; AZA 500 mg/d; CS 20 mg/d | CCS 200 mg/d; CS 10 mg/d |
| Date of PCP | 16 March 2015 | 11 May 2015 | 15 July 2015 | 28 July 2015 | 6 Aug 2015 | 24 Aug 2015 | 8 Sept 2015 |
| Posttransplant period, da | 135 | 124 | 898 | 1383 | 260 | 103 | 1098 |
| Symptoms | Fever, cough, asthenia | Dyspnea, cough, asthenia | Fever, cough | Dyspnea, cough | No clinical symptoms; interstitial syndrome (CXR) | Chest pain, fever, dyspnea | Respiratory distress syndrome |
| Direct microscopic examination on BAL | Negative | Positive | Negative | Negative | Positive | Negative | Negative |
| PCR on IS (gene copies/mL), ×103 | 1.2 | 68.000 | … | … | … | … | … |
| PCR on BAL (gene copies/mL), ×103 | 6.7 | 125.000 | 30.000 | 180 | 1.430 | 1.700 | 2.100 |
Abbreviations: ATQ, atovaquone; AZA, azathioprine; BAL, bronchoalveolar lavage; CCS, cyclosporine; COPD, chronic obstructive pulmonary disease; CRD, chronic renal disease; CS, corticosteroids; CXR, chest radiograph; eGFR, estimated glomerular filtration rate; HBV, hepatitis B virus; HIV, human immunodeficiency virus; IS, induced sputum; MMF, mycophenolate mofetil; PCP, Pneumocystis jirovecii pneumonia; PCR, polymerase chain reaction; RTR, renal transplant recipient; TCL, tacrolimus.
aPosttransplant period: time between day of transplantation and the diagnosis of PCP.
Characteristics of the 7 Heart Transplant Recipients During an Outbreak of Pneumocystis jirovecii Pneumonia
| Characteristic . | Case 1 . | Case 2 . | Case 3 . | Case 4 . | Case 5 . | Case 6 . | Case 7 . |
|---|---|---|---|---|---|---|---|
| Sex | M | F | M | M | M | F | M |
| Age, y | 43 | 34 | 68 | 64 | 49 | 68 | 37 |
| Comorbidities | HIV/HBV (CD4 = 20), RTR, hypertension, CRD (eGFR <30 mL/min) | Hodgkin lymphoma, atrial flutter, CRD (eGFR<30) | Diabetes, hypertension, COPD, CRD (eGFR <30 mL/min) | Hypertension, CRD (eGFR <50 mL/min) | Hypertension, CRD (eGFR <50 mL/min) | Legionella pneumonia (July 2015), CRD, (eGFR <30 mL/ min) | Diabetes, nephrectomy, hemodialysis |
| Date of transplantation | 1 Nov 2014 | 7 Jan 2015 | 28 Jan 2013 | 1 Oct 2011 | 19 Nov 2014 | 13 May 2015 | 5 Sept 2012 |
| PCP prophylaxis | ATQ | ATQ | ATQ | None | ATQ | ATQ | ATQ |
| Immunosuppressive therapy | CCS 350 mg/d; MMF 3 g/d; CS 15 mg/d | TCL 2.5 mg/d; MMF 1 g/d; CS 20 mg/d | MMF 1 g/d; CS 10 mg/d | MMF 2 g/d; CS 10 mg/d | MMF 2 g/d; CS 15 mg/d | TCL 6 mg/d; AZA 500 mg/d; CS 20 mg/d | CCS 200 mg/d; CS 10 mg/d |
| Date of PCP | 16 March 2015 | 11 May 2015 | 15 July 2015 | 28 July 2015 | 6 Aug 2015 | 24 Aug 2015 | 8 Sept 2015 |
| Posttransplant period, da | 135 | 124 | 898 | 1383 | 260 | 103 | 1098 |
| Symptoms | Fever, cough, asthenia | Dyspnea, cough, asthenia | Fever, cough | Dyspnea, cough | No clinical symptoms; interstitial syndrome (CXR) | Chest pain, fever, dyspnea | Respiratory distress syndrome |
| Direct microscopic examination on BAL | Negative | Positive | Negative | Negative | Positive | Negative | Negative |
| PCR on IS (gene copies/mL), ×103 | 1.2 | 68.000 | … | … | … | … | … |
| PCR on BAL (gene copies/mL), ×103 | 6.7 | 125.000 | 30.000 | 180 | 1.430 | 1.700 | 2.100 |
| Characteristic . | Case 1 . | Case 2 . | Case 3 . | Case 4 . | Case 5 . | Case 6 . | Case 7 . |
|---|---|---|---|---|---|---|---|
| Sex | M | F | M | M | M | F | M |
| Age, y | 43 | 34 | 68 | 64 | 49 | 68 | 37 |
| Comorbidities | HIV/HBV (CD4 = 20), RTR, hypertension, CRD (eGFR <30 mL/min) | Hodgkin lymphoma, atrial flutter, CRD (eGFR<30) | Diabetes, hypertension, COPD, CRD (eGFR <30 mL/min) | Hypertension, CRD (eGFR <50 mL/min) | Hypertension, CRD (eGFR <50 mL/min) | Legionella pneumonia (July 2015), CRD, (eGFR <30 mL/ min) | Diabetes, nephrectomy, hemodialysis |
| Date of transplantation | 1 Nov 2014 | 7 Jan 2015 | 28 Jan 2013 | 1 Oct 2011 | 19 Nov 2014 | 13 May 2015 | 5 Sept 2012 |
| PCP prophylaxis | ATQ | ATQ | ATQ | None | ATQ | ATQ | ATQ |
| Immunosuppressive therapy | CCS 350 mg/d; MMF 3 g/d; CS 15 mg/d | TCL 2.5 mg/d; MMF 1 g/d; CS 20 mg/d | MMF 1 g/d; CS 10 mg/d | MMF 2 g/d; CS 10 mg/d | MMF 2 g/d; CS 15 mg/d | TCL 6 mg/d; AZA 500 mg/d; CS 20 mg/d | CCS 200 mg/d; CS 10 mg/d |
| Date of PCP | 16 March 2015 | 11 May 2015 | 15 July 2015 | 28 July 2015 | 6 Aug 2015 | 24 Aug 2015 | 8 Sept 2015 |
| Posttransplant period, da | 135 | 124 | 898 | 1383 | 260 | 103 | 1098 |
| Symptoms | Fever, cough, asthenia | Dyspnea, cough, asthenia | Fever, cough | Dyspnea, cough | No clinical symptoms; interstitial syndrome (CXR) | Chest pain, fever, dyspnea | Respiratory distress syndrome |
| Direct microscopic examination on BAL | Negative | Positive | Negative | Negative | Positive | Negative | Negative |
| PCR on IS (gene copies/mL), ×103 | 1.2 | 68.000 | … | … | … | … | … |
| PCR on BAL (gene copies/mL), ×103 | 6.7 | 125.000 | 30.000 | 180 | 1.430 | 1.700 | 2.100 |
Abbreviations: ATQ, atovaquone; AZA, azathioprine; BAL, bronchoalveolar lavage; CCS, cyclosporine; COPD, chronic obstructive pulmonary disease; CRD, chronic renal disease; CS, corticosteroids; CXR, chest radiograph; eGFR, estimated glomerular filtration rate; HBV, hepatitis B virus; HIV, human immunodeficiency virus; IS, induced sputum; MMF, mycophenolate mofetil; PCP, Pneumocystis jirovecii pneumonia; PCR, polymerase chain reaction; RTR, renal transplant recipient; TCL, tacrolimus.
aPosttransplant period: time between day of transplantation and the diagnosis of PCP.
Among the 117 HTR without PCP, prophylaxis status could be retrieved in 86; 24 received TMP-SMX, 4 received atovaquone, and 58 did not receive any prophylaxis.
Among the 34 HTR needing prophylactic treatment (28 without PCP, 6 with PCP), PCP occurred more frequently to patients under atovaquone prophylaxis (6/10 [60%]) compared to patients under TMP-SMX (0/24 [0%]) (P < .001).
Screening of Heart Transplant Recipients for Colonization
Among the 117 HTR without PCP followed at the outpatient clinic, 100 were screened for P. jirovecii. Three of 100 nasopharyngeal swabs (3%) were positive by PCR for P. jirovecii, and all 3 were classified as colonized.
Genotyping of Pneumocystis jirovecii Strains
Positive results of P. jirovecii SOD typing were obtained in specimens from 7 HTR patients (5/7 patients with PCP and 2/3 patients with pulmonary colonization) and 11 patients of the control group (Table 2). Allele SOD1 was identified in 7 HTR patients and 7 of the 11 patients in the control group (P = .12). Positive results of P. jirovecii mtLSUrRNA and CYB typing were obtained in specimens from 9 HTR patients (7/7 patients with PCP and 2/3 patients with pulmonary colonization) and 11 patients of the control group. A same mtLSUrRNA-CYB haplotype (4-CYB2) was identified in 9 HTR patients and 2 of the 11 patients of the control group (P < .001). Taking into account mtLSUrRNA, CYB, and SOD typing, a same multilocus genotype (4-CYB2-SOD1) was identified in the 7 HTR patients (5/7 patients with PCP and 2/3 patients with pulmonary colonization) for whom genotyping was successful at the 3 loci, and in 2 of the 11 patients of the control group (P < .01). Moreover, the 9 HTR patients harbored a previously unreported C350T mutation at the CYB gene, whereas none of the control patients carried this mutation.
Pneumocystis jirovecii Genotypes Obtained From Mitochondrial Large Subunit Ribosomal RNA, Superoxide Dismutase, and Cytochrome b and Sequences in 7 P. jirovecii Pneumonia Cases, 2 Colonized Patients, and 11 Unrelated Immunocompromised Control Patients
| Patient . | Sample . | Date . | Type of Immunosuppression . | mtLSUrRNA . | CYBa . | SOD . |
|---|---|---|---|---|---|---|
| Ctl1 | IS | 8 Jan 2015 | Immunosuppressive treatment | 1 + 2 | CYB1 | SOD1 |
| Ctl2 | BAL | 22 Jan 2015 | LTR | 4 | CYB2 | SOD1 |
| Ctl3 | BAL | 26 Jan 2015 | HIV | 3 | CYB1 | SOD1 |
| Ctl4 | BAL | 5 Feb 2015 | Immunosuppressive treatment | 1 | CYB1 | SOD1 |
| Ctl5 | BAL | 24 Feb 2015 | Immunosuppressive treatment | 1 + 2 | CYB1 + CYB7 | SOD1 |
| Ctl6 | IS | 9 Mar 2015 | HIV | 1 | CYB1 | SOD2 |
| Ctl7 | BAL | 21 Mar 2015 | LTR | 4 | CYB2 | SOD1 |
| Ctl8 | BAL | 31 Mar 2015 | Immunosuppressive treatment + neoplasia | 1 + 4 | mix | mix |
| Ctl9 | BAL | 13 Apr 2015 | Neoplasia | 2 + 3 | CYB1 + CYB2 | mix |
| Ctl10 | IS | 5 May 2015 | Immunosuppressive treatment + neoplasia | 3 | CYB6 | SOD1 |
| Ctl11 | BAL | 7 May 2015 | Immunosuppressive treatment | mix | mix | mix |
| Case 1 | BAL | 17 Mar 2015 | HIV + RTR + HTR | 4 | CYB2 | |
| Case 2 | IS | 12 May 2015 | HTR | 4 | CYB2 | SOD1 |
| Case 3 | BAL | 15 July 2015 | HTR | 4 | CYB2 | SOD1 |
| Case 4 | BAL | 28 July 2015 | HTR | 4 | CYB2 | |
| Case 5 | BAL | 06 Aug 2015 | HTR | 4 | CYB2 | SOD1 |
| Case 6 | BAL | 24 Aug 2015 | HTR | 4 | CYB2 | SOD1 |
| Case 7 | BAL | 2 Sept 2015 | HTR | 4 | CYB2 | SOD1 |
| Col 8 | NS | 31 Aug 2015 | HTR | 4 | CYB2 | SOD1 |
| Col 9 | NS | 2 Sept 2015 | HTR | 4 | CYB2 | SOD1 |
| Col 10 | NS | 31 Aug 2015 | HTR | NAb | NAb | NAb |
| Patient . | Sample . | Date . | Type of Immunosuppression . | mtLSUrRNA . | CYBa . | SOD . |
|---|---|---|---|---|---|---|
| Ctl1 | IS | 8 Jan 2015 | Immunosuppressive treatment | 1 + 2 | CYB1 | SOD1 |
| Ctl2 | BAL | 22 Jan 2015 | LTR | 4 | CYB2 | SOD1 |
| Ctl3 | BAL | 26 Jan 2015 | HIV | 3 | CYB1 | SOD1 |
| Ctl4 | BAL | 5 Feb 2015 | Immunosuppressive treatment | 1 | CYB1 | SOD1 |
| Ctl5 | BAL | 24 Feb 2015 | Immunosuppressive treatment | 1 + 2 | CYB1 + CYB7 | SOD1 |
| Ctl6 | IS | 9 Mar 2015 | HIV | 1 | CYB1 | SOD2 |
| Ctl7 | BAL | 21 Mar 2015 | LTR | 4 | CYB2 | SOD1 |
| Ctl8 | BAL | 31 Mar 2015 | Immunosuppressive treatment + neoplasia | 1 + 4 | mix | mix |
| Ctl9 | BAL | 13 Apr 2015 | Neoplasia | 2 + 3 | CYB1 + CYB2 | mix |
| Ctl10 | IS | 5 May 2015 | Immunosuppressive treatment + neoplasia | 3 | CYB6 | SOD1 |
| Ctl11 | BAL | 7 May 2015 | Immunosuppressive treatment | mix | mix | mix |
| Case 1 | BAL | 17 Mar 2015 | HIV + RTR + HTR | 4 | CYB2 | |
| Case 2 | IS | 12 May 2015 | HTR | 4 | CYB2 | SOD1 |
| Case 3 | BAL | 15 July 2015 | HTR | 4 | CYB2 | SOD1 |
| Case 4 | BAL | 28 July 2015 | HTR | 4 | CYB2 | |
| Case 5 | BAL | 06 Aug 2015 | HTR | 4 | CYB2 | SOD1 |
| Case 6 | BAL | 24 Aug 2015 | HTR | 4 | CYB2 | SOD1 |
| Case 7 | BAL | 2 Sept 2015 | HTR | 4 | CYB2 | SOD1 |
| Col 8 | NS | 31 Aug 2015 | HTR | 4 | CYB2 | SOD1 |
| Col 9 | NS | 2 Sept 2015 | HTR | 4 | CYB2 | SOD1 |
| Col 10 | NS | 31 Aug 2015 | HTR | NAb | NAb | NAb |
Abbreviations: BAL, bronchoalveolar lavage; Ctl, control patient; Col, colonized patient; CYB, cytochrome b; HIV, human immunodeficiency virus; HTR, heart transplant recipient; IS, induced sputum; LTR, liver transplant recipient; mtLSUrRNA, mitochondrial large subunit ribosomal RNA; NA, not available; NS, nasopharyngeal swab; RTR, renal transplant recipient; SOD, superoxide dismutase.
aThe 9 HTR patients harbored a previously unreported C350T mutation at the CYB gene, whereas none of the control patients harbored this punctual mutation.
bThe absence of genotyping data was due to genotype analysis failure for patient 10 (Col 10).
Pneumocystis jirovecii Genotypes Obtained From Mitochondrial Large Subunit Ribosomal RNA, Superoxide Dismutase, and Cytochrome b and Sequences in 7 P. jirovecii Pneumonia Cases, 2 Colonized Patients, and 11 Unrelated Immunocompromised Control Patients
| Patient . | Sample . | Date . | Type of Immunosuppression . | mtLSUrRNA . | CYBa . | SOD . |
|---|---|---|---|---|---|---|
| Ctl1 | IS | 8 Jan 2015 | Immunosuppressive treatment | 1 + 2 | CYB1 | SOD1 |
| Ctl2 | BAL | 22 Jan 2015 | LTR | 4 | CYB2 | SOD1 |
| Ctl3 | BAL | 26 Jan 2015 | HIV | 3 | CYB1 | SOD1 |
| Ctl4 | BAL | 5 Feb 2015 | Immunosuppressive treatment | 1 | CYB1 | SOD1 |
| Ctl5 | BAL | 24 Feb 2015 | Immunosuppressive treatment | 1 + 2 | CYB1 + CYB7 | SOD1 |
| Ctl6 | IS | 9 Mar 2015 | HIV | 1 | CYB1 | SOD2 |
| Ctl7 | BAL | 21 Mar 2015 | LTR | 4 | CYB2 | SOD1 |
| Ctl8 | BAL | 31 Mar 2015 | Immunosuppressive treatment + neoplasia | 1 + 4 | mix | mix |
| Ctl9 | BAL | 13 Apr 2015 | Neoplasia | 2 + 3 | CYB1 + CYB2 | mix |
| Ctl10 | IS | 5 May 2015 | Immunosuppressive treatment + neoplasia | 3 | CYB6 | SOD1 |
| Ctl11 | BAL | 7 May 2015 | Immunosuppressive treatment | mix | mix | mix |
| Case 1 | BAL | 17 Mar 2015 | HIV + RTR + HTR | 4 | CYB2 | |
| Case 2 | IS | 12 May 2015 | HTR | 4 | CYB2 | SOD1 |
| Case 3 | BAL | 15 July 2015 | HTR | 4 | CYB2 | SOD1 |
| Case 4 | BAL | 28 July 2015 | HTR | 4 | CYB2 | |
| Case 5 | BAL | 06 Aug 2015 | HTR | 4 | CYB2 | SOD1 |
| Case 6 | BAL | 24 Aug 2015 | HTR | 4 | CYB2 | SOD1 |
| Case 7 | BAL | 2 Sept 2015 | HTR | 4 | CYB2 | SOD1 |
| Col 8 | NS | 31 Aug 2015 | HTR | 4 | CYB2 | SOD1 |
| Col 9 | NS | 2 Sept 2015 | HTR | 4 | CYB2 | SOD1 |
| Col 10 | NS | 31 Aug 2015 | HTR | NAb | NAb | NAb |
| Patient . | Sample . | Date . | Type of Immunosuppression . | mtLSUrRNA . | CYBa . | SOD . |
|---|---|---|---|---|---|---|
| Ctl1 | IS | 8 Jan 2015 | Immunosuppressive treatment | 1 + 2 | CYB1 | SOD1 |
| Ctl2 | BAL | 22 Jan 2015 | LTR | 4 | CYB2 | SOD1 |
| Ctl3 | BAL | 26 Jan 2015 | HIV | 3 | CYB1 | SOD1 |
| Ctl4 | BAL | 5 Feb 2015 | Immunosuppressive treatment | 1 | CYB1 | SOD1 |
| Ctl5 | BAL | 24 Feb 2015 | Immunosuppressive treatment | 1 + 2 | CYB1 + CYB7 | SOD1 |
| Ctl6 | IS | 9 Mar 2015 | HIV | 1 | CYB1 | SOD2 |
| Ctl7 | BAL | 21 Mar 2015 | LTR | 4 | CYB2 | SOD1 |
| Ctl8 | BAL | 31 Mar 2015 | Immunosuppressive treatment + neoplasia | 1 + 4 | mix | mix |
| Ctl9 | BAL | 13 Apr 2015 | Neoplasia | 2 + 3 | CYB1 + CYB2 | mix |
| Ctl10 | IS | 5 May 2015 | Immunosuppressive treatment + neoplasia | 3 | CYB6 | SOD1 |
| Ctl11 | BAL | 7 May 2015 | Immunosuppressive treatment | mix | mix | mix |
| Case 1 | BAL | 17 Mar 2015 | HIV + RTR + HTR | 4 | CYB2 | |
| Case 2 | IS | 12 May 2015 | HTR | 4 | CYB2 | SOD1 |
| Case 3 | BAL | 15 July 2015 | HTR | 4 | CYB2 | SOD1 |
| Case 4 | BAL | 28 July 2015 | HTR | 4 | CYB2 | |
| Case 5 | BAL | 06 Aug 2015 | HTR | 4 | CYB2 | SOD1 |
| Case 6 | BAL | 24 Aug 2015 | HTR | 4 | CYB2 | SOD1 |
| Case 7 | BAL | 2 Sept 2015 | HTR | 4 | CYB2 | SOD1 |
| Col 8 | NS | 31 Aug 2015 | HTR | 4 | CYB2 | SOD1 |
| Col 9 | NS | 2 Sept 2015 | HTR | 4 | CYB2 | SOD1 |
| Col 10 | NS | 31 Aug 2015 | HTR | NAb | NAb | NAb |
Abbreviations: BAL, bronchoalveolar lavage; Ctl, control patient; Col, colonized patient; CYB, cytochrome b; HIV, human immunodeficiency virus; HTR, heart transplant recipient; IS, induced sputum; LTR, liver transplant recipient; mtLSUrRNA, mitochondrial large subunit ribosomal RNA; NA, not available; NS, nasopharyngeal swab; RTR, renal transplant recipient; SOD, superoxide dismutase.
aThe 9 HTR patients harbored a previously unreported C350T mutation at the CYB gene, whereas none of the control patients harbored this punctual mutation.
bThe absence of genotyping data was due to genotype analysis failure for patient 10 (Col 10).
Transmission Map
The transmission map revealed that inter-human transmission of P. jirovecii might have been possible on multiple occasions during outpatient clinic visits (Figure 1). Based on visual investigation, either patient 1 or patient 5 could be the index patient of the outbreak, and several chains of transmission may exist. PCP and colonized HTR cases were linked with 23 and 21 possible transmission chains from patient 1 (Figure 1A) and patient 5 (Figure 1B), respectively. The median time between a possible transmission and PCP diagnosis was 89 (range, 11–224) days, and 91 (range, 11–313) days from patient 1 and patient 5, respectively. No other potential index case was identified in other departments of our hospital.
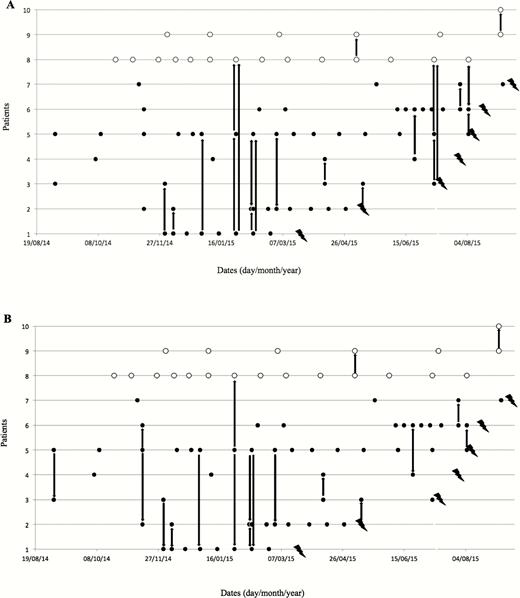
Transmission chain map considering index case patient 1 as the index case (A) or index case patient 5 as the index case (B). Black circles: visit of Pneumocystis jirovecii pneumonia (PCP) cases in the outpatient clinic. White circles: visit of colonized heart transplant recipients (HTR) in the outpatient clinic. Lightning symbols: date of PCP diagnosis. Arrows: possible transmission between PCP cases or colonized HTR patients in the outpatient clinic.
Among patients without TMP-SMX prophylaxis (n = 62), the median number of visits in the outpatient clinic was 6.5 (range, 3–13) in PCP cases and 2 (range, 0–13) in noncases (P = .03) with patient 1 as the index case. The same figure was 6.5 (range, 4–11) in PCP cases and 3 (range, 0–15) in noncases (P = .06) with patient 5 as the index case.
Serum Concentration of Atovaquone for Pneumocystis jirovecii Pneumonia Prophylaxis
Determination of serum atovaquone (regardless of time interval between last drug intake and sampling) was performed in 6 PCP cases and 5 noninfected HTR patients receiving atovaquone prophylaxis. The median of atovaquone serum concentration was 8.25 (range, <0.050–30.5) µg/mL in PCP patients and 10.9 (range, 5.8–21.8) µg/mL in the control group. The difference was not significant (P = .67).
DISCUSSION
Pneumocystis jirovecii infection has become an increasing problem in non-HIV-infected immunocompromised patients [1]. We report the first outbreak of PCP in HTR over a 6-month period in the cardiology outpatient clinic, with successful control [8]. The investigation using molecular analysis and transmission map evidenced interhuman transmission. Whereas the ecology of the fungus remains partly unknown, airborne transmission was highly suspected from infected and also colonized patients considered as a reservoir [5, 9, 23, 24]. Based on this type of transmission, several outbreaks of PCP have been reported, mainly in renal transplant recipients [8, 9, 12, 25, 26] but also in liver transplant recipients [26, 27] or in patients with a long-term immunosuppressive therapy [28].
Pneumocystis jirovecii cross-transmission among HTR patients was hypothesized. The identification of the same strain of P. jirovecii in 7 PCP cases and in 2 colonized patients among the 124 HTR patients combined with the construction of 2 possible transmission maps, revealing multiple contacts between cases, suggested patient-to-patient transmission of the pathogen favored by a higher number of visits in the outpatient clinic, rather than an environmental source. Transmission from patient 1 was more plausible than from patient 5. Indeed, patient 1 was HIV infected with a low CD4 count and therefore a possible longer period of colonization with P. jirovecii before infection; furthermore, the duration of possible contamination of secondary cases was limited to 4 months in patient 1, compared with >6 months in patient 5.
Chemoprophylaxis is recommended for solid organ transplant recipients, especially during the first 6–12 months after transplantation [29]. TMP-SMX is the preferred agent for the prevention of PCP [29, 30]. However, adverse events—especially renal failure and skin adverse effect—are frequent, and less effective alternatives drugs are proposed such as atovaquone [29]. In this cluster, 6 PCP cases received chemoprophylaxis with atovaquone. Only 3 (1 PCP case and 2 controls) of 11 measured serum patients had adequate atovaquone serum concentration, but serum concentrations of atovaquone between PCP patients and control group were not significantly different. Patients receiving atovaquone prophylaxis, however, did not receive information about the need of taking prophylaxis with a fatty meal, as should be performed. Atovaquone prophylaxis was statistically related to the occurrence of PCP infection, strengthening the recommendation for TMP-SMX as frontline PCP prevention and caution to use atovaquone without measuring drug levels. The small number of cases, however, did not allow to perform a multivariate analysis to identify other risk factors for PCP.
Moreover, a decrease in sensitivity of P. jirovecii organisms to atovaquone was suspected as a previously unreported mutation was identified on the CYB gene in the 9 HTR patients. This mutation corresponds to a transition from C to T at nucleotide position 350 of the CYB gene, target of atovaquone. This mutation was not identified in the 11 unrelated control patients, strengthening the hypothesis of P. jirovecii nosocomial acquisition and interhuman transmission.
The screening and management of HTR also represents an original approach of our investigation [11]. Screening of colonization/infection with molecular methods on nasopharyngeal swabs allowed the identification of 3 additional colonized HTR, 2 of whom had the outbreak strain. Reproducible, noninvasive, and easy-to-sample nasopharyngeal swabs were preferred in our study for screening compared to more invasive and nonreproducible samples [28, 31]. However, as already stated for upper respiratory tract specimens [31], PCR on nasopharyngeal swabs may be less sensitive than lower respiratory samples, thus resulting in possible false-negative results. In addition, because colonization and infection may be difficult to delineate using PCR on nasopharyngeal swab, invasive investigations in case of high suspicion of PCP could improve active screening of PCP-infected patients.
Early systematic administration of prophylaxis with TMP-SMX in all HTR patients was decided to interrupt the transmission chain, as was recently reported [32]. No new PCP case was observed during the year after September 2015. This approach has to be balanced with the potential risk of adverse events due to long-term TMP-SMX, and the difficulties to decide when to stop the universal prophylaxis after outbreak control.
In conclusion, our investigation strongly suggests interhuman transmission among HTR in the outpatient clinic. Transmission was favored by sharing the same waiting room during the visits. Trimethoprim-sulfamethoxazole is still the preferred agent for the prevention and treatment of PCP. The use of atovaquone should remain a secondary choice and should be prescribed only after educational measures of recipients. Although it is recognized that HTR should be placed in single rooms to prevent airborne transmission of various infectious agents during inpatient stay, whether these patients should be placed in a single room in all circumstance and should systematically wear a surgical mask during visits in the outpatient clinic remains a matter of debate.
Notes
Acknowledgments. The authors thank Michèle Virmaux for her technical contribution.
Financial support. This work was supported in part by the European Commission’s ERANet-LAC program (award number CAPRI-PC HID-0254).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




