-
PDF
- Split View
-
Views
-
Cite
Cite
Emily L Ho, Clare L Maxwell, Shelia B Dunaway, Sharon K Sahi, Lauren C Tantalo, Sheila A Lukehart, Christina M Marra, Neurosyphilis Increases Human Immunodeficiency Virus (HIV)-associated Central Nervous System Inflammation but Does Not Explain Cognitive Impairment in HIV-infected Individuals With Syphilis, Clinical Infectious Diseases, Volume 65, Issue 6, 15 September 2017, Pages 943–948, https://doi.org/10.1093/cid/cix473
Close - Share Icon Share
Abstract
Individuals infected with human immunodeficiency virus (HIV) who have previously had syphilis may have cognitive impairment. We tested the hypothesis that neurosyphilis causes cognitive impairment in HIV by amplifying HIV-related central nervous system (CNS) inflammation.
HIV-infected participants enrolled in a study of cerebrospinal fluid (CSF) abnormalities in syphilis underwent the mental alternation test (MAT), venipuncture, and lumbar puncture. CSF concentrations of chemokine (C-X-C motif) ligand 10 (CXCL10), chemokine (C-C motif) ligand 2 (CCL2), and neurofilament light (NFL) were determined by commercial assays. The proportion of peripheral blood mononuclear cells (PBMCs) and of CSF white blood cells (WBCs) that were activated monocytes (CD14+CD16+) was determined by flow cytometry. Neurosyphilis was defined as detection of Treponema pallidum 16S RNA in CSF or CSF white blood cells (WBCs) >20/uL or a reactive CSF-Venereal Disease Research Laboratory (VDRL) test; uncomplicated syphilis was defined as undetectable CSF T. pallidum, CSF WBCs ≤5/uL and nonreactive CSF-VDRL. MAT <18 was considered low.
Median proportion of PBMCs that were activated monocytes (16.6 vs. 5.3), and median CSF CXCL10 (10658 vs. 2530 units), CCL2 (519 vs. 337 units) and HIV RNA (727 vs. 50 c/mL) were higher in neurosyphilis than in uncomplicated syphilis (P ≤ .001 for all comparisons). Neurosyphilis was not related to low MAT scores. Participants with low MAT scores had higher median CSF CXCL10 (10299 vs. 3650 units, P = .008) and CCL2 (519 vs. 365 units, P = .04) concentrations than those with high MAT scores.
Neurosyphilis may augment HIV-associated CNS inflammation, but it does not explain cognitive impairment in HIV-infected individuals with syphilis.
The rate of primary and secondary syphilis in the United States has progressively increased since 2001, with a 19% increase between 2014 and 2015 [1]. In 2015, most cases were in men who have sex with men, and 50% of them were infected with human immunodeficiency virus (HIV) [1]. Treponema pallidum subsp pallidum (hereafter T. pallidum) is highly neuroinvasive. Neuroinvasion, defined by identifying living organisms or their nucleic acid in cerebrospinal fluid (CSF), may be seen in as many as 40% of patients with early syphilis [2] and does not differ in those who are HIV-infected compared to those who are not [3]. However, asymptomatic or symptomatic neurosyphilis defined as reactive CSF-Venereal Disease Research Laboratory (VDRL), CSF pleocytosis, or both, without or with neurological abnormalities, may be more common in HIV-infected compared to HIV-uninfected individuals with syphilis [4].
We previously showed that HIV-infected individuals with a prior episode of syphilis had neurocognitive impairment compared to appropriate HIV-infected controls [5]. Similar findings have been reported by others [6, 7], even among individuals with acute HIV infection [8]. Six of 100 individuals in our study had a reactive CSF-VDRL, and they had significantly higher CSF white blood cell (WBC) and CSF HIV RNA concentrations than those with a nonreactive CSF-VDRL. There was no significant relationship between reactive CSF-VDRL and neuropsychological test performance, but the number of individuals with reactive CSF-VDRL was small [5]. Among HIV-infected individuals with detectable plasma HIV RNA, an earlier retrospective study showed that CSF WBC and CSF HIV RNA were significantly higher in those with past neurosyphilis than in those with previous uncomplicated syphilis or no history of syphilis [9]. No neuropsychological testing was performed. The authors suggested that immune activation due to neurosyphilis could amplify central nervous system (CNS) HIV infection and predispose affected individuals to future cognitive impairment [9].
Cognitive impairment is common in HIV-infected individuals, even in the setting of control of peripheral viremia [10]. Its pathogenesis remains incompletely understood, but ongoing inflammation in the CNS compartment may play a role [11]. We hypothesized that neurosyphilis causes cognitive impairment in HIV-infected individuals, and that greater cognitive impairment in HIV-infected individuals with a history of syphilis is due to amplification of HIV-related CNS inflammation by neurosyphilis. We aimed to test this hypothesis in a cohort of HIV-infected patients with uncomplicated syphilis and neurosyphilis.
METHODS
Characteristics of Study Participants
Participants were enrolled in a study of CSF abnormalities in syphilis conducted in Seattle, Washington [12]. Study eligibility included clinical or serological evidence of syphilis, and assessment by the referring provider that the patient was at risk for neurosyphilis. Reasons for referral to the study included but were not restricted to (1) neurological symptoms or signs, (2) serum rapid plasma reagin (RPR) titer ≥ 1:32, and (3) in HIV-infected individuals, peripheral blood CD4+ T-cell count ≤ 350/mL. The 2 latter criteria increase risk of neurosyphilis [12–14]. Participants underwent a structured history and examination that included the Mental Alternation Test (MAT, an oral version of the Trails B test) [15], venipuncture, and lumbar puncture. The study protocol was reviewed and approved by the University of Washington Institutional Review Board, and human experimentation guidelines were followed in the conduct of this research. Written informed consent was obtained from all participants.
Laboratory Methods
General Methods
Plasma and cell-free CSF HIV RNA, CSF-VDRL tests, and enumeration of CSF red blood cells (RBCs) and WBCs were performed in a Clinical Laboratory Improvement Amendments (CLIA)-approved hospital clinical laboratory. Serum RPR tests were performed in a research laboratory using published methods [16]. Identification of T. pallidum 16S ribosomal RNA (16S RNA) in CSF was performed using reverse transcriptase polymerase chain reaction (RT-PCR) as previously described [12].
Measurement of Cerebrospinal Fluid Chemokines and Neurofilament Light
Cell-free CSF concentrations of chemokine (C-X-C motif) ligand 10 (CXCL10; also known as interferon-gamma inducible protein 10 [IP-10]) and chemokine (C-C motif) ligand 2 (CCL2; also known as monocyte chemoattractant protein [MCP]-1) were determined by Meso Scale Discovery multiplex system (Meso Scale Diagnostics, Rockville, Maryland). Cell-free CSF concentrations of neurofilament light (NFL) were determined by NF-light ELISA (UmanDiagnostics AB, Umea, Sweden) according to the manufacturer’s instructions.
Peripheral Blood Mononuclear Cells and Cerebrospinal Fluid Preparation
Paired peripheral blood mononuclear cells (PBMCs) and CSF WBCs were collected from individual study participants. PBMCs were purified from blood collected in EDTA tubes using Ficoll-Paque PLUS density gradient media (Sigma-Aldrich, St. Louis, Missouri) according to manufacturer’s instructions. CSF cells were collected by centrifugation of 5–8 mL of CSF at 200 g for 10 min. Both were preserved in a solution of 90% fetal bovine serum (FBS) and 10% dimethyl sulfoxide (DMSO) in liquid nitrogen until use.
Flow Cytometry
CD45+ mononuclear cells were gated to define monocytes (CD3-CD4+). Activated monocytes were gated for a CD14+CD16+ population. Antibodies included anti-CD45 conjugated with VioBlue; anti-CD16 conjugated with PECy5; anti-CD14 conjugated with ECD (Beckman Coulter, Brea, California); anti-CD8 conjugated with QDot605; anti-CD4 conjugated with PE; anti-CD3 conjugated with AmCyan. All antibodies were purchased from BD Biosciences, San Jose, California, except where specifically noted. Unstained PBMCs and CSF cells, as well as unstained and single stained BDTM CompBeads (BD Biosciences, San Jose, California) controls were prepared for optimizing fluorescence compensation settings for flow cytometric analyses. Fluorescent minus one (FMO) controls were prepared on PBMC samples by omitting 1 antibody and were used to evaluate fluorescence spill-over and assist with setting gates. Stained samples were acquired on a customized LSRII flow cytometer (BD Biosciences, San Jose, California) within 2 hours of staining. For PBMC samples, 100000 events were acquired; for CSF samples, the entire cell suspension was acquired. Analysis and compensation of data was performed using FlowJo (Treestar, Ashland, Oregon).
Statistical Methods
Neurosyphilis was defined as detection of T. pallidum 16S RNA in CSF or CSF WBCs >20/uL or a reactive CSF-VDRL. Uncomplicated syphilis was defined as undetectable T. pallidum 16S RNA in CSF and CSF WBCs ≤5/uL and nonreactive CSF-VDRL. A cut-off of 18, the cut-off of the lower quartile of values in our participants, was used to define abnormal performance on the MAT. Peripheral blood CD4+ T cells and plasma HIV RNA concentration were obtained from medical records and were included in analyses if they were determined within 90 days of the study visit. Plasma and CSF HIV RNA concentrations ≤50 copies/mL were assigned a value of 50 copies/mL. Descriptive statistics are reported as number (n) (%) or median (interquartile range [IQR]). Associations between categorical variables were assessed by χ2 or Fisher exact tests; associations between continuous and categorical variables were determined by the Mann-Whitney U test; and associations between continuous variables were assessed using the Spearman rank correlation coefficient. Multivariate associations were assessed using logistic regression. SPSS version 19 was used for all analyses. All tests were 2-tailed. P values < .05 were considered statistically significant.
RESULTS
The characteristics of the 132 HIV-infected study participants are shown in Table 1. Sixty-eight individuals met our definition of neurosyphilis: 52 had CSF WBC >20/uL, and this was the only CSF abnormality in 18; 28 had detection of T. pallidum 16S RNA in CSF, and this was the only CSF abnormality in 6; and 36 had a reactive CSF-VDRL, and this was the only CSF abnormality in 8. Consistent with other studies, individuals with neurosyphilis had higher serum RPR titers, were less likely to be taking antiretroviral agents, and had higher plasma HIV RNA concentrations than those with uncomplicated syphilis [12–14, 17, 18]. Contrary to our hypothesis, there was no difference in the proportion with an abnormal score (<18) on the MAT in the 2 groups (17 [25.0%] of 68 vs. 13 [21.0%] of 62, P = .59; Table 1).
Characteristics of the 132 Human Immunodeficiency Virus-infected Study Participants
| Characteristic . | Uncomplicated Syphilis, n = 64 . | Neurosyphilis, n = 68 . | P-value . |
|---|---|---|---|
| Male | 63 (98.4) | 68 (100.0) | NS |
| Race | |||
| Black | 11 (17.2) | 9 (13.2) | NS |
| White | 47 (73.4) | 55 (80.9) | |
| Other | 6 (9.4) | 4 (5.9) | |
| Age | 37 (32–43) | 41 (33–48) | NS |
| 1/serum RPR titer | 64 (8–128) | 128 (64–512) | <.001 |
| On antiretrovirals | 41 (68.3), n = 60 | 27 (45.0), n = 60 | .01 |
| Peripheral blood CD4 T cells/uL within 90 days | 445 (227–627), n = 56 | 432 (303–567), n = 63 | NS |
| Plasma HIV RNA copies/mL within 90 days | 60 (50–4582), n = 57 | 10765 (50–92938), n = 64 | .006 |
| Previous AIDS diagnosis | 28 (43.8) | 25 (37.3), n = 67 | NS |
| Mental alternation test score <18 | 13 (21.0), n = 62 | 17 (25.0) | NS |
| Characteristic . | Uncomplicated Syphilis, n = 64 . | Neurosyphilis, n = 68 . | P-value . |
|---|---|---|---|
| Male | 63 (98.4) | 68 (100.0) | NS |
| Race | |||
| Black | 11 (17.2) | 9 (13.2) | NS |
| White | 47 (73.4) | 55 (80.9) | |
| Other | 6 (9.4) | 4 (5.9) | |
| Age | 37 (32–43) | 41 (33–48) | NS |
| 1/serum RPR titer | 64 (8–128) | 128 (64–512) | <.001 |
| On antiretrovirals | 41 (68.3), n = 60 | 27 (45.0), n = 60 | .01 |
| Peripheral blood CD4 T cells/uL within 90 days | 445 (227–627), n = 56 | 432 (303–567), n = 63 | NS |
| Plasma HIV RNA copies/mL within 90 days | 60 (50–4582), n = 57 | 10765 (50–92938), n = 64 | .006 |
| Previous AIDS diagnosis | 28 (43.8) | 25 (37.3), n = 67 | NS |
| Mental alternation test score <18 | 13 (21.0), n = 62 | 17 (25.0) | NS |
Results are expressed as n (%) or median (interquartile range).
Abbreviations: HIV, human immunodeficiency virus; NS, not significant; RPR, rapid plasma reagin.
Characteristics of the 132 Human Immunodeficiency Virus-infected Study Participants
| Characteristic . | Uncomplicated Syphilis, n = 64 . | Neurosyphilis, n = 68 . | P-value . |
|---|---|---|---|
| Male | 63 (98.4) | 68 (100.0) | NS |
| Race | |||
| Black | 11 (17.2) | 9 (13.2) | NS |
| White | 47 (73.4) | 55 (80.9) | |
| Other | 6 (9.4) | 4 (5.9) | |
| Age | 37 (32–43) | 41 (33–48) | NS |
| 1/serum RPR titer | 64 (8–128) | 128 (64–512) | <.001 |
| On antiretrovirals | 41 (68.3), n = 60 | 27 (45.0), n = 60 | .01 |
| Peripheral blood CD4 T cells/uL within 90 days | 445 (227–627), n = 56 | 432 (303–567), n = 63 | NS |
| Plasma HIV RNA copies/mL within 90 days | 60 (50–4582), n = 57 | 10765 (50–92938), n = 64 | .006 |
| Previous AIDS diagnosis | 28 (43.8) | 25 (37.3), n = 67 | NS |
| Mental alternation test score <18 | 13 (21.0), n = 62 | 17 (25.0) | NS |
| Characteristic . | Uncomplicated Syphilis, n = 64 . | Neurosyphilis, n = 68 . | P-value . |
|---|---|---|---|
| Male | 63 (98.4) | 68 (100.0) | NS |
| Race | |||
| Black | 11 (17.2) | 9 (13.2) | NS |
| White | 47 (73.4) | 55 (80.9) | |
| Other | 6 (9.4) | 4 (5.9) | |
| Age | 37 (32–43) | 41 (33–48) | NS |
| 1/serum RPR titer | 64 (8–128) | 128 (64–512) | <.001 |
| On antiretrovirals | 41 (68.3), n = 60 | 27 (45.0), n = 60 | .01 |
| Peripheral blood CD4 T cells/uL within 90 days | 445 (227–627), n = 56 | 432 (303–567), n = 63 | NS |
| Plasma HIV RNA copies/mL within 90 days | 60 (50–4582), n = 57 | 10765 (50–92938), n = 64 | .006 |
| Previous AIDS diagnosis | 28 (43.8) | 25 (37.3), n = 67 | NS |
| Mental alternation test score <18 | 13 (21.0), n = 62 | 17 (25.0) | NS |
Results are expressed as n (%) or median (interquartile range).
Abbreviations: HIV, human immunodeficiency virus; NS, not significant; RPR, rapid plasma reagin.
Forty-two individuals were treated for uncomplicated syphilis before LP. There was no significant difference in the proportion with an abnormal MAT score between those who were not and those who were treated for uncomplicated syphilis before LP (20 [22.7%] of 88 vs. 10 [23.8%] of 42, P = .89). Similarly, there was no difference in the proportion with an abnormal MAT score between those without and with a prior episode of syphilis (27 [25.2%] of 107 vs. 3 [13.0%] of 23, P = .21) or among those not currently on antiretrovirals compared to those currently on antiretrovirals (13 [25.5%] of 51 vs. 15 (22.4%) of 67 P = .70).
The proportion of PBMCs that were activated monocytes (CD14+CD16+) was significantly higher in those with neurosyphilis compared to those with uncomplicated syphilis (Figure 1). CSF concentrations of CXCL10, CCL2, and HIV RNA were also significantly higher in those with neurosyphilis (Figure 1), but the proportion of CSF WBCs that were activated monocytes and the CSF concentration of NFL did not differ between the 2 groups. Because CSF NFL concentration increases with age [19], the latter analysis was repeated taking into account participant age, and the results were unchanged. Results were unchanged when we restricted the analysis to individuals with plasma HIV RNA ≤ 50 copies/mL, except that CSF NFL concentration was significantly higher in those with neurosyphilis (data not shown). The number of samples tested was too small to allow us to take age into account.
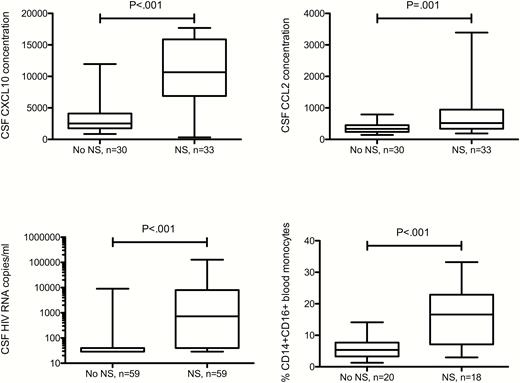
Concentrations of CSF and peripheral blood measures in patients with uncomplicated syphilis (no NS) and neurosyphilis (NS). CXCL10 and CCL2 concentrations are in arbitrary units. Abbreviation: CSF, cerebrospinal fluid; HIV, human immunodeficiency virus.
Among all study participants, CSF concentrations of CXCL10, CCL2, and HIV RNA were significantly higher in samples with higher CSF WBC concentrations (Table 2). We were not able to test for association between CXCL10 or CCL2 and neurosyphilis taking into account WBC concentration because of our sample size and collinearity. Taking into account CSF WBCs, CSF concentration of HIV RNA did not remain significantly higher in individuals with neurosyphilis.
Correlationsa Between Cerebrospinal Fluid; and Blood Measures in All Study Participants
| Measure . | WBC . | CXCL10 . | CCL2 . | NFL . | CSF HIV RNA . | CSF CD14+CD16+ . |
|---|---|---|---|---|---|---|
| CXCL10 | .637c | |||||
| CCL2 | .515c | .598c | ||||
| NFL | NS | NS | NS | |||
| CSF HIV RNA | .497c | .585c | .403b | NS | ||
| CSF CD14+CD16+ | NS | NS | NS | NS | NS | |
| BLOOD CD14+CD16+ | .521b | NS | NS | NS | NS | NS |
| Measure . | WBC . | CXCL10 . | CCL2 . | NFL . | CSF HIV RNA . | CSF CD14+CD16+ . |
|---|---|---|---|---|---|---|
| CXCL10 | .637c | |||||
| CCL2 | .515c | .598c | ||||
| NFL | NS | NS | NS | |||
| CSF HIV RNA | .497c | .585c | .403b | NS | ||
| CSF CD14+CD16+ | NS | NS | NS | NS | NS | |
| BLOOD CD14+CD16+ | .521b | NS | NS | NS | NS | NS |
Abbreviations: CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; NFL, neurofilament light; NS, not significant; WBC, white blood cell.
aSpearman’s rho.
bP < .01.
cP < .001.
Correlationsa Between Cerebrospinal Fluid; and Blood Measures in All Study Participants
| Measure . | WBC . | CXCL10 . | CCL2 . | NFL . | CSF HIV RNA . | CSF CD14+CD16+ . |
|---|---|---|---|---|---|---|
| CXCL10 | .637c | |||||
| CCL2 | .515c | .598c | ||||
| NFL | NS | NS | NS | |||
| CSF HIV RNA | .497c | .585c | .403b | NS | ||
| CSF CD14+CD16+ | NS | NS | NS | NS | NS | |
| BLOOD CD14+CD16+ | .521b | NS | NS | NS | NS | NS |
| Measure . | WBC . | CXCL10 . | CCL2 . | NFL . | CSF HIV RNA . | CSF CD14+CD16+ . |
|---|---|---|---|---|---|---|
| CXCL10 | .637c | |||||
| CCL2 | .515c | .598c | ||||
| NFL | NS | NS | NS | |||
| CSF HIV RNA | .497c | .585c | .403b | NS | ||
| CSF CD14+CD16+ | NS | NS | NS | NS | NS | |
| BLOOD CD14+CD16+ | .521b | NS | NS | NS | NS | NS |
Abbreviations: CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; NFL, neurofilament light; NS, not significant; WBC, white blood cell.
aSpearman’s rho.
bP < .01.
cP < .001.
Among all study participants, those with scores <18 had higher CSF CXCL10 and CCL2 concentrations, lower peripheral blood CD4+ T cells (Figure 2) and were more likely to have a previous AIDS diagnosis (17 [56.7%] of 30 vs. 35 [35.4%] of 99, P = .04) than those with MAT scores ≥18. Low scores on the MAT were not significantly related to CSF concentrations of WBC, HIV RNA, or NFL (even taking into account age); or to age, plasma HIV RNA concentration or use of antiretroviral medications.
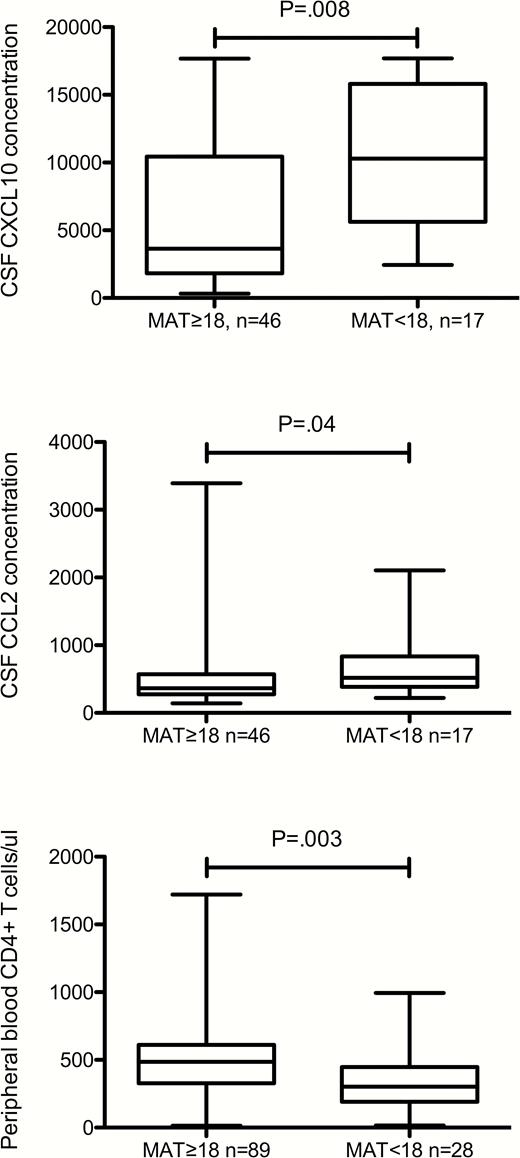
Concentrations of CSF and peripheral blood measures in all patients dichotomized by MAT scores at or above 18 (≥18) vs. below 18 (<18), which is the score defining the lowest quartile for the study population. CXCL10 and CCL2 concentrations are in arbitrary units. Abbreviations: CSF, cerebrospinal fluid; MAT, mental alternation test.
DISCUSSION
The goal of this study was to test the hypothesis that neurosyphilis causes cognitive impairment in HIV-infected individuals, and that greater cognitive impairment in HIV-infected individuals with a history of syphilis is due to amplification of HIV-related CNS inflammation by neurosyphilis. We focused on assessment of a small panel of mediators that are associated with cognitive impairment and CSF inflammation in patients infected with HIV, including peripheral blood CD14+CD16+ cells and CSF concentrations of CXCL10, CCL2, and NFL.
The number of circulating peripheral blood CD14+CD16+ monocytes is higher in HIV-infected individuals with dementia than in AIDS patients without dementia [20], and perivascular CD14+CD16+ cells, many of which are HIV-infected, accumulate in brains of HIV-infected individuals with dementia [21]. Higher CSF concentrations of CXCL10 and CCL2 are seen in HIV-infected patients with cognitive impairment, even in the setting of modern antiretroviral therapy [22], and higher CSF concentrations are seen in HIV-infected individuals with evidence of neuronal injury by MR spectroscopy, suggesting that CXCL10 and CCR2 may be neurotoxic in vivo [23]. Higher CSF CXCL10 concentrations are seen in HIV-infected individuals with higher CSF WBC and CSF HIV RNA concentrations. This finding suggests that CXCL10 contributes to CSF pleocytosis and amplifies local HIV infection [24]. Moreover, CCL2 may promote ingress of CD16+ cells into the CNS [25]. The CSF concentration of NFL is elevated in untreated HIV-infected patients with dementia [26]; the association with cognitive impairment is attenuated in those with controlled plasma viremia [19].
Our results are in accord with our hypothesis that neurosyphilis augments HIV-associated CNS inflammation. In patients with neurosyphilis, the proportion of peripheral blood monocytes that were CD14+CD16+ was significantly higher than in those with uncomplicated syphilis, and patients with higher proportion of peripheral blood CD14+CD16+ monocytes had higher CSF WBC concentrations. The finding that the proportion of CSF WBCs that were CD14+CD16+ monocytes did not differ between the groups does not weaken our conclusion, as these cells likely migrate to perivascular locations and thus would not necessarily be reflected in the CSF cellular profile.
In addition to the above, CSF concentrations of HIV RNA, CXCL10, and CCL2 were significantly higher in those with neurosyphilis compared to individuals with uncomplicated syphilis. Higher CSF concentrations of CXCL10 and CCL2 were seen in individuals with higher CSF WBC concentrations. CNS infection by T. pallidum causes CSF pleocytosis, and CSF pleocytosis could be the source of increased CSF concentrations of HIV RNA or chemokines. CXCL10 stimulates HIV replication, and it is neurotoxic in vitro [27]. Induction of CSF CXCL10 by neurosyphilis thus has biologic plausibility for augmenting CNS HIV-related inflammation. Our finding that CSF CXCL10 and CCL2 concentrations remained higher in individuals with neurosyphilis when the analysis was restricted to those with plasma HIV RNA ≤50 copies/mL supports the contention that higher CSF chemokine concentrations were due to neurosyphilis and not to HIV infection.
A recent study in HIV-uninfected patients with uncomplicated syphilis and neurosyphilis also documented elevated CSF CXCL10 concentrations in patients with neurosyphilis but did not demonstrate an association between CSF WBC and CSF CXCL10 concentrations [28], suggesting that CSF pleocytosis in our patients could reflect both T. pallidum and HIV infection. Moreover, that study did not identify an increase in CSF CCL2 concentration in HIV-uninfected patients with neurosyphilis compared to uncomplicated syphilis as we did, suggesting that upregulation of CSF CCL2 in neurosyphilis may be unique to HIV-infected patients.
However, we were not able to support our hypothesis that neurosyphilis causes cognitive impairment in HIV-infected individuals. We did not identify a relationship between neurosyphilis and abnormal cognition. However, among all study participants, CSF concentrations of CXCL10 and CCL2 were significantly higher in those with MAT scores <18 compared to those with scores of ≥18. These findings further support the contention that CXCL10 and CCL2 are neurotoxic and raise the question of whether simply having uncomplicated syphilis is sufficient to adversely impact cognition. Because we did not have a control group of HIV-infected individuals without syphilis, we cannot address this issue.
Additional limitations of our study should be acknowledged. Study participants included in this analysis were chosen as an unmatched convenience sample. We were not able to determine the concentration of all measures in all study participants, which limits the power of some of our analyses. Because CSF CXCL10 and CCL2 were measured by Meso Scale technology, which provides concentrations in arbitrary units, we cannot compare our CSF values to published literature. We used the MAT as our single formal assessment of cognition [15], and we did not employ a comprehensive neuropsychological test battery that might have been more sensitive in detecting cognitive impairment. However, imprecision in our cognitive assessment would have weakened, rather than strengthened the associations that we identified. We used a cut-off of 18 for abnormal cognition, defined by the lower quartile among our participants, rather than a cut-off of 15 that was established for a diagnosis of dementia [15]. Our reasoning was that the cut-off of 15 was established in a cohort of largely hospitalized HIV-infected patients, many of whom had AIDS, who were studied before the advent of combination antiretroviral therapy [15]. Thus, the applicability of that cut-off to our participant population is limited, and we opted to rely on an internal standard. We also wanted to identify cognitive impairment, not just frank dementia. Our finding that low MAT scores were more common among study participants with lower peripheral blood CD4+ T cells and among those with a prior AIDS diagnosis supports the validity of our use of the MAT, as similar associations have been seen in HIV-infected individuals tested with more comprehensive neuropsychological batteries [10, 29]. Finally, we explored whether neurosyphilis amplifies HIV-related CNS inflammation, rather than the opposite, or whether the effects might be additive. Our findings support our supposition but do not exclude other possibilities. Our results should thus be considered as hypothesis generating.
Our study leaves unanswered the question of whether uncomplicated syphilis contributes to cognitive impairment in HIV-infected individuals. There is a precedent for the ability of peripheral processes to affect cognition. For example, a study of community dwelling individuals with Alzheimer disease showed that those who experienced an acute systemic inflammatory event such as urinary tract infection, myocardial infarction, or accidental trauma had a higher rate of cognitive decline over the ensuing 6 months compared to those without acute systemic inflammatory events [30]. Additionally, among HIV-infected individuals, chronic infections, for example, hepatitis C virus or even seropositivity for toxoplasmosis without evidence of CNS disease, may adversely affect cognition [31, 32]. Future study of HIV-infected individuals with and without syphilis and neurosyphilis is required to better understand the etiology of cognitive impairment in HIV-infected individuals with syphilis.
Notes
Acknowledgements. The authors thank Dr. Mark Wurfel (University of Washington) for technical assistance with Mesoscale Discovery System, and Dr. Xiaoping Wu (Bloodworks Northwest Research Institute) for technical assistance with flow cytometry.
Financial support. This work was supported by grants from the National Institutes of Health (NIH) (NS34235 to C. M. M.) and by the University of Washington Center for AIDS Research (to E. L. H.). E. L. H. was supported by National Institutes of Health training grant T32AI07140.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




