-
PDF
- Split View
-
Views
-
Cite
Cite
Samuel M Jenness, Kevin M Weiss, Steven M Goodreau, Thomas Gift, Harrell Chesson, Karen W Hoover, Dawn K Smith, Albert Y Liu, Patrick S Sullivan, Eli S Rosenberg, Incidence of Gonorrhea and Chlamydia Following Human Immunodeficiency Virus Preexposure Prophylaxis Among Men Who Have Sex With Men: A Modeling Study, Clinical Infectious Diseases, Volume 65, Issue 5, 1 September 2017, Pages 712–718, https://doi.org/10.1093/cid/cix439
Close - Share Icon Share
Abstract
Preexposure prophylaxis (PrEP) is highly effective for preventing human immunodeficiency virus (HIV) infection, but risk compensation (RC) in men who have sex with men (MSM) raises concerns about increased sexually transmitted infections (STIs). The Center for Disease Control and Prevention’s (CDC’s) PrEP guidelines recommend biannual STI screening, which may reduce incidence by treating STIs that would otherwise remain undiagnosed. We investigated these two counteracting phenomena.
With a network-based mathematical model of HIV, Neisseria gonorrhoeae (NG), and Chlamydia trachomatis (CT) transmission dynamics among MSM in the United States, we simulated PrEP uptake following the prescription indications and HIV/STI screening recommendations in the CDC guidelines. Scenarios varied PrEP coverage (the proportion of MSM indicated for PrEP who received it), RC (a reduction in the per-act probability of condom use), and the STI screening interval.
In our reference scenario (40% coverage, 40% RC), 42% of NG and 40% of CT infections would be averted over the next decade. A doubling of RC would still result in net STI prevention relative to no PrEP. STIs declined because PrEP-related STI screening resulted in a 17% and 16% absolute increase in the treatment of asymptomatic and rectal STIs, respectively. Screening and timely treatment at quarterly vs biannual intervals would reduce STI incidence an additional 50%.
Implementation of the CDC PrEP guidelines while scaling up PrEP coverage could result in a significant decline in STI incidence among MSM. Our study highlights the design of PrEP not only as antiretroviral medication but as combination HIV/STI prevention incorporating STI screening.
Preexposure prophylaxis (PrEP) can reduce the risk of human immunodeficiency virus (HIV) infection by more than 95% among men who have sex with men (MSM) when taken consistently [1]. Adherence to PrEP has been strong in open-label studies, including a clinical cohort in California in which no incident HIV infections were observed among active PrEP users [2]. However, 50% of men on PrEP in that cohort were diagnosed with a sexually transmitted infection (STI) within 12 months of starting medication. High levels of STI incidence were also observed in the PrEP Demo Project, where the overall STI incidence rate was 90 per 100 person-years [3]. A recent metaanalysis compared the STI incidence among MSM in PrEP cohorts to MSM in non-PrEP cohorts, estimating that incidence among PrEP users was 33.3 per 100 person-years at risk (PYAR) higher for Neisseria gonorrhoeae (NG) and 31.4 per 100 PYAR higher for Chlamydia trachomatis (CT) [4]. There are at least 3 noncausal explanations for these excess rates: increased STI detection (if STIs were more frequently screened for in the PrEP cohorts), selection bias (if the PrEP cohorts recruited higher-risk samples of MSM), and secular trends (STI incidence has increased over time, and the PrEP cohorts included more recent samples).
The primary causal mechanism by which PrEP use could lead to higher STI incidence would be behavioral risk compensation (RC); MSM who initiate PrEP may reduce their use of other disease prevention strategies [5]. Estimates of whether RC occurs among MSM on PrEP, and by how much, have been mixed. The PrEP Demo Project saw no increase in condomless anal intercourse (AI) [3], consistent with the iPrEx trial [6], whereas 41% of PrEP users in the California clinic-based cohort had reduced condom use [2]. In the PROUD trial that tested immediate vs deferred PrEP initiation, there were increases in reported risk behavior for those on PrEP but no differences in the STI incidence rates between the arms [7]. In that population, STI rates were high at baseline and continued to rise during the study in both arms [8]. Similar behavioral and epidemiological trends were observed in the iPERGAY open-label extension for on-demand PrEP [9]. Understanding whether RC alone could reproduce the STI incidence differential observed in the metaanalysis would help provide evidence for the causal explanation [4].
The Centers for Disease Control and Prevention (CDC) clinical practice guidelines for PrEP outline behavioral indications for prescription and recommend biannual STI screening after PrEP initiation [10]. Although PrEP medication does not biologically lower bacterial STI acquisition risk, STI screening that accompanies PrEP may detect and facilitate treatment of STIs that would otherwise go undetected, including asymptomatic rectal NG and CT, thus preventing onward transmission of STIs [11]. Questions remain about how to optimize the guidelines with respect to the STI screening interval, since an estimated 34%–41% of incident STI cases could be missed if screened biannually compared to quarterly [12].
In this study, we used mathematical modeling to address these questions about the potential causal effects and the public health impact of PrEP on bacterial STI incidence among MSM. Our research aim was to estimate how 2 potentially counteracting phenomena surrounding PrEP use—behavioral change and ongoing STI screening—could interact to either increase or decrease the incidence of rectal and urogenital NG and CT in this population.
METHODS
This study extends our mathematical models investigating HIV transmission dynamics among MSM in the United States [13] and the impact of PrEP on preventing new infections [14, 15]. We developed this model within the EpiModel software platform (www.epimodel.org), which provides tools for modeling HIV over complex sexual networks with the statistical framework of temporal exponential random graph models [16]. For this study, our additions were to build the model structure, parameterization, and analysis methods for rectal and urogenital NG and CT infection, alongside HIV infection. Full details on the methods for this model are provided in the Appendix.
Human Immunodeficiency Virus Transmission and Progression
Our model simulated the dynamics of main, casual, and one-time MSM sexual partnerships, with behavioral model parameters estimated from sexual network data [17, 18]. Predictors of partnership formation and dissolution varied by partnership type, number of current ongoing partnerships, age mixing, and sorting by receptive vs insertive sexual position. For main and casual partnerships, there was a hazard of relationship dissolution that reflected their median durations. The model simulated HIV seroadaptive behavior by which men changed their rates of condom use depending on HIV testing histories and disclosure of HIV status within partnerships.
HIV progression followed the natural history of disease, including modifications by antiretroviral therapy (ART) [19]. Persons progressed through disease stages in the absence of ART with evolving HIV viral loads that modified the rate of HIV transmission in discordant pairs [20]. Other factors that modified the transmission probability of HIV per sexual act included current NG or CT infection, condom use [21], receptive vs insertive sexual position [22], and circumcision for an HIV-negative insertive partner [23]. After infection, persons were assigned into clinical care trajectories controlling rates of HIV diagnosis, ART initiation, and HIV viral suppression to match empirical estimates [24]. ART was associated with decreased viral load [25] and extended lifespan [26].
Sexually Transmitted Infection Transmission and Recovery
NG and CT transmission was simulated along the same partnership network as HIV, but with disease recovery through either natural clearance or antibiotic treatment [27]. STI transmission was directional and site-specific during AI. For example, receptive AI with a partner infected with urethral NG was necessary for a new rectal NG infection. Men could be infected at both anatomical sites and with both NG and CT. The symptomatic status of the newly acquired infections depended on site of infection, with most rectal infections asymptomatic and most urethral infections symptomatic [28]. STI symptoms influenced the probability of testing and treatment, modifying the recovery rate from infection [29]. The base (no PrEP) models assumed no routine, interval-based screening for asymptomatic STIs, although we relaxed that assumption in sensitivity analyses. Treatment for an STI at one anatomical site resulted in effective treatment at the anatomical site for men with dual-site infections. The reference intervention models assumed no explicit treatment failure, but another sensitivity analysis explored suboptimal treatment completion. Given uncertainty in estimates for parameters that govern site- and disease-specific transmission risks, rates of clinical encounters, and duration of infection with and without treatment, we used a Bayesian approach [30] to define prior distributions for these model parameters and fit the model to empirical estimates of NG/CT incidence among MSM in the non-PrEP cohorts of the metaanalysis [4].
Preexposure Prophylaxis Uptake and Clinical Encounters
PrEP implementation was simulated following the CDC guidelines [10], which indicate HIV-uninfected MSM for PrEP on 4 conditions: condomless AI (CAI) in a monogamous but HIV status–unknown partnership; CAI outside of a monogamous partnership; AI in a known HIV-discordant partnership; and a recent STI diagnosis [14]. Exhibiting any one of these within the 6 months prior to assessment clinically indicated a man for PrEP. PrEP adherence was based on data for US MSM; 21.1% of men were categorized as nonadherent (0 pills/week), 7.0% as taking fewer than 2 pills/week (low adherence), 10.0% at 2–3 pills/week (moderate adherence), and 61.9% at 4+ pills/week (higher adherence) [3]. PrEP use reduced HIV infection probability in these adherence groups by 0%, 31%, 81%, and 95%, respectively [31]. Consistent with the guidelines, men were reassessed every 12 months for indications and discontinued from PrEP if they no longer exhibited any indications or if they were diagnosed with HIV.
Our primary analysis varied 2 PrEP parameters: coverage level and behavioral RC. Coverage was the proportion of PrEP-indicated men currently using PrEP at any time, with a reference value of 40% consistent with previous models [14, 15, 32]. Probability of condom use in a given sexual act was driven by the PrEP user when RC, the reduction in the probability of condom use during AI following initiation of PrEP, occurred. RC was modeled continuously from 0% (no reduction) to 100% (full elimination of condoms), with a reference value of 40% [2].
HIV and STI screening were simulated based on the CDC PrEP guidelines, that is, HIV testing every 3 months and STI testing every 6 months after initiating PrEP. We varied the STI screening interval from 1 to 12 months in sensitivity analyses, and the fraction of screened men who successfully completed their STI treatment from 100% (reference) to 0%. To isolate the performance of the PrEP indications as a method to target STI screening, additional scenarios simulated random screening of asymptomatic STIs. Over all PrEP scenarios, men with symptomatic STIs continued to receive treatment at the same rates as the base (no-PrEP) scenario.
Simulation and Analysis
We established pre-PrEP equilibrium in disease prevalence and incidence in an open population of 10000 MSM by calibrating the model to NG and CT incidence from the non-PrEP cohorts in the metaanalysis (4.2 and 6.6 per 100 PYAR, respectively) [4], and HIV prevalence (26%) estimated from one of the cohorts with available baseline prevalence data, consistent with our previous model [14]. Each model scenario, including the base no-PrEP model, was then simulated 250 times over 10 years. Primary outcomes were NG and CT incidence per 100 PYAR and hazard ratios, both of which were calculated across the final year of the time series; percent of infections averted (PIA) compared to base model, which was a function of cumulative incidence over the full 10-year time series; and the number needed to treat (NNT) on PrEP to prevent 1 new NG or CT infection. Given the model stochasticity, we calculated the medians and interquartile range of the simulated data for each outcome.
RESULTS
Table 1 and Figure 1 show NG and CT incidence across levels of PrEP coverage and RC. In Table 1, scenarios varying coverage hold RC constant at its 40% reference value, while scenarios varying RC hold coverage constant at its 40% reference value. Higher PrEP coverage was associated with lower incidence of both NG and CT. Incidence followed similar relative declines for both infections by coverage levels. Over 10 years, PrEP was predicted to prevent 42% and 40% of cumulative NG and CT infections, respectively.
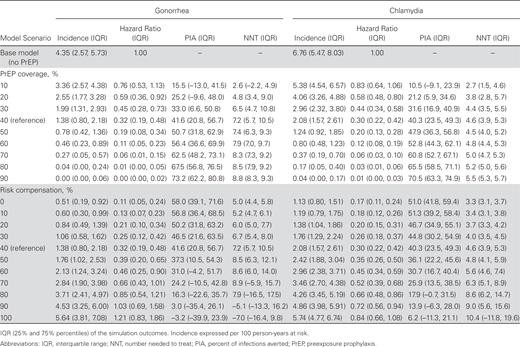
Gonorrhea and Chlamydia Incidence Rates, Hazard Ratios, Percent of Infections Averted, and Number Needed to Treat on Preexposure Prophylaxis (PrEP) by PrEP Coverage Level and Behavioral Risk Compensation Level among Men Who Have Sex With Men in the United States
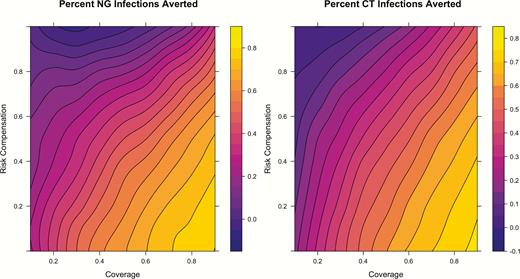
Percent of infections averted for gonorrhea and chlamydia under varying preexposure prophylaxis coverage and risk compensation levels among men who have sex with men in the United States over 10 years of 250 simulations. Abbreviations: CT, chlamydia; NG, gonorrhea.
At RC levels lower than 40%, STI incidence declined further, to hazard ratios of 0.11 and 0.17 for NG and CT under 0% RC. Increasing RC would partially offset the STI prevention benefits associated with PrEP but more so for NG than CT. At extreme levels of RC, the PIA for NG became negative in some simulations as the incidence approached or exceeded incidence in the base model. In the interaction (Figure 1), RC would need to be nearly 90% at 40% coverage for no NG incidence reduction, whereas CT incidence always remained lower than in the base model except for extreme levels of RC and coverage. Complete RC (total elimination of condoms) always resulted in a net reduction in NG and CT incidence when PrEP coverage exceeded 50%.
In the reference scenario, PrEP was associated with an NNT of 7.2 for NG and 4.6 for CT. The NNT grew with increasing PrEP coverage; the efficiency of the intervention (PrEP-related STI screening) decreased as fewer PrEP users were infected with STIs over time. At extreme levels of RC, the NNT became negative for NG as the incidence approached or exceeded the levels in the base model.
Because PrEP medication was assumed to have no biological effect on NG and CT acquisition risk, the observed prevention benefits were attributable only to the increased STI screening and treatment associated with ongoing PrEP use. Table 2 and Figure 2 demonstrate these mechanisms. Here we aggregated NG and CT incidence and held both PrEP coverage and RC constant at the values in the reference analyses (both 40%). With biannual screening, the PrEP intervention treated 17% more asymptomatic infections and 16% more rectal infections compared to the base model. This form of STI screening was associated with a 67% decline in combined STI incidence (1.77 vs 5.32) among all MSM, despite the higher behavioral risk resulting from RC in PrEP users. Reducing the STI screening interval from 6 months to 3 months would increase the number of asymptomatic urethral and rectal cases treated. This increase in STI screening and treatment would result in a further 50% reduction in STI incidence (from 1.77 to 0.89).
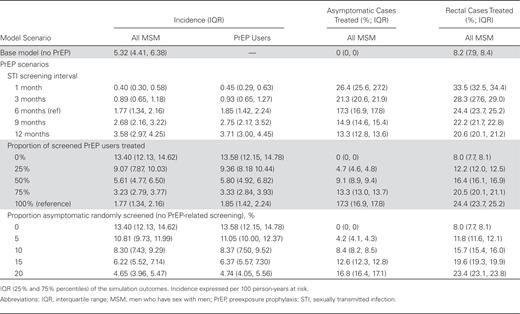
Combined Gonorrhea and Chlamydia Incidence Among All Men Who Have Sex With Men and Preexposure Prophylaxis (PrEP) Users, and Proportion of Asymptomatic Sexually Transmitted Infection (STI) Cases and Rectal STI Cases Treated by PrEP STI Screening Interval, Proportion Successfully Treated, and Random Asymptomatic Screening Outside of PrEP
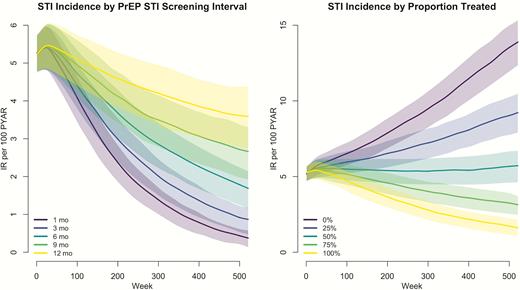
Incidence rates, per 100 person-years at risk, of combined gonorrhea and chlamydia infections under varying preexposure prophylaxis (PrEP)–associated sexually transmitted infection screening intervals and proportion of PrEP users screened and treated among men who have sex with men in the United States over 10 years of 250 simulations. Abbreviations: IR, incidence rate; PrEP, preexposure prophylaxis; PYAR, person-years at risk; STI, sexually transmitted infection.
Reductions in the proportion of MSM who successfully completed STI treatment under PrEP STI screening (held fixed at 100% above) increased STI incidence. At the extreme, where no MSM on PrEP completed STI treatment, combined STI incidence was 13.40. This 2.5-fold hazard compared to the base model was attributable to RC in the PrEP users. Under a more realistic scenario where 75% successfully completed treatment, STI incidence at 3.23 would still be 39% lower than in the base model.
Finally, in the reference PrEP scenario, 17.3% of men with asymptomatic infection would be screened and treated, resulting in a population-level STI incidence of 1.77. Even if slightly more asymptomatic men were screened (20% vs 17%), but randomly within the population instead of concentrated among PrEP users, STI incidence was 4.65, 2.6 times as high, but still lower than in the base model. Random STI screening confers STI prevention benefits, but not nearly as much as using the bio-behavioral indications for targeting PrEP recommended within the CDC guidelines.
DISCUSSION
Implementation of the CDC PrEP guidelines could result in a significant decline in STI incidence among MSM in the United States, with 42% of NG infections and 40% of CT infections predicted to be prevented over the next decade. As PrEP has no biological effect on bacterial STI risk, the incidence reduction forecast by our model was attributable only to the recommended ongoing screening and treatment of STIs as part of the broader PrEP intervention. This screening would result in a substantial increase in treatment for STIs that often remain undiagnosed, that is, asymptomatic rectal NG and CT.
The potential for PrEP to increase STI incidence was a public health concern even before the completion of the clinical trials that established its efficacy [33]. The hypothesized cause for increased STI incidence would be different forms of RC, including an increase in the number of partners, greater clustering of higher-risk men, or a reduction in condom use with existing partners [1]. Few studies have found behavioral evidence for RC [3], but one that has suggested the predominant form of RC is reduced condom use [2]. In our model, we adopted their upper bound of observed condom-related RC in our PrEP scenarios as a reference value to predict the impact of this phenomenon on STI incidence. Our models demonstrate that even a doubling of this observed RC value among PrEP users would result in a net prevention benefit for STIs; that benefit increases as PrEP coverage grows.
We were unable to reproduce, even under extreme levels of RC, the NG and CT incidence rates approaching 40 per 100 PY for PrEP users estimated in the metaanalysis [4]. Therefore, our results suggest that the considerably higher STI incidence rates in the PrEP cohorts compared to non-PrEP cohorts were not a causal effect of RC but more likely an issue of study design whereby the MSM in the 2 comparison groups were not sampled from the same underlying MSM population. This selection bias may have resulted from PrEP studies’ need to recruit higher-risk MSM to achieve sufficient power to test efficacy hypotheses in clinical trials and maximize PrEP benefit in demonstration projects. Secular trends in STI incidence and increased STI detection in the PrEP cohorts could also bias the comparisons [1, 3, 7]. A recent commentary by Harawa et al. on the meta-analysis discusses these potential biases and others in further detail [34].
Our model results have important implications for the role of PrEP as an intervention for STI prevention. Building on prior models that estimated the impact of CDC’s PrEP guidelines for HIV incidence [14], we aimed to assess the potentially counteracting forces between higher behavioral risk and more STI screening after initiation of PrEP. Prevention of NG and CT among MSM is complicated by the high prevalence of asymptomatic rectal infection [35], which allows for reinfection within ongoing sexual partnerships even if symptomatic urogenital infection is controlled [36].
Previous models have explored the impact and cost-effectiveness of NG/CT screening outside of PrEP on disease incidence among MSM [37]. Our study suggests that CDC’s recommended indications for PrEP initiation may be well suited for targeting STI screening to high-risk MSM. Consistent with empirical data [12], we found that decreasing the STI screening interval from 6 months to 3 months could identify even more incident infections that would otherwise go undiagnosed and untreated, suggesting more frequent PrEP-related screening may be needed. These results underscore the critical role of clinicians in performing the STI screening and treatment recommendations among their current PrEP patients, as we predicted STI incidence could increase if the proportion of PrEP users treated for STIs fell below 50%.
The study limitations include modeling urogenital and rectal NG and CT infections (along with HIV) but no other STIs among MSM, such as syphilis [38]. Syphilis incidence has increased among US MSM over the past 5 years [39], but rates are still substantially lower than GC and CT incidence according to case surveillance data as well as comparisons in the PrEP metaanalysis, where NG/CT incidence was 6 times higher than syphilis in the non-PrEP cohorts and 3 times higher in the PrEP cohorts. NG and CT were modeled only as uncomplicated infections of the urethra and rectum, missing pharyngeal and disseminated infections. Pharyngeal-only NG and CT infections are less common in this population [35, 40] but they may serve as a reservoir for rectal and urogenital infections modeled in our analysis [41]. Our models also did not explicitly simulate STI treatment failure, although the analysis on proportion successfully treated could be a proxy. We only modeled RC as a reduction in condom use, a decision based on the best empirical data for US MSM [2], but modeling other forms of RC could lead to different results. Finally, some of the parameters for the dynamic network models were derived from empirical studies of MSM in Atlanta, Georgia, that, although similar to national data [42], may not be fully representative.
CONCLUSIONS
This study highlights the design of PrEP not only as daily antiretroviral medication but as a combination HIV/STI prevention package that incorporates STI screening and treatment. MSM who are at substantial risk for HIV and therefore indicated for PrEP are also at substantial risk for STIs through the same sexual partnership networks and behaviors. PrEP, as a package prescribed and administered following CDC guidelines that include ongoing STI screening, could be an effective STI prevention intervention.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the US National Institutes of Health or Centers for Disease Control and Prevention (CDC).
Financial support. This work was supported by the CDC (grant U38 PS004646), the National Institutes of Health (R21 MH112449; R21 HD075662), the Center for AIDS Research at Emory (grant P30 AI050409), and the University of Washington (grant P30 AI027757).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




