-
PDF
- Split View
-
Views
-
Cite
Cite
Offianan Andre Toure, Victor Mwapasa, Issaka Sagara, Oumar Gaye, Ricardo Thompson, Aishwarya V Maheshwar, Pitabas Mishra, Narendra Behra, Antoinette K Tshefu, Rashmi R Das, Anupkumar R Anvikar, Pradeep Sharma, Arjun Roy, Sanjay K Sharma, Amit Nasa, Rajinder K Jalali, Neena Valecha, for the Arterolane Maleate-Piperaquine Phosphate (AM-PQP) Study Team , Assessment of Efficacy and Safety of Arterolane Maleate–Piperaquine Phosphate Dispersible Tablets in Comparison With Artemether-Lumefantrine Dispersible Tablets in Pediatric Patients With Acute Uncomplicated Plasmodium falciparum Malaria: A Phase 3, Randomized, Multicenter Trial in India and Africa, Clinical Infectious Diseases, Volume 65, Issue 10, 15 November 2017, Pages 1711–1720, https://doi.org/10.1093/cid/cix617
Close - Share Icon Share
Abstract
Administration of artemisinin-based combination therapy (ACT) to infant and young children can be challenging. A formulation with accurate dose and ease of administration will improve adherence and compliance in children. The fixed-dose combination dispersible tablet of arterolane maleate (AM) 37.5 mg and piperaquine phosphate (PQP) 187.5 mg can make dosing convenient in children.
This multicenter (India and Africa), comparative, parallel-group trial enrolled 859 patients aged 6 months to 12 years with Plasmodium falciparum malaria. Patients were randomized in a ratio of 2:1 to AM-PQP (571 patients) once daily and artemether-lumefantrine (AL) (288 patients) twice daily for 3 days and followed for 42 days.
The cure rate (ie, polymerase chain reaction–corrected adequate clinical and parasitological response) in the per-protocol population at day 28 was 100.0% and 98.5% (difference, 1.48% [95% confidence interval {CI}, .04%–2.91%]) in the AM-PQP and AL arms, respectively, and 96.0% and 95.8% (difference, 0.14% [95% CI, –2.68% to 2.95%]) in the intention-to-treat (ITT) population. The cure rate was comparable at day 42 in the ITT population (AM-PQP, 94.4% vs AL, 93.1%). The median parasite clearance time was 24 hours in both the arms. The median fever clearance time was 6 hours in AM-PQP and 12 hours in the AL arm. Both the treatments were found to be safe and well tolerated. Overall, safety profile of both the treatments was similar.
The efficacy and safety of fixed-dose combination of AM and PQP was comparable to AL for the treatment of uncomplicated P. falciparum malaria in pediatric patients.
CTRI/2014/07/004764.
The World Health Organization (WHO) African region accounts for about 70% of all deaths in children <5 years of age as per the WHO estimates [1]. Artemisinin-based combination therapies (ACTs) were given to these children. ACTs are the WHO-recommended first-line treatment for uncomplicated Plasmodium falciparum malaria [2, 3]. However, it is difficult to administer ACT to infants and small children [2, 4]. Therefore, a new fixed-dose combination (FDC) of arterolane maleate (AM) 37.5 mg and piperaquine phosphate (PQP) 187.5 mg dispersible tablet has been formulated with easy administration which may enhance patient adherence and compliance.
Arterolane is a synthetic trioxolane with proven efficacy and safety when combined with PQP for the treatment of P. falciparum malaria [5–7]. A phase 2 study in pediatric patients (n = 141) with uncomplicated P. falciparum malaria has demonstrated the efficacy and safety of the FDC of AM and PQP in pediatric patients with P. falciparum malaria [6].
The primary aim of this phase 3 study was to compare the efficacy, safety, and tolerability of FDC of AM 37.5 mg and PQP 187.5 mg dispersible tablets (AM-PQP) and artemether 20 mg and lumefantrine 120 mg dispersible tablets (AL) in the treatment of uncomplicated P. falciparum malaria in a larger pediatric population.
METHODS
Study Design
This study was a randomized, comparative, active-controlled, parallel-group, multicenter trial of 42 days duration including 3 days of treatment period, conducted in India, Democratic Republic of Congo (DRC), Ivory Coast, Malawi, Mali, Mozambique, and Senegal. This study was conducted from October 2014 to January 2016.
Patients
Male and female patients of 6 months to 12 years of age, with minimum body weight of 5 kg, hemoglobin >8 g/dL, absence of severe malnutrition, acute symptomatic uncomplicated P. falciparum malaria (parasite density, 1000–100000 asexual parasites/μL), fever (axillary temperature ≥ 37.5°C), or a history of fever in the past 24 hours and nonlactating and nonpregnant females were enrolled in the study.
Randomization and Blinding
Eligible patients were randomly assigned in a 2:1 ratio to either AM-PQP or AL arm. A randomization schedule was prepared for each site using Statistical Analysis System (SAS, version 9.1.3) software. Permuted block size of 6 was considered to maintain equal number of patients across centers. Treatment allocation scratch cards were provided to each site for each individual patient to prevent treatment bias.
Treatment
Each hospitalized patient received AM-PQP (37.5 + 187.5 mg) dispersible tablets, as a single daily dose once daily for 3 days, or AL (20 + 120 mg) dispersible tablets twice a day for 3 days. Each tablet was dissolved in 10 mL of drinking water.
According to the age of the patients, the number of AM-PQP tablets in a single dose was administered irrespective of meals: 6 months to <2 years, 1 tablet; 2 years to <6 years, 2 tablets; and 6–12 years, 3 tablets.
According to the body weight of the patients, the required number of AL tablets was administered followed by food or drinks rich in fat (eg, milk): 5 to <15 kg, 1 tablet; 15 to <25 kg, 2 tablets; and 25 to <35 kg, 3 tablets.
If the vomiting occurred within 30 minutes of the study drug intake, a repeat dose of study drug was given only once.
Assessments
Assessments (vital signs, physical examination signs and symptoms of P. falciparum malaria, body temperature, and parasite count) were performed at days 0 (screening and treatment), 1, 2, 3, 7, 14, 28, and 42. Postdose, body temperature (°C) was recorded at 6-hour intervals till temperature normalized and remained normal for 24 hours. The parasitological assessments were performed according to WHO guidelines [8]. Genotyping by polymerase chain reaction (PCR) analysis and quality control of the parasitology slides (if not performed by 2 independent microscopists at site) were conducted at the National Institute of Malaria Research, New Delhi. The average of the 2 parasite counts were used for efficacy assessments.
Other assessments such as laboratory tests (hematology, biochemistry, and urinalysis including urine pregnancy test for females 8–12 years of age), 12-lead electrocardiogram (at screening and between 2 and 4 hours after the third dose of AM-PQP and fifth dose of AL), and pharmacokinetic analysis were also performed.
For pharmacokinetic analysis, blood samples were withdrawn and collected from patients. The compounds (arterolane, piperaquine, artemether, dihydroartemisinin, lumefantrine) were measured in compliance with Good Laboratory Practice regulations using liquid chromatography tandem mass spectrometry methods validated as per US Food and Drug Administration Guidance for Industry: Bioanalytical Method Validation [9].
Independent Data Monitoring Committee
An Independent Data Monitoring Committee (IDMC) was constituted before initiation of the study to keep a close vigil on efficacy and safety data emerging during the trial. Four IDMC meetings were conducted during the course of the study wherein the data were discussed.
Outcomes
The primary efficacy outcome was PCR corrected adequate clinical and parasitological response (ACPR) on day 28. Secondary efficacy outcome was proportion of patients with PCR-uncorrected ACPR on day 28 and day 42, PCR-corrected ACPR on day 42, parasite clearance time (PCT), and fever clearance time (FCT).
Statistical Analysis
Approximately 840 patients (560 patients in the AM-PQP arm and 280 patients in the AL arm) were planned to be enrolled in the study considering the expected cure rate (PCR-corrected ACPR at day 28) of 95%, a noninferiority margin of 5%, power 80%, α 5%, treatment allocation ratio of 2:1, and attrition rate of 20%. This sample size was expected to provide at least 672 evaluable patients (448 patients in the AM-PQP arm and 224 patients in the AL arm).
Primary efficacy analysis was based on per-protocol (PP) population: PCR-corrected ACPR at day 28. Intention-to-treat (ITT) analysis was used as supportive evidence. In addition, life-table analysis using Kaplan-Meier graph was done. Unless otherwise specified, all statistical tests were performed using a 2-sided, 5% level of significance. Comparison between the treatments was done using Wald 95% confidence interval (CI) of difference in proportion of patients with PCR-corrected ACPR. Proportion of patients with PCR-corrected ACPR was compared between regimen groups using a logistic regression model including treatment and site as factors and baseline parasite count as covariate. Analysis of PCT was done on data for all patients in ITT population. Kaplan-Meier survival probabilities for parasite clearance at each visit was estimated along with 95% CI for both the treatments. Kaplan-Meier graphs were plotted and survival probabilities between 2 regimens were assessed either by log-rank test or Wilcoxon-Gehan test. The effect of treatment on PCT was investigated using Cox proportional hazards regression analysis, including factors for regimen and study center, as applicable. Hazard ratio between 2 regimens was calculated along with their confidence intervals. Analysis of FCT was done in a similar manner to PCT. The safety analysis was performed on the safety population. The safety assessments were performed using summary statistics. Statistical tests were performed using SAS, version 9.1.3 and Data Extraction, Analysis and Reporting tool (DEAR).
Ethics
This clinical trial was conducted as per the Good Clinical Practices, Declaration of Helsinki and applicable regulatory requirements. The protocol was approved by ethics committees and applicable regulatory authorities. Written informed consent and assent were obtained. This study is registered with Clinical Trial Registry India (CTRI), number CTRI/2014/07/004764.
RESULTS
Eight hundred fifty-nine patients were enrolled from India (101) and countries in Africa (758) (DRC, 15; Ivory Coast, 332; Malawi, 161; Mali, 114; Mozambique, 64; and Senegal, 72). Of these 859 patients, 571 patients received AM-PQP and 288 patients received AL. All patients were included in the safety and ITT analysis. A total of 546 patients in the AM-PQP arm and 271 patients in AL arm were included in PP analysis (Figure 1).
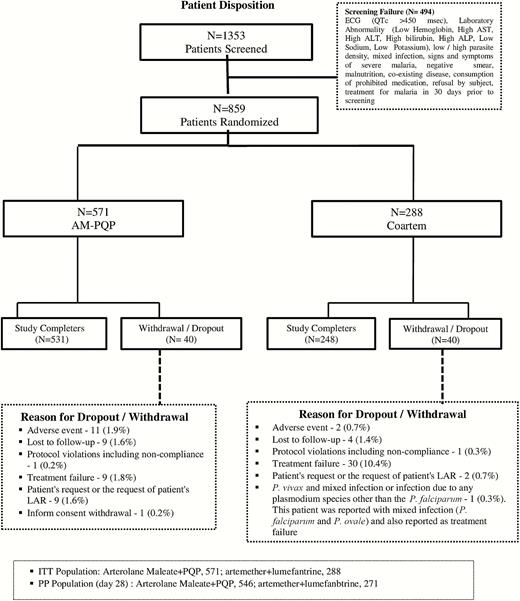
Patient disposition. Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AM-PQP, arterolane maleate–piperaquine phosphate; AST, aspartate aminotransferase; ECG, electrocardiography; ITT, intention to treat; LAR, legally acceptable representative; PP, per protocol.
The baseline characteristics are presented in Table 1.
| Variable . | Arterolane Maleate–Piperaquine Phosphate (n = 571) . | Artemether- Lumefantrine (n = 288) . |
|---|---|---|
| Sex, No. (%) | ||
| Male | 286 (50.1) | 161 (55.9) |
| Female | 285 (49.9) | 127 (44.1) |
| Age, mo | ||
| Mean | 70.49 | 86.53 |
| Range | 6.0–144 | 7–149 |
| Age 6 mo to <2 y | ||
| No. | 86 | 15 |
| Mean, mo | 16.93 | 16.73 |
| Range, mo | 6–23 | 7–22 |
| Age 2 y to <6 y | ||
| No. | 212 | 88 |
| Mean ± SD, mo | 47.54 ± 13.80 | 44.67 |
| Range, mo | 24–71 | 24–70 |
| Age 6–12 y | ||
| No. | 273 | 185 |
| Mean, mo | 105.17 | 112.10 |
| Range, mo | 72–144 | 72–149 |
| Race | ||
| Asian | 65 (11.4) | 36 (12.5) |
| African | 506 (88.6) | 252 (87.5) |
| Weight, kg | ||
| Mean | 18.5 | 20.9 |
| Range | 6.3–38.6 | 6–34.9 |
| Weight 5 kg to <15 kg | ||
| No. | 198 | 75 |
| Mean ± SD | 11.4 | 11.8 |
| Range | 6.3–14.9 | 6–14.8 |
| Weight 15 kg to <25 kg | ||
| No. | 250 | 107 |
| Mean ± SD | 18.9 | 19.3 |
| Range | 15–24.9 | 15–24.6 |
| Weight 25 kg to <35 kg | ||
| No. | 120 | 106 |
| Mean ± SD | 29.0 | 29.0 |
| Range | 25–34.9 | 25–34.9 |
| Weight ≥35 kg | ||
| No. | 3 | 0 |
| Mean ± SD | 6.8 | 0 |
| Range | 35–38.60 | 0 |
| Height, cm | ||
| Mean ± SD | 111.4 ± 21.96 | 117.5 ± 20.39 |
| Range | 60–193 | 66–160 |
| P. falciparum asexual parasites/µL at day 0 screening | ||
| Mean ± SD | 33973.0 ± 30466.05 | 33927.4 ± 30666.20 |
| Median | 24623.0 | 23920.0 |
| Temperature, °C, at day 0 screening | ||
| Mean ± SD | 38.4 ± 1.07 | 38.3 ± 1.03 |
| Median | 38.4 | 38.3 |
| History of fever at day 0 screening | ||
| Yes | 567 (99.3) | 286 (99.3) |
| No | 4 (0.7) | 2 (0.7) |
| P. falciparum gametocytes/uL at day 0 screening | ||
| Mean ± SD | 37.53 ± 520.13 | 13.72 ± 103.41 |
| Zero count of gametocytes | ||
| No. (%) | 535 (93.7) | 278 (96.5) |
| Variable . | Arterolane Maleate–Piperaquine Phosphate (n = 571) . | Artemether- Lumefantrine (n = 288) . |
|---|---|---|
| Sex, No. (%) | ||
| Male | 286 (50.1) | 161 (55.9) |
| Female | 285 (49.9) | 127 (44.1) |
| Age, mo | ||
| Mean | 70.49 | 86.53 |
| Range | 6.0–144 | 7–149 |
| Age 6 mo to <2 y | ||
| No. | 86 | 15 |
| Mean, mo | 16.93 | 16.73 |
| Range, mo | 6–23 | 7–22 |
| Age 2 y to <6 y | ||
| No. | 212 | 88 |
| Mean ± SD, mo | 47.54 ± 13.80 | 44.67 |
| Range, mo | 24–71 | 24–70 |
| Age 6–12 y | ||
| No. | 273 | 185 |
| Mean, mo | 105.17 | 112.10 |
| Range, mo | 72–144 | 72–149 |
| Race | ||
| Asian | 65 (11.4) | 36 (12.5) |
| African | 506 (88.6) | 252 (87.5) |
| Weight, kg | ||
| Mean | 18.5 | 20.9 |
| Range | 6.3–38.6 | 6–34.9 |
| Weight 5 kg to <15 kg | ||
| No. | 198 | 75 |
| Mean ± SD | 11.4 | 11.8 |
| Range | 6.3–14.9 | 6–14.8 |
| Weight 15 kg to <25 kg | ||
| No. | 250 | 107 |
| Mean ± SD | 18.9 | 19.3 |
| Range | 15–24.9 | 15–24.6 |
| Weight 25 kg to <35 kg | ||
| No. | 120 | 106 |
| Mean ± SD | 29.0 | 29.0 |
| Range | 25–34.9 | 25–34.9 |
| Weight ≥35 kg | ||
| No. | 3 | 0 |
| Mean ± SD | 6.8 | 0 |
| Range | 35–38.60 | 0 |
| Height, cm | ||
| Mean ± SD | 111.4 ± 21.96 | 117.5 ± 20.39 |
| Range | 60–193 | 66–160 |
| P. falciparum asexual parasites/µL at day 0 screening | ||
| Mean ± SD | 33973.0 ± 30466.05 | 33927.4 ± 30666.20 |
| Median | 24623.0 | 23920.0 |
| Temperature, °C, at day 0 screening | ||
| Mean ± SD | 38.4 ± 1.07 | 38.3 ± 1.03 |
| Median | 38.4 | 38.3 |
| History of fever at day 0 screening | ||
| Yes | 567 (99.3) | 286 (99.3) |
| No | 4 (0.7) | 2 (0.7) |
| P. falciparum gametocytes/uL at day 0 screening | ||
| Mean ± SD | 37.53 ± 520.13 | 13.72 ± 103.41 |
| Zero count of gametocytes | ||
| No. (%) | 535 (93.7) | 278 (96.5) |
Abbreviation: SD, standard deviation.
| Variable . | Arterolane Maleate–Piperaquine Phosphate (n = 571) . | Artemether- Lumefantrine (n = 288) . |
|---|---|---|
| Sex, No. (%) | ||
| Male | 286 (50.1) | 161 (55.9) |
| Female | 285 (49.9) | 127 (44.1) |
| Age, mo | ||
| Mean | 70.49 | 86.53 |
| Range | 6.0–144 | 7–149 |
| Age 6 mo to <2 y | ||
| No. | 86 | 15 |
| Mean, mo | 16.93 | 16.73 |
| Range, mo | 6–23 | 7–22 |
| Age 2 y to <6 y | ||
| No. | 212 | 88 |
| Mean ± SD, mo | 47.54 ± 13.80 | 44.67 |
| Range, mo | 24–71 | 24–70 |
| Age 6–12 y | ||
| No. | 273 | 185 |
| Mean, mo | 105.17 | 112.10 |
| Range, mo | 72–144 | 72–149 |
| Race | ||
| Asian | 65 (11.4) | 36 (12.5) |
| African | 506 (88.6) | 252 (87.5) |
| Weight, kg | ||
| Mean | 18.5 | 20.9 |
| Range | 6.3–38.6 | 6–34.9 |
| Weight 5 kg to <15 kg | ||
| No. | 198 | 75 |
| Mean ± SD | 11.4 | 11.8 |
| Range | 6.3–14.9 | 6–14.8 |
| Weight 15 kg to <25 kg | ||
| No. | 250 | 107 |
| Mean ± SD | 18.9 | 19.3 |
| Range | 15–24.9 | 15–24.6 |
| Weight 25 kg to <35 kg | ||
| No. | 120 | 106 |
| Mean ± SD | 29.0 | 29.0 |
| Range | 25–34.9 | 25–34.9 |
| Weight ≥35 kg | ||
| No. | 3 | 0 |
| Mean ± SD | 6.8 | 0 |
| Range | 35–38.60 | 0 |
| Height, cm | ||
| Mean ± SD | 111.4 ± 21.96 | 117.5 ± 20.39 |
| Range | 60–193 | 66–160 |
| P. falciparum asexual parasites/µL at day 0 screening | ||
| Mean ± SD | 33973.0 ± 30466.05 | 33927.4 ± 30666.20 |
| Median | 24623.0 | 23920.0 |
| Temperature, °C, at day 0 screening | ||
| Mean ± SD | 38.4 ± 1.07 | 38.3 ± 1.03 |
| Median | 38.4 | 38.3 |
| History of fever at day 0 screening | ||
| Yes | 567 (99.3) | 286 (99.3) |
| No | 4 (0.7) | 2 (0.7) |
| P. falciparum gametocytes/uL at day 0 screening | ||
| Mean ± SD | 37.53 ± 520.13 | 13.72 ± 103.41 |
| Zero count of gametocytes | ||
| No. (%) | 535 (93.7) | 278 (96.5) |
| Variable . | Arterolane Maleate–Piperaquine Phosphate (n = 571) . | Artemether- Lumefantrine (n = 288) . |
|---|---|---|
| Sex, No. (%) | ||
| Male | 286 (50.1) | 161 (55.9) |
| Female | 285 (49.9) | 127 (44.1) |
| Age, mo | ||
| Mean | 70.49 | 86.53 |
| Range | 6.0–144 | 7–149 |
| Age 6 mo to <2 y | ||
| No. | 86 | 15 |
| Mean, mo | 16.93 | 16.73 |
| Range, mo | 6–23 | 7–22 |
| Age 2 y to <6 y | ||
| No. | 212 | 88 |
| Mean ± SD, mo | 47.54 ± 13.80 | 44.67 |
| Range, mo | 24–71 | 24–70 |
| Age 6–12 y | ||
| No. | 273 | 185 |
| Mean, mo | 105.17 | 112.10 |
| Range, mo | 72–144 | 72–149 |
| Race | ||
| Asian | 65 (11.4) | 36 (12.5) |
| African | 506 (88.6) | 252 (87.5) |
| Weight, kg | ||
| Mean | 18.5 | 20.9 |
| Range | 6.3–38.6 | 6–34.9 |
| Weight 5 kg to <15 kg | ||
| No. | 198 | 75 |
| Mean ± SD | 11.4 | 11.8 |
| Range | 6.3–14.9 | 6–14.8 |
| Weight 15 kg to <25 kg | ||
| No. | 250 | 107 |
| Mean ± SD | 18.9 | 19.3 |
| Range | 15–24.9 | 15–24.6 |
| Weight 25 kg to <35 kg | ||
| No. | 120 | 106 |
| Mean ± SD | 29.0 | 29.0 |
| Range | 25–34.9 | 25–34.9 |
| Weight ≥35 kg | ||
| No. | 3 | 0 |
| Mean ± SD | 6.8 | 0 |
| Range | 35–38.60 | 0 |
| Height, cm | ||
| Mean ± SD | 111.4 ± 21.96 | 117.5 ± 20.39 |
| Range | 60–193 | 66–160 |
| P. falciparum asexual parasites/µL at day 0 screening | ||
| Mean ± SD | 33973.0 ± 30466.05 | 33927.4 ± 30666.20 |
| Median | 24623.0 | 23920.0 |
| Temperature, °C, at day 0 screening | ||
| Mean ± SD | 38.4 ± 1.07 | 38.3 ± 1.03 |
| Median | 38.4 | 38.3 |
| History of fever at day 0 screening | ||
| Yes | 567 (99.3) | 286 (99.3) |
| No | 4 (0.7) | 2 (0.7) |
| P. falciparum gametocytes/uL at day 0 screening | ||
| Mean ± SD | 37.53 ± 520.13 | 13.72 ± 103.41 |
| Zero count of gametocytes | ||
| No. (%) | 535 (93.7) | 278 (96.5) |
Abbreviation: SD, standard deviation.
Efficacy
The primary outcome was cure rate (PCR-corrected ACPR) at day 28. In AM-PQP and AL arms, the cure rates were 100% and 98.5%, respectively, in the PP population (treatment difference, 1.48% [95% CI, .04%–2.91%]; Table 2) and 96.0% and 95.8% in the ITT population (treatment difference, 0.14% [95% CI, –2.68% to 2.95%]; Table 3).
Adequate Clinical and Parasitological Response by Time-point (Per-Protocol Population)
| Response . | Arterolane Maleate–PQP . | Artemether- Lumefantrine . | Difference (Wald 95% CI) . |
|---|---|---|---|
| Day 28 (n = 827) | 548 | 279 | |
| PCR-uncorrected ACPR | 546 (99.6) | 267 (95.7) | 3.94 (1.5–6.37) |
| Total failures | 2 (0.4) | 12 (4.3) | |
| Late clinical failure | 1 (0.2) | 5 (1.8) | |
| Late parasitological failure | 1 (0.2) | 7 (2.5) | |
| Day 28 (n = 817) | 546 | 271 | |
| PCR-corrected ACPR | 546 (100.0) | 267 (98.5) | 1.48 (.04–2.91) |
| Total failures | 0 | 4 (1.5) | |
| Recrudescence | 0 | 4 (1.5) | |
| Day 42 (n = 805) | 538 | 267 | |
| PCR-uncorrected ACPR | 531 (98.7) | 248 (92.9) | 5.81 (2.59–9.04) |
| Total failures | 7 (1.3) | 19 (7.1) | |
| Late clinical failure | 2 (0.4) | 10 (3.7) | |
| Late parasitological failure | 5 (0.9) | 8 (3.0) | |
| P. vivax and mixed infection or infection due to any Plasmodium species other than P. falciparum | 0 | 1 (0.4) | |
| Day 42 (n = 783) | 532 | 251 | 1.01 (–.39 to 2.4) |
| PCR-corrected ACPR | 531 (99.8) | 248 (98.8) | |
| Total failures | 1 (0.2) | 3 (1.2) | |
| Recrudescence | 1 (0.2) | 3 (1.2) |
| Response . | Arterolane Maleate–PQP . | Artemether- Lumefantrine . | Difference (Wald 95% CI) . |
|---|---|---|---|
| Day 28 (n = 827) | 548 | 279 | |
| PCR-uncorrected ACPR | 546 (99.6) | 267 (95.7) | 3.94 (1.5–6.37) |
| Total failures | 2 (0.4) | 12 (4.3) | |
| Late clinical failure | 1 (0.2) | 5 (1.8) | |
| Late parasitological failure | 1 (0.2) | 7 (2.5) | |
| Day 28 (n = 817) | 546 | 271 | |
| PCR-corrected ACPR | 546 (100.0) | 267 (98.5) | 1.48 (.04–2.91) |
| Total failures | 0 | 4 (1.5) | |
| Recrudescence | 0 | 4 (1.5) | |
| Day 42 (n = 805) | 538 | 267 | |
| PCR-uncorrected ACPR | 531 (98.7) | 248 (92.9) | 5.81 (2.59–9.04) |
| Total failures | 7 (1.3) | 19 (7.1) | |
| Late clinical failure | 2 (0.4) | 10 (3.7) | |
| Late parasitological failure | 5 (0.9) | 8 (3.0) | |
| P. vivax and mixed infection or infection due to any Plasmodium species other than P. falciparum | 0 | 1 (0.4) | |
| Day 42 (n = 783) | 532 | 251 | 1.01 (–.39 to 2.4) |
| PCR-corrected ACPR | 531 (99.8) | 248 (98.8) | |
| Total failures | 1 (0.2) | 3 (1.2) | |
| Recrudescence | 1 (0.2) | 3 (1.2) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ACPR, adequate clinical and parasitological response; CI, confidence interval; PCR, polymerase chain reaction; PQP, piperaquine phosphate.
Adequate Clinical and Parasitological Response by Time-point (Per-Protocol Population)
| Response . | Arterolane Maleate–PQP . | Artemether- Lumefantrine . | Difference (Wald 95% CI) . |
|---|---|---|---|
| Day 28 (n = 827) | 548 | 279 | |
| PCR-uncorrected ACPR | 546 (99.6) | 267 (95.7) | 3.94 (1.5–6.37) |
| Total failures | 2 (0.4) | 12 (4.3) | |
| Late clinical failure | 1 (0.2) | 5 (1.8) | |
| Late parasitological failure | 1 (0.2) | 7 (2.5) | |
| Day 28 (n = 817) | 546 | 271 | |
| PCR-corrected ACPR | 546 (100.0) | 267 (98.5) | 1.48 (.04–2.91) |
| Total failures | 0 | 4 (1.5) | |
| Recrudescence | 0 | 4 (1.5) | |
| Day 42 (n = 805) | 538 | 267 | |
| PCR-uncorrected ACPR | 531 (98.7) | 248 (92.9) | 5.81 (2.59–9.04) |
| Total failures | 7 (1.3) | 19 (7.1) | |
| Late clinical failure | 2 (0.4) | 10 (3.7) | |
| Late parasitological failure | 5 (0.9) | 8 (3.0) | |
| P. vivax and mixed infection or infection due to any Plasmodium species other than P. falciparum | 0 | 1 (0.4) | |
| Day 42 (n = 783) | 532 | 251 | 1.01 (–.39 to 2.4) |
| PCR-corrected ACPR | 531 (99.8) | 248 (98.8) | |
| Total failures | 1 (0.2) | 3 (1.2) | |
| Recrudescence | 1 (0.2) | 3 (1.2) |
| Response . | Arterolane Maleate–PQP . | Artemether- Lumefantrine . | Difference (Wald 95% CI) . |
|---|---|---|---|
| Day 28 (n = 827) | 548 | 279 | |
| PCR-uncorrected ACPR | 546 (99.6) | 267 (95.7) | 3.94 (1.5–6.37) |
| Total failures | 2 (0.4) | 12 (4.3) | |
| Late clinical failure | 1 (0.2) | 5 (1.8) | |
| Late parasitological failure | 1 (0.2) | 7 (2.5) | |
| Day 28 (n = 817) | 546 | 271 | |
| PCR-corrected ACPR | 546 (100.0) | 267 (98.5) | 1.48 (.04–2.91) |
| Total failures | 0 | 4 (1.5) | |
| Recrudescence | 0 | 4 (1.5) | |
| Day 42 (n = 805) | 538 | 267 | |
| PCR-uncorrected ACPR | 531 (98.7) | 248 (92.9) | 5.81 (2.59–9.04) |
| Total failures | 7 (1.3) | 19 (7.1) | |
| Late clinical failure | 2 (0.4) | 10 (3.7) | |
| Late parasitological failure | 5 (0.9) | 8 (3.0) | |
| P. vivax and mixed infection or infection due to any Plasmodium species other than P. falciparum | 0 | 1 (0.4) | |
| Day 42 (n = 783) | 532 | 251 | 1.01 (–.39 to 2.4) |
| PCR-corrected ACPR | 531 (99.8) | 248 (98.8) | |
| Total failures | 1 (0.2) | 3 (1.2) | |
| Recrudescence | 1 (0.2) | 3 (1.2) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ACPR, adequate clinical and parasitological response; CI, confidence interval; PCR, polymerase chain reaction; PQP, piperaquine phosphate.
Adequate Clinical and Parasitological Response by Time-point (Intention-to-Treat Population)
| Response . | Arterolane Maleate–PQP . | Artemether-Lumefantrine . | Difference (Wald 95% CI) . |
|---|---|---|---|
| Day 28 (n = 859) | 571 | 288 | |
| PCR-uncorrected ACPR | 546 (95.6) | 269 (93.4) | 2.22 (–1.1 to 5.54) |
| Total failures | 25 (4.4) | 19 (6.6) | |
| Late parasitological failure | 1 (0.2) | 7 (2.4) | |
| Late clinical failure | 1 (0.2) | 5 (1.7) | |
| Adverse event/severe malaria/SAE | 11 (1.9) | 2 (0.7) | |
| Protocol violation including noncompliance | 1 (0.2) | 1 (0.3) | |
| Patient`s request or the request of patient`s LAR for withdrawal | 8 (1.4) | 2 (0.7) | |
| Inform consent withdrawal | 1 (0.2) | 0 | |
| Lost to follow-up | 2 (0.4) | 2 (0.7) | |
| Day 28 (n = 859) | 571 | 288 | |
| PCR-corrected ACPR | 548 (96.0) | 276 (95.8) | 0.14 (–2.68 to 2.95) |
| Total failures | 23 (4.0) | 12 (4.2) | |
| Recrudescence | 0 | 4 (1.4) | |
| Sample not amplified | 0 | 1 (0.3) | |
| Adverse event/severe malaria/SAE | 11 (1.9) | 2 (0.7) | |
| Protocol violation including non-compliance | 1 (0.2) | 1 (0.3) | |
| Patient`s request or the request of LAR for withdrawal | 8 (1.4) | 2 (0.7) | |
| Inform consent withdrawal | 1 (0.2) | 0 | |
| Lost to follow-up | 2 (0.4) | 2 (0.7) | |
| Day 42 (n = 859) | 571 | 288 | |
| PCR-uncorrected ACPR | 531 (93.0) | 248 (86.1) | 6.88 (2.37–11.39) |
| Total failures | 40 (7.0) | 40 (13.9) | |
| Late clinical failure | 3 (0.5) | 16 (5.6) | |
| Late parasitological failure | 6 (1.1) | 14 (4.9) | |
| Adverse event/severe malaria/SAE | 11 (1.9) | 2 (0.7) | |
| Protocol violation including noncompliance | 1 (0.2) | 1 (0.3) | |
| P. vivax and mixed infection or infection due to any plasmodium species other than the P. falciparum | 0 | 1 (0.3) | |
| Patient`s request or the request of patient`s LAR for withdrawal | 9 (1.6) | 2 (0.7) | |
| Lost to follow-up | 9 (1.6) | 4 (1.4) | |
| Other | 1 (0.2) | 0 | |
| Day 42 (n = 859) | 571 | 288 | |
| PCR-corrected ACPR | 539 (94.4) | 268 (93.1) | 1.34 (–2.15 to 4.83) |
| Total failures | 32 (5.6) | 20 (6.9) | |
| Sample not amplified | 0 | 4 (1.4) | |
| Recrudescence | 1 (0.2) | 7 (2.4) | |
| Adverse event/severe malaria/SAE | 11 (1.9) | 2 (0.7) | |
| Protocol violation including noncompliance | 1 (0.2) | 1 (0.3) | |
| Patient`s request or the request of patient’s LAR | 9 (1.6) | 2 (0.7) | |
| Lost to follow-up | 9 (1.6) | 4 (1.4) | |
| Other | 1 (0.2) | 0 |
| Response . | Arterolane Maleate–PQP . | Artemether-Lumefantrine . | Difference (Wald 95% CI) . |
|---|---|---|---|
| Day 28 (n = 859) | 571 | 288 | |
| PCR-uncorrected ACPR | 546 (95.6) | 269 (93.4) | 2.22 (–1.1 to 5.54) |
| Total failures | 25 (4.4) | 19 (6.6) | |
| Late parasitological failure | 1 (0.2) | 7 (2.4) | |
| Late clinical failure | 1 (0.2) | 5 (1.7) | |
| Adverse event/severe malaria/SAE | 11 (1.9) | 2 (0.7) | |
| Protocol violation including noncompliance | 1 (0.2) | 1 (0.3) | |
| Patient`s request or the request of patient`s LAR for withdrawal | 8 (1.4) | 2 (0.7) | |
| Inform consent withdrawal | 1 (0.2) | 0 | |
| Lost to follow-up | 2 (0.4) | 2 (0.7) | |
| Day 28 (n = 859) | 571 | 288 | |
| PCR-corrected ACPR | 548 (96.0) | 276 (95.8) | 0.14 (–2.68 to 2.95) |
| Total failures | 23 (4.0) | 12 (4.2) | |
| Recrudescence | 0 | 4 (1.4) | |
| Sample not amplified | 0 | 1 (0.3) | |
| Adverse event/severe malaria/SAE | 11 (1.9) | 2 (0.7) | |
| Protocol violation including non-compliance | 1 (0.2) | 1 (0.3) | |
| Patient`s request or the request of LAR for withdrawal | 8 (1.4) | 2 (0.7) | |
| Inform consent withdrawal | 1 (0.2) | 0 | |
| Lost to follow-up | 2 (0.4) | 2 (0.7) | |
| Day 42 (n = 859) | 571 | 288 | |
| PCR-uncorrected ACPR | 531 (93.0) | 248 (86.1) | 6.88 (2.37–11.39) |
| Total failures | 40 (7.0) | 40 (13.9) | |
| Late clinical failure | 3 (0.5) | 16 (5.6) | |
| Late parasitological failure | 6 (1.1) | 14 (4.9) | |
| Adverse event/severe malaria/SAE | 11 (1.9) | 2 (0.7) | |
| Protocol violation including noncompliance | 1 (0.2) | 1 (0.3) | |
| P. vivax and mixed infection or infection due to any plasmodium species other than the P. falciparum | 0 | 1 (0.3) | |
| Patient`s request or the request of patient`s LAR for withdrawal | 9 (1.6) | 2 (0.7) | |
| Lost to follow-up | 9 (1.6) | 4 (1.4) | |
| Other | 1 (0.2) | 0 | |
| Day 42 (n = 859) | 571 | 288 | |
| PCR-corrected ACPR | 539 (94.4) | 268 (93.1) | 1.34 (–2.15 to 4.83) |
| Total failures | 32 (5.6) | 20 (6.9) | |
| Sample not amplified | 0 | 4 (1.4) | |
| Recrudescence | 1 (0.2) | 7 (2.4) | |
| Adverse event/severe malaria/SAE | 11 (1.9) | 2 (0.7) | |
| Protocol violation including noncompliance | 1 (0.2) | 1 (0.3) | |
| Patient`s request or the request of patient’s LAR | 9 (1.6) | 2 (0.7) | |
| Lost to follow-up | 9 (1.6) | 4 (1.4) | |
| Other | 1 (0.2) | 0 |
Data are presented as No. (%) unless otherwise indicated. Patients with missing data were regarded as having treatment failure.
Abbreviations: ACPR, adequate clinical and parasitological response; CI, confidence interval; LAR, legally acceptable representative;
PCR, polymerase chain reaction; PQP, piperaquine phosphate; SAE, serious adverse event.
Adequate Clinical and Parasitological Response by Time-point (Intention-to-Treat Population)
| Response . | Arterolane Maleate–PQP . | Artemether-Lumefantrine . | Difference (Wald 95% CI) . |
|---|---|---|---|
| Day 28 (n = 859) | 571 | 288 | |
| PCR-uncorrected ACPR | 546 (95.6) | 269 (93.4) | 2.22 (–1.1 to 5.54) |
| Total failures | 25 (4.4) | 19 (6.6) | |
| Late parasitological failure | 1 (0.2) | 7 (2.4) | |
| Late clinical failure | 1 (0.2) | 5 (1.7) | |
| Adverse event/severe malaria/SAE | 11 (1.9) | 2 (0.7) | |
| Protocol violation including noncompliance | 1 (0.2) | 1 (0.3) | |
| Patient`s request or the request of patient`s LAR for withdrawal | 8 (1.4) | 2 (0.7) | |
| Inform consent withdrawal | 1 (0.2) | 0 | |
| Lost to follow-up | 2 (0.4) | 2 (0.7) | |
| Day 28 (n = 859) | 571 | 288 | |
| PCR-corrected ACPR | 548 (96.0) | 276 (95.8) | 0.14 (–2.68 to 2.95) |
| Total failures | 23 (4.0) | 12 (4.2) | |
| Recrudescence | 0 | 4 (1.4) | |
| Sample not amplified | 0 | 1 (0.3) | |
| Adverse event/severe malaria/SAE | 11 (1.9) | 2 (0.7) | |
| Protocol violation including non-compliance | 1 (0.2) | 1 (0.3) | |
| Patient`s request or the request of LAR for withdrawal | 8 (1.4) | 2 (0.7) | |
| Inform consent withdrawal | 1 (0.2) | 0 | |
| Lost to follow-up | 2 (0.4) | 2 (0.7) | |
| Day 42 (n = 859) | 571 | 288 | |
| PCR-uncorrected ACPR | 531 (93.0) | 248 (86.1) | 6.88 (2.37–11.39) |
| Total failures | 40 (7.0) | 40 (13.9) | |
| Late clinical failure | 3 (0.5) | 16 (5.6) | |
| Late parasitological failure | 6 (1.1) | 14 (4.9) | |
| Adverse event/severe malaria/SAE | 11 (1.9) | 2 (0.7) | |
| Protocol violation including noncompliance | 1 (0.2) | 1 (0.3) | |
| P. vivax and mixed infection or infection due to any plasmodium species other than the P. falciparum | 0 | 1 (0.3) | |
| Patient`s request or the request of patient`s LAR for withdrawal | 9 (1.6) | 2 (0.7) | |
| Lost to follow-up | 9 (1.6) | 4 (1.4) | |
| Other | 1 (0.2) | 0 | |
| Day 42 (n = 859) | 571 | 288 | |
| PCR-corrected ACPR | 539 (94.4) | 268 (93.1) | 1.34 (–2.15 to 4.83) |
| Total failures | 32 (5.6) | 20 (6.9) | |
| Sample not amplified | 0 | 4 (1.4) | |
| Recrudescence | 1 (0.2) | 7 (2.4) | |
| Adverse event/severe malaria/SAE | 11 (1.9) | 2 (0.7) | |
| Protocol violation including noncompliance | 1 (0.2) | 1 (0.3) | |
| Patient`s request or the request of patient’s LAR | 9 (1.6) | 2 (0.7) | |
| Lost to follow-up | 9 (1.6) | 4 (1.4) | |
| Other | 1 (0.2) | 0 |
| Response . | Arterolane Maleate–PQP . | Artemether-Lumefantrine . | Difference (Wald 95% CI) . |
|---|---|---|---|
| Day 28 (n = 859) | 571 | 288 | |
| PCR-uncorrected ACPR | 546 (95.6) | 269 (93.4) | 2.22 (–1.1 to 5.54) |
| Total failures | 25 (4.4) | 19 (6.6) | |
| Late parasitological failure | 1 (0.2) | 7 (2.4) | |
| Late clinical failure | 1 (0.2) | 5 (1.7) | |
| Adverse event/severe malaria/SAE | 11 (1.9) | 2 (0.7) | |
| Protocol violation including noncompliance | 1 (0.2) | 1 (0.3) | |
| Patient`s request or the request of patient`s LAR for withdrawal | 8 (1.4) | 2 (0.7) | |
| Inform consent withdrawal | 1 (0.2) | 0 | |
| Lost to follow-up | 2 (0.4) | 2 (0.7) | |
| Day 28 (n = 859) | 571 | 288 | |
| PCR-corrected ACPR | 548 (96.0) | 276 (95.8) | 0.14 (–2.68 to 2.95) |
| Total failures | 23 (4.0) | 12 (4.2) | |
| Recrudescence | 0 | 4 (1.4) | |
| Sample not amplified | 0 | 1 (0.3) | |
| Adverse event/severe malaria/SAE | 11 (1.9) | 2 (0.7) | |
| Protocol violation including non-compliance | 1 (0.2) | 1 (0.3) | |
| Patient`s request or the request of LAR for withdrawal | 8 (1.4) | 2 (0.7) | |
| Inform consent withdrawal | 1 (0.2) | 0 | |
| Lost to follow-up | 2 (0.4) | 2 (0.7) | |
| Day 42 (n = 859) | 571 | 288 | |
| PCR-uncorrected ACPR | 531 (93.0) | 248 (86.1) | 6.88 (2.37–11.39) |
| Total failures | 40 (7.0) | 40 (13.9) | |
| Late clinical failure | 3 (0.5) | 16 (5.6) | |
| Late parasitological failure | 6 (1.1) | 14 (4.9) | |
| Adverse event/severe malaria/SAE | 11 (1.9) | 2 (0.7) | |
| Protocol violation including noncompliance | 1 (0.2) | 1 (0.3) | |
| P. vivax and mixed infection or infection due to any plasmodium species other than the P. falciparum | 0 | 1 (0.3) | |
| Patient`s request or the request of patient`s LAR for withdrawal | 9 (1.6) | 2 (0.7) | |
| Lost to follow-up | 9 (1.6) | 4 (1.4) | |
| Other | 1 (0.2) | 0 | |
| Day 42 (n = 859) | 571 | 288 | |
| PCR-corrected ACPR | 539 (94.4) | 268 (93.1) | 1.34 (–2.15 to 4.83) |
| Total failures | 32 (5.6) | 20 (6.9) | |
| Sample not amplified | 0 | 4 (1.4) | |
| Recrudescence | 1 (0.2) | 7 (2.4) | |
| Adverse event/severe malaria/SAE | 11 (1.9) | 2 (0.7) | |
| Protocol violation including noncompliance | 1 (0.2) | 1 (0.3) | |
| Patient`s request or the request of patient’s LAR | 9 (1.6) | 2 (0.7) | |
| Lost to follow-up | 9 (1.6) | 4 (1.4) | |
| Other | 1 (0.2) | 0 |
Data are presented as No. (%) unless otherwise indicated. Patients with missing data were regarded as having treatment failure.
Abbreviations: ACPR, adequate clinical and parasitological response; CI, confidence interval; LAR, legally acceptable representative;
PCR, polymerase chain reaction; PQP, piperaquine phosphate; SAE, serious adverse event.
No recrudescence was reported in the AM-PQP arm compared with 4 patients (1.5%) in the AL arm who developed recrudescence at or before day 28.
In the ITT population, cure rate at day 42 was 94.4% vs 93.1% (1.34 [95% CI, –2.15 to 4.83]) in AM-PQP and AL arms, respectively. The survival probability at day 28 and day 42 was found to be higher in the AM-PQP arm as compared to AL arm in the ITT population (log-rank P = .0018, day 28, Figure 2; and log-rank P = .0001, day 42, Figure 3).
![Survival analysis at day 28 (polymerase chain reaction [PCR] corrected) in the intention-to-treat population. Drug A: arterolane maleate–piperaquine phosphate; drug B: artemether-lumefantrine.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/65/10/10.1093_cid_cix617/1/m_cix61702.jpeg?Expires=1749864658&Signature=PRZBRWaOxU9phLzkxgASBto~sNxzEMvx8zW0SKpZ61BYvUqG6chDSHkp2Z2xbMmoXEz1nOO-v2apxzqFjwtZW6PScxx0h-bLjEfC2w4S3SiSa9blShxxNDnpV9qSwyQJWLYaSs47X084D-ijT3Hc5vZoSxjEtj7DEo9ttsOwcuZpPUTISjtGRB5YWxbkdDsDTPkqHp9azEA-7Yh-FuE0trxdtFJYogZYKtFDtQBWulKCpxarQbMuxq4Q8~WMvAun0geMSBYRA3gSqUcSg-85hpSBG~sOsiAznAQVGju~o0r0Vze4DHRMhp6-xxPErOzH9MCv~QFovh2R3lsvIBCm4g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Survival analysis at day 28 (polymerase chain reaction [PCR] corrected) in the intention-to-treat population. Drug A: arterolane maleate–piperaquine phosphate; drug B: artemether-lumefantrine.
![Survival analysis at day 28 (polymerase chain reaction [PCR] uncorrected) in the intention-to-treat population. Drug A: arterolane maleate–piperaquine phosphate; drug B: artemether-lumefantrine.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/65/10/10.1093_cid_cix617/1/m_cix61703.jpeg?Expires=1749864658&Signature=AiykaM71pM5vyju~Jr1oRYAdRqFVTr2EpPJ~QAReUGOK7fHLfFuTJdpAuxkzSrq3eN7xVLZWgu9o5orc5L7PgdPMXITAeP9U1TnmBfsbs1~KqJYTYWlOeUs~InXAHjNhsnqzSVwtqaYgUNYlvKVm6eYX-TcZ2ze6MQ8fr4ahJG5cOlGlAITuKcQNaWsi0DM7fVoea4pkqTlj2FikHLfZBH4dQoCxIBLe2Pk3UjBAHmCxtCK-x6POUrMqnYK6SPEG3RoMburtxUy-LvtFcPtglf5fbe424qTXQFfosrTHwrcRLW~77RHbvmTLr-VeLpozgfG50lCMeZheEll-1kq4pw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Survival analysis at day 28 (polymerase chain reaction [PCR] uncorrected) in the intention-to-treat population. Drug A: arterolane maleate–piperaquine phosphate; drug B: artemether-lumefantrine.
There was no difference in the median PCT (24 hours) in the 2 treatment arms (Supplementary Table 1). Median FCT was 6 hours in the AM–PQP arm and 12 hours in the AL arm. The gametocyte clearance was also comparable in both the treatment arm (Table 4).
Proportion of Patients Having Zero Gametocyte Counts (Intention-to-Treat Population)
| Time Point . | Arterolane Maleate–Piperaquine Phosphate . | Artemether-Lumefantrine . | ||
|---|---|---|---|---|
| No. . | No. (%) of Patients With Zero Gametocytes . | No. . | No. (%) of Patients With Zero Gametocytes . | |
| Baseline | 571 | 538 (94.2) | 288 | 274 (95.1) |
| Day 7 | 539 | 512 (95.0) | 279 | 276 (98.9) |
| Day 14 | 540 | 527 (97.6) | 272 | 271 (99.6) |
| Day 28 | 544 | 543 (99.8) | 269 | 267 (99.3) |
| Day 42 | 534 | 534 (100.0) | 255 | 255 (100.0) |
| Time Point . | Arterolane Maleate–Piperaquine Phosphate . | Artemether-Lumefantrine . | ||
|---|---|---|---|---|
| No. . | No. (%) of Patients With Zero Gametocytes . | No. . | No. (%) of Patients With Zero Gametocytes . | |
| Baseline | 571 | 538 (94.2) | 288 | 274 (95.1) |
| Day 7 | 539 | 512 (95.0) | 279 | 276 (98.9) |
| Day 14 | 540 | 527 (97.6) | 272 | 271 (99.6) |
| Day 28 | 544 | 543 (99.8) | 269 | 267 (99.3) |
| Day 42 | 534 | 534 (100.0) | 255 | 255 (100.0) |
Proportion of Patients Having Zero Gametocyte Counts (Intention-to-Treat Population)
| Time Point . | Arterolane Maleate–Piperaquine Phosphate . | Artemether-Lumefantrine . | ||
|---|---|---|---|---|
| No. . | No. (%) of Patients With Zero Gametocytes . | No. . | No. (%) of Patients With Zero Gametocytes . | |
| Baseline | 571 | 538 (94.2) | 288 | 274 (95.1) |
| Day 7 | 539 | 512 (95.0) | 279 | 276 (98.9) |
| Day 14 | 540 | 527 (97.6) | 272 | 271 (99.6) |
| Day 28 | 544 | 543 (99.8) | 269 | 267 (99.3) |
| Day 42 | 534 | 534 (100.0) | 255 | 255 (100.0) |
| Time Point . | Arterolane Maleate–Piperaquine Phosphate . | Artemether-Lumefantrine . | ||
|---|---|---|---|---|
| No. . | No. (%) of Patients With Zero Gametocytes . | No. . | No. (%) of Patients With Zero Gametocytes . | |
| Baseline | 571 | 538 (94.2) | 288 | 274 (95.1) |
| Day 7 | 539 | 512 (95.0) | 279 | 276 (98.9) |
| Day 14 | 540 | 527 (97.6) | 272 | 271 (99.6) |
| Day 28 | 544 | 543 (99.8) | 269 | 267 (99.3) |
| Day 42 | 534 | 534 (100.0) | 255 | 255 (100.0) |
Safety
The overall incidence of treatment-emergent adverse events (AEs) was comparable in the 2 treatment arms (AM-PQP, 93.5% and AL, 92.7%). The most commonly observed adverse events were anemia, vomiting, and cough (Table 5). One serious adverse event (sepsis requiring prolongation of hospitalization) was reported in a patient in AM-PQP arm, which was not related to study treatment and resolved without sequelae. No death was reported. Most of the AEs were “mild” in intensity and “resolved without sequelae” in both the treatment arms. The relationship of majority of the AEs was judged as “possibly related” (approximately 50.0%) to the study medication. The number of patients withdrawn from the study due to vomiting was 1.8% vs 0.7% in the AM-PQP and AL arms, respectively.
| System Organ Class Preferred Term . | Arterolane Maleate– Piperaquine Phosphate (n = 571) . | Artemether- Lumefantrine (n = 288) . |
|---|---|---|
| Patients with at least 1 adverse event | 534 (93.5) | 267 (92.7) |
| Clinical adverse events | ||
| Anemia | 279 (48.9) | 145 (50.3) |
| Ear pain | 0 | 1 (0.3) |
| Blepharitis | 1 (0.2) | 0 |
| Conjunctival hyperemia | 1 (0.2) | 0 |
| Abdominal distension | 6 (1.1) | 1 (0.3) |
| Abdominal pain | 5 (0.9) | 1 (0.3) |
| Constipation | 2 (0.4) | 0 |
| Diarrhea | 12 (2.1) | 4 (1.4) |
| Dyspepsia | 1 (0.2) | 1 (0.3) |
| Flatulence | 1 (0.2) | 0 |
| Mouth ulceration | 1 (0.2) | 0 |
| Nausea | 9 (1.6) | 6 (2.1) |
| Vomiting | 96 (16.8) | 20 (6.9) |
| Chills | 6 (1.1) | 6 (2.1) |
| Fatigue | 6 (1.1) | 9 (3.1) |
| Malaise | 5 (0.9) | 5 (1.7) |
| Pyrexia | 27 (4.7) | 28 (9.7) |
| Abscess | 1 (0.2) | 1 (0.3) |
| Body tinea | 1 (0.2) | 0 |
| Bronchitis | 1 (0.2) | 2 (0.7) |
| Bronchopneumonia | 0 | 1 (0.3) |
| Candida infection | 1 (0.2) | 0 |
| Ear infection | 1 (0.2) | 1 (0.3) |
| Gastroenteritis | 2 (0.4) | 2 (0.7) |
| Lice infestation | 1 (0.2) | 1 (0.3) |
| Mumps | 0 | 1 (0.3) |
| Pharyngitis | 4 (0.7) | 1 (0.3) |
| Rhinitis | 1 (0.2) | 4 (1.4) |
| Sepsis | 1 (0.2) | 0 |
| Tinea capitis | 2 (0.4) | 0 |
| Tonsillitis | 5 (0.9) | 2 (0.7) |
| Upper respiratory tract infection | 6 (1.1) | 2 (0.7) |
| Urinary tract infection | 1 (0.2) | 0 |
| Limb injury | 1 (0.2) | 0 |
| Decreased appetite | 11 (1.9) | 11 (3.8) |
| Headache | 15 (2.6) | 18 (6.3) |
| Cough | 63 (11.0) | 21 (7.3) |
| Nasal congestion | 1 (0.2) | 0 |
| Pneumonitis | 1 (0.2) | 0 |
| Rhinorrhea | 12 (2.1) | 7 (2.4) |
| Snoring | 2 (0.4) | 0 |
| Tachypnea | 1 (0.2) | 0 |
| Wheezing | 1 (0.2) | 0 |
| Dermatitis | 1 (0.2) | 0 |
| Hyperhidrosis | 4 (0.7) | 6 (2.1) |
| Rash | 1 (0.2) | 0 |
| Skin hypopigmentation | 1 (0.2) | 0 |
| Pallor | 2 (0.4) | 0 |
| System Organ Class Preferred Term . | Arterolane Maleate– Piperaquine Phosphate (n = 571) . | Artemether- Lumefantrine (n = 288) . |
|---|---|---|
| Patients with at least 1 adverse event | 534 (93.5) | 267 (92.7) |
| Clinical adverse events | ||
| Anemia | 279 (48.9) | 145 (50.3) |
| Ear pain | 0 | 1 (0.3) |
| Blepharitis | 1 (0.2) | 0 |
| Conjunctival hyperemia | 1 (0.2) | 0 |
| Abdominal distension | 6 (1.1) | 1 (0.3) |
| Abdominal pain | 5 (0.9) | 1 (0.3) |
| Constipation | 2 (0.4) | 0 |
| Diarrhea | 12 (2.1) | 4 (1.4) |
| Dyspepsia | 1 (0.2) | 1 (0.3) |
| Flatulence | 1 (0.2) | 0 |
| Mouth ulceration | 1 (0.2) | 0 |
| Nausea | 9 (1.6) | 6 (2.1) |
| Vomiting | 96 (16.8) | 20 (6.9) |
| Chills | 6 (1.1) | 6 (2.1) |
| Fatigue | 6 (1.1) | 9 (3.1) |
| Malaise | 5 (0.9) | 5 (1.7) |
| Pyrexia | 27 (4.7) | 28 (9.7) |
| Abscess | 1 (0.2) | 1 (0.3) |
| Body tinea | 1 (0.2) | 0 |
| Bronchitis | 1 (0.2) | 2 (0.7) |
| Bronchopneumonia | 0 | 1 (0.3) |
| Candida infection | 1 (0.2) | 0 |
| Ear infection | 1 (0.2) | 1 (0.3) |
| Gastroenteritis | 2 (0.4) | 2 (0.7) |
| Lice infestation | 1 (0.2) | 1 (0.3) |
| Mumps | 0 | 1 (0.3) |
| Pharyngitis | 4 (0.7) | 1 (0.3) |
| Rhinitis | 1 (0.2) | 4 (1.4) |
| Sepsis | 1 (0.2) | 0 |
| Tinea capitis | 2 (0.4) | 0 |
| Tonsillitis | 5 (0.9) | 2 (0.7) |
| Upper respiratory tract infection | 6 (1.1) | 2 (0.7) |
| Urinary tract infection | 1 (0.2) | 0 |
| Limb injury | 1 (0.2) | 0 |
| Decreased appetite | 11 (1.9) | 11 (3.8) |
| Headache | 15 (2.6) | 18 (6.3) |
| Cough | 63 (11.0) | 21 (7.3) |
| Nasal congestion | 1 (0.2) | 0 |
| Pneumonitis | 1 (0.2) | 0 |
| Rhinorrhea | 12 (2.1) | 7 (2.4) |
| Snoring | 2 (0.4) | 0 |
| Tachypnea | 1 (0.2) | 0 |
| Wheezing | 1 (0.2) | 0 |
| Dermatitis | 1 (0.2) | 0 |
| Hyperhidrosis | 4 (0.7) | 6 (2.1) |
| Rash | 1 (0.2) | 0 |
| Skin hypopigmentation | 1 (0.2) | 0 |
| Pallor | 2 (0.4) | 0 |
Data are presented as No. (%) unless otherwise indicated.
| System Organ Class Preferred Term . | Arterolane Maleate– Piperaquine Phosphate (n = 571) . | Artemether- Lumefantrine (n = 288) . |
|---|---|---|
| Patients with at least 1 adverse event | 534 (93.5) | 267 (92.7) |
| Clinical adverse events | ||
| Anemia | 279 (48.9) | 145 (50.3) |
| Ear pain | 0 | 1 (0.3) |
| Blepharitis | 1 (0.2) | 0 |
| Conjunctival hyperemia | 1 (0.2) | 0 |
| Abdominal distension | 6 (1.1) | 1 (0.3) |
| Abdominal pain | 5 (0.9) | 1 (0.3) |
| Constipation | 2 (0.4) | 0 |
| Diarrhea | 12 (2.1) | 4 (1.4) |
| Dyspepsia | 1 (0.2) | 1 (0.3) |
| Flatulence | 1 (0.2) | 0 |
| Mouth ulceration | 1 (0.2) | 0 |
| Nausea | 9 (1.6) | 6 (2.1) |
| Vomiting | 96 (16.8) | 20 (6.9) |
| Chills | 6 (1.1) | 6 (2.1) |
| Fatigue | 6 (1.1) | 9 (3.1) |
| Malaise | 5 (0.9) | 5 (1.7) |
| Pyrexia | 27 (4.7) | 28 (9.7) |
| Abscess | 1 (0.2) | 1 (0.3) |
| Body tinea | 1 (0.2) | 0 |
| Bronchitis | 1 (0.2) | 2 (0.7) |
| Bronchopneumonia | 0 | 1 (0.3) |
| Candida infection | 1 (0.2) | 0 |
| Ear infection | 1 (0.2) | 1 (0.3) |
| Gastroenteritis | 2 (0.4) | 2 (0.7) |
| Lice infestation | 1 (0.2) | 1 (0.3) |
| Mumps | 0 | 1 (0.3) |
| Pharyngitis | 4 (0.7) | 1 (0.3) |
| Rhinitis | 1 (0.2) | 4 (1.4) |
| Sepsis | 1 (0.2) | 0 |
| Tinea capitis | 2 (0.4) | 0 |
| Tonsillitis | 5 (0.9) | 2 (0.7) |
| Upper respiratory tract infection | 6 (1.1) | 2 (0.7) |
| Urinary tract infection | 1 (0.2) | 0 |
| Limb injury | 1 (0.2) | 0 |
| Decreased appetite | 11 (1.9) | 11 (3.8) |
| Headache | 15 (2.6) | 18 (6.3) |
| Cough | 63 (11.0) | 21 (7.3) |
| Nasal congestion | 1 (0.2) | 0 |
| Pneumonitis | 1 (0.2) | 0 |
| Rhinorrhea | 12 (2.1) | 7 (2.4) |
| Snoring | 2 (0.4) | 0 |
| Tachypnea | 1 (0.2) | 0 |
| Wheezing | 1 (0.2) | 0 |
| Dermatitis | 1 (0.2) | 0 |
| Hyperhidrosis | 4 (0.7) | 6 (2.1) |
| Rash | 1 (0.2) | 0 |
| Skin hypopigmentation | 1 (0.2) | 0 |
| Pallor | 2 (0.4) | 0 |
| System Organ Class Preferred Term . | Arterolane Maleate– Piperaquine Phosphate (n = 571) . | Artemether- Lumefantrine (n = 288) . |
|---|---|---|
| Patients with at least 1 adverse event | 534 (93.5) | 267 (92.7) |
| Clinical adverse events | ||
| Anemia | 279 (48.9) | 145 (50.3) |
| Ear pain | 0 | 1 (0.3) |
| Blepharitis | 1 (0.2) | 0 |
| Conjunctival hyperemia | 1 (0.2) | 0 |
| Abdominal distension | 6 (1.1) | 1 (0.3) |
| Abdominal pain | 5 (0.9) | 1 (0.3) |
| Constipation | 2 (0.4) | 0 |
| Diarrhea | 12 (2.1) | 4 (1.4) |
| Dyspepsia | 1 (0.2) | 1 (0.3) |
| Flatulence | 1 (0.2) | 0 |
| Mouth ulceration | 1 (0.2) | 0 |
| Nausea | 9 (1.6) | 6 (2.1) |
| Vomiting | 96 (16.8) | 20 (6.9) |
| Chills | 6 (1.1) | 6 (2.1) |
| Fatigue | 6 (1.1) | 9 (3.1) |
| Malaise | 5 (0.9) | 5 (1.7) |
| Pyrexia | 27 (4.7) | 28 (9.7) |
| Abscess | 1 (0.2) | 1 (0.3) |
| Body tinea | 1 (0.2) | 0 |
| Bronchitis | 1 (0.2) | 2 (0.7) |
| Bronchopneumonia | 0 | 1 (0.3) |
| Candida infection | 1 (0.2) | 0 |
| Ear infection | 1 (0.2) | 1 (0.3) |
| Gastroenteritis | 2 (0.4) | 2 (0.7) |
| Lice infestation | 1 (0.2) | 1 (0.3) |
| Mumps | 0 | 1 (0.3) |
| Pharyngitis | 4 (0.7) | 1 (0.3) |
| Rhinitis | 1 (0.2) | 4 (1.4) |
| Sepsis | 1 (0.2) | 0 |
| Tinea capitis | 2 (0.4) | 0 |
| Tonsillitis | 5 (0.9) | 2 (0.7) |
| Upper respiratory tract infection | 6 (1.1) | 2 (0.7) |
| Urinary tract infection | 1 (0.2) | 0 |
| Limb injury | 1 (0.2) | 0 |
| Decreased appetite | 11 (1.9) | 11 (3.8) |
| Headache | 15 (2.6) | 18 (6.3) |
| Cough | 63 (11.0) | 21 (7.3) |
| Nasal congestion | 1 (0.2) | 0 |
| Pneumonitis | 1 (0.2) | 0 |
| Rhinorrhea | 12 (2.1) | 7 (2.4) |
| Snoring | 2 (0.4) | 0 |
| Tachypnea | 1 (0.2) | 0 |
| Wheezing | 1 (0.2) | 0 |
| Dermatitis | 1 (0.2) | 0 |
| Hyperhidrosis | 4 (0.7) | 6 (2.1) |
| Rash | 1 (0.2) | 0 |
| Skin hypopigmentation | 1 (0.2) | 0 |
| Pallor | 2 (0.4) | 0 |
Data are presented as No. (%) unless otherwise indicated.
An initial decrease in hemoglobin (<10 g/dL) was observed, which restored to the baseline levels by day 28 in both treatment arms; 14.2% of patients in the AM-PQP arm and 18.7% in the AL arm had at least 1 incidence of reduced platelet count during the trial. Severe thrombocytopenia (<50000/µL) was reported in 18 (3.2%) patients in the AM-PQP arm and 11 (3.8%) patients in the AL arm. At least 1 incidence of decreased neutrophil count was reported in 42.6% in the AM-PQP arm and 46.5% in the AL arm. All the events were mild in intensity. No patient was withdrawn due to decrease in neutrophil counts. All of these cases were resolved without sequelae. There were no differences in laboratory measurements between arms. The mean increase in alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase values were similar in both the arms. Most of the increase in hepatic enzymes levels was mild to moderate in severity.
The mean (standard deviation [SD]) increase from baseline in QTc interval (Fridericia correction) at day 2 in the AM-PQP and AL arms was 27.9 (SD, 33.70) msec and 14.8 (SD, 28.62) msec, respectively. The prolongation of QTc >500 msec at day 2 was seen in 4 patients (0.7%) in the AM-PQP arm and none in the AL arm. More than 60 msec increase in QTc interval over baseline at day 2 was reported in 80 patients (14%) and 12 patients (4.17%) in the AM-PQP and AL arms, respectively. However, no clinical cardiac events were reported in any of these patients.
Pharmacokinetics
The mean maximum plasma concentration (Cmax) for arterolane was 57.90, 87.52, and 93.08 ng/mL and mean exposure (AUClast) of arterolane was 985.19, 2157.36, and 2158.47 h × ng/mL across age groups of 6 months to <2 years, 2 to <6 years, and 6–12 years, respectively. Similarly, the mean Cmax of piperaquine was 315.08, 550.27, and 523.75 ng/mL and mean exposure was 38018.82, 74148.42, and 77032.99 h × ng/mL across age groups of 6 months to <2 years, 2 to <6 years, and 6–12 years, respectively.
Mean pharmacokinetic parameters for arterolane, piperaquine, artemether, dihydroartemisinin, and lumefantrine are presented in Tables 6–8.
Mean Pharmacokinetic Parameters of Arterolane, Piperaquine, Artemether, Dihydroartemisinin, and Lumefantrine in Children Aged 6 Months to <2 Years With Plasmodium falciparum Malaria Across Sites in Africa and India
| Parameter . | 6 mo to <2 y (1 Tablet Once Daily) . | 5 kg to <15 kg (1 Tablet Twice Daily) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arterolane . | Piperaquine . | Artemether . | Dihydroartemisinin . | Lumefantrine . | ||||||
| Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (µg/mL) . | AUClast (h × µg/mL) . | |
| No. | 83 | 83 | 83 | 83 | 58 | 58 | 38 | 38 | 62 | 62 |
| Mean | 57.90 | 985.19 | 315.08 | 38018.82 | 76.24 | 1614.48 | 59.39 | 1336.20 | 4.365 | 417.91 |
| SD | 112.15 | 1269.23 | 426.81 | 33873.31 | 72.13 | 1608.49 | 70.29 | 1788.10 | 3.903 | 1205.99 |
| CV% | 193.70 | 128.83 | 135.46 | 89.10 | 94.61 | 99.63 | 118.34 | 133.82 | 89.42 | 288.57 |
| Parameter . | 6 mo to <2 y (1 Tablet Once Daily) . | 5 kg to <15 kg (1 Tablet Twice Daily) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arterolane . | Piperaquine . | Artemether . | Dihydroartemisinin . | Lumefantrine . | ||||||
| Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (µg/mL) . | AUClast (h × µg/mL) . | |
| No. | 83 | 83 | 83 | 83 | 58 | 58 | 38 | 38 | 62 | 62 |
| Mean | 57.90 | 985.19 | 315.08 | 38018.82 | 76.24 | 1614.48 | 59.39 | 1336.20 | 4.365 | 417.91 |
| SD | 112.15 | 1269.23 | 426.81 | 33873.31 | 72.13 | 1608.49 | 70.29 | 1788.10 | 3.903 | 1205.99 |
| CV% | 193.70 | 128.83 | 135.46 | 89.10 | 94.61 | 99.63 | 118.34 | 133.82 | 89.42 | 288.57 |
Pharmacokinetic parameters determined based on sparse sampling times and from patients who had ≥2 non-below limit of quantitation concentrations. Cmax may not be “true” Cmax.
Abbreviations: AUClast, the area under the plasma concentration versus time curve from time zero to the last measurable concentration; Cmax, maximum observed concentration; CV%, coefficient of variation is reported as a percentage; SD, standard deviation.
Mean Pharmacokinetic Parameters of Arterolane, Piperaquine, Artemether, Dihydroartemisinin, and Lumefantrine in Children Aged 6 Months to <2 Years With Plasmodium falciparum Malaria Across Sites in Africa and India
| Parameter . | 6 mo to <2 y (1 Tablet Once Daily) . | 5 kg to <15 kg (1 Tablet Twice Daily) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arterolane . | Piperaquine . | Artemether . | Dihydroartemisinin . | Lumefantrine . | ||||||
| Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (µg/mL) . | AUClast (h × µg/mL) . | |
| No. | 83 | 83 | 83 | 83 | 58 | 58 | 38 | 38 | 62 | 62 |
| Mean | 57.90 | 985.19 | 315.08 | 38018.82 | 76.24 | 1614.48 | 59.39 | 1336.20 | 4.365 | 417.91 |
| SD | 112.15 | 1269.23 | 426.81 | 33873.31 | 72.13 | 1608.49 | 70.29 | 1788.10 | 3.903 | 1205.99 |
| CV% | 193.70 | 128.83 | 135.46 | 89.10 | 94.61 | 99.63 | 118.34 | 133.82 | 89.42 | 288.57 |
| Parameter . | 6 mo to <2 y (1 Tablet Once Daily) . | 5 kg to <15 kg (1 Tablet Twice Daily) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arterolane . | Piperaquine . | Artemether . | Dihydroartemisinin . | Lumefantrine . | ||||||
| Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (µg/mL) . | AUClast (h × µg/mL) . | |
| No. | 83 | 83 | 83 | 83 | 58 | 58 | 38 | 38 | 62 | 62 |
| Mean | 57.90 | 985.19 | 315.08 | 38018.82 | 76.24 | 1614.48 | 59.39 | 1336.20 | 4.365 | 417.91 |
| SD | 112.15 | 1269.23 | 426.81 | 33873.31 | 72.13 | 1608.49 | 70.29 | 1788.10 | 3.903 | 1205.99 |
| CV% | 193.70 | 128.83 | 135.46 | 89.10 | 94.61 | 99.63 | 118.34 | 133.82 | 89.42 | 288.57 |
Pharmacokinetic parameters determined based on sparse sampling times and from patients who had ≥2 non-below limit of quantitation concentrations. Cmax may not be “true” Cmax.
Abbreviations: AUClast, the area under the plasma concentration versus time curve from time zero to the last measurable concentration; Cmax, maximum observed concentration; CV%, coefficient of variation is reported as a percentage; SD, standard deviation.
Mean Pharmacokinetic Parameters of Arterolane, Piperaquine, Artemether, Dihydroartemisinin, and Lumefantrine in Children Aged 2 Years to <6 Years With Plasmodium falciparum Malaria Across Sites in Africa and India
| Parameter . | 2 y to <6 y (2 Tablets Once Daily) . | 15 kg to <25 kg (2 Tablets Twice Daily) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arterolane . | Piperaquine . | Artemether . | Dihydroartemisinin . | Lumefantrine . | ||||||
| Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (µg/mL) . | AUClast (h × µg/mL) . | |
| No. | 192 | 192 | 192 | 192 | 98 | 98 | 80 | 80 | 98 | 98 |
| Mean | 87.52 | 2157.36 | 550.27 | 74148.42 | 122.51 | 2815.68 | 75.63 | 4083.47 | 6.920 | 647.71 |
| SD | 85.50 | 2272.55 | 417.00 | 51013.81 | 92.63 | 4404.91 | 72.28 | 10381.97 | 5.013 | 727.50 |
| CV% | 97.70 | 105.34 | 75.78 | 68.80 | 75.61 | 156.44 | 95.57 | 254.24 | 72.43 | 112.32 |
| Parameter . | 2 y to <6 y (2 Tablets Once Daily) . | 15 kg to <25 kg (2 Tablets Twice Daily) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arterolane . | Piperaquine . | Artemether . | Dihydroartemisinin . | Lumefantrine . | ||||||
| Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (µg/mL) . | AUClast (h × µg/mL) . | |
| No. | 192 | 192 | 192 | 192 | 98 | 98 | 80 | 80 | 98 | 98 |
| Mean | 87.52 | 2157.36 | 550.27 | 74148.42 | 122.51 | 2815.68 | 75.63 | 4083.47 | 6.920 | 647.71 |
| SD | 85.50 | 2272.55 | 417.00 | 51013.81 | 92.63 | 4404.91 | 72.28 | 10381.97 | 5.013 | 727.50 |
| CV% | 97.70 | 105.34 | 75.78 | 68.80 | 75.61 | 156.44 | 95.57 | 254.24 | 72.43 | 112.32 |
Pharmacokinetic parameters determined based on sparse sampling times and from patients who had ≥2 non-below limit of quantitation concentrations. Cmax may not be “true” Cmax.
Abbreviations: AUClast, the area under the plasma concentration versus time curve from time zero to the last measurable concentration; Cmax, maximum observed concentration; CV%, coefficient of variation is reported as a percentage; SD, standard deviation.
Mean Pharmacokinetic Parameters of Arterolane, Piperaquine, Artemether, Dihydroartemisinin, and Lumefantrine in Children Aged 2 Years to <6 Years With Plasmodium falciparum Malaria Across Sites in Africa and India
| Parameter . | 2 y to <6 y (2 Tablets Once Daily) . | 15 kg to <25 kg (2 Tablets Twice Daily) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arterolane . | Piperaquine . | Artemether . | Dihydroartemisinin . | Lumefantrine . | ||||||
| Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (µg/mL) . | AUClast (h × µg/mL) . | |
| No. | 192 | 192 | 192 | 192 | 98 | 98 | 80 | 80 | 98 | 98 |
| Mean | 87.52 | 2157.36 | 550.27 | 74148.42 | 122.51 | 2815.68 | 75.63 | 4083.47 | 6.920 | 647.71 |
| SD | 85.50 | 2272.55 | 417.00 | 51013.81 | 92.63 | 4404.91 | 72.28 | 10381.97 | 5.013 | 727.50 |
| CV% | 97.70 | 105.34 | 75.78 | 68.80 | 75.61 | 156.44 | 95.57 | 254.24 | 72.43 | 112.32 |
| Parameter . | 2 y to <6 y (2 Tablets Once Daily) . | 15 kg to <25 kg (2 Tablets Twice Daily) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arterolane . | Piperaquine . | Artemether . | Dihydroartemisinin . | Lumefantrine . | ||||||
| Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (µg/mL) . | AUClast (h × µg/mL) . | |
| No. | 192 | 192 | 192 | 192 | 98 | 98 | 80 | 80 | 98 | 98 |
| Mean | 87.52 | 2157.36 | 550.27 | 74148.42 | 122.51 | 2815.68 | 75.63 | 4083.47 | 6.920 | 647.71 |
| SD | 85.50 | 2272.55 | 417.00 | 51013.81 | 92.63 | 4404.91 | 72.28 | 10381.97 | 5.013 | 727.50 |
| CV% | 97.70 | 105.34 | 75.78 | 68.80 | 75.61 | 156.44 | 95.57 | 254.24 | 72.43 | 112.32 |
Pharmacokinetic parameters determined based on sparse sampling times and from patients who had ≥2 non-below limit of quantitation concentrations. Cmax may not be “true” Cmax.
Abbreviations: AUClast, the area under the plasma concentration versus time curve from time zero to the last measurable concentration; Cmax, maximum observed concentration; CV%, coefficient of variation is reported as a percentage; SD, standard deviation.
Mean Pharmacokinetic Parameters of Arterolane, Piperaquine, Artemether, Dihydroartemisinin, and Lumefantrine in Children Aged 6–12 Years With Plasmodium falciparum Malaria Across Sites in Africa and India
| Parameter . | 6–12 y (3 Tablets Once Daily) . | 25 to <35 kg (3 Tablets Twice Daily) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arterolane . | Piperaquine . | Artemether . | Dihydroartemisinin . | Lumefantrine . | ||||||
| Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (µg/mL) . | AUClast (h × µg/mL) . | |
| No. | 257 | 257 | 257 | 257 | 94 | 94 | 84 | 84 | 96 | 96 |
| Mean | 93.08 | 2158.47 | 523.75 | 77032.99 | 122.02 | 2815.47 | 94.83 | 4384.85 | 7.317 | 682.19 |
| SD | 73.44 | 1770.97 | 369.91 | 56648.59 | 97.20 | 4614.53 | 74.58 | 8207.18 | 5.952 | 893.76 |
| CV% | 78.90 | 82.05 | 70.63 | 73.54 | 79.66 | 163.90 | 78.65 | 187.17 | 81.35 | 131.01 |
| Parameter . | 6–12 y (3 Tablets Once Daily) . | 25 to <35 kg (3 Tablets Twice Daily) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arterolane . | Piperaquine . | Artemether . | Dihydroartemisinin . | Lumefantrine . | ||||||
| Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (µg/mL) . | AUClast (h × µg/mL) . | |
| No. | 257 | 257 | 257 | 257 | 94 | 94 | 84 | 84 | 96 | 96 |
| Mean | 93.08 | 2158.47 | 523.75 | 77032.99 | 122.02 | 2815.47 | 94.83 | 4384.85 | 7.317 | 682.19 |
| SD | 73.44 | 1770.97 | 369.91 | 56648.59 | 97.20 | 4614.53 | 74.58 | 8207.18 | 5.952 | 893.76 |
| CV% | 78.90 | 82.05 | 70.63 | 73.54 | 79.66 | 163.90 | 78.65 | 187.17 | 81.35 | 131.01 |
Pharmacokinetic parameters determined based on sparse sampling times and from patients who had ≥2 non-below limit of quantitation concentrations. Cmax may not be “true” Cmax.
Abbreviations: AUClast, the area under the plasma concentration versus time curve from time zero to the last measurable concentration; Cmax, maximum observed concentration; CV%, coefficient of variation is reported as a percentage; SD, standard deviation.
Mean Pharmacokinetic Parameters of Arterolane, Piperaquine, Artemether, Dihydroartemisinin, and Lumefantrine in Children Aged 6–12 Years With Plasmodium falciparum Malaria Across Sites in Africa and India
| Parameter . | 6–12 y (3 Tablets Once Daily) . | 25 to <35 kg (3 Tablets Twice Daily) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arterolane . | Piperaquine . | Artemether . | Dihydroartemisinin . | Lumefantrine . | ||||||
| Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (µg/mL) . | AUClast (h × µg/mL) . | |
| No. | 257 | 257 | 257 | 257 | 94 | 94 | 84 | 84 | 96 | 96 |
| Mean | 93.08 | 2158.47 | 523.75 | 77032.99 | 122.02 | 2815.47 | 94.83 | 4384.85 | 7.317 | 682.19 |
| SD | 73.44 | 1770.97 | 369.91 | 56648.59 | 97.20 | 4614.53 | 74.58 | 8207.18 | 5.952 | 893.76 |
| CV% | 78.90 | 82.05 | 70.63 | 73.54 | 79.66 | 163.90 | 78.65 | 187.17 | 81.35 | 131.01 |
| Parameter . | 6–12 y (3 Tablets Once Daily) . | 25 to <35 kg (3 Tablets Twice Daily) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arterolane . | Piperaquine . | Artemether . | Dihydroartemisinin . | Lumefantrine . | ||||||
| Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (ng/mL) . | AUClast (h × ng/mL) . | Cmax (µg/mL) . | AUClast (h × µg/mL) . | |
| No. | 257 | 257 | 257 | 257 | 94 | 94 | 84 | 84 | 96 | 96 |
| Mean | 93.08 | 2158.47 | 523.75 | 77032.99 | 122.02 | 2815.47 | 94.83 | 4384.85 | 7.317 | 682.19 |
| SD | 73.44 | 1770.97 | 369.91 | 56648.59 | 97.20 | 4614.53 | 74.58 | 8207.18 | 5.952 | 893.76 |
| CV% | 78.90 | 82.05 | 70.63 | 73.54 | 79.66 | 163.90 | 78.65 | 187.17 | 81.35 | 131.01 |
Pharmacokinetic parameters determined based on sparse sampling times and from patients who had ≥2 non-below limit of quantitation concentrations. Cmax may not be “true” Cmax.
Abbreviations: AUClast, the area under the plasma concentration versus time curve from time zero to the last measurable concentration; Cmax, maximum observed concentration; CV%, coefficient of variation is reported as a percentage; SD, standard deviation.
DISCUSSION
As per the WHO world malaria report 2016, majority of deaths reported are due to P. falciparum malaria. Also, it has been estimated that malaria mortality rates between 2000 and 2015 have declined by 62% globally and between 2010 and 2015 by 29%. ACTs were given to children at public health facilities or via community health workers [1].
The FDC of AM 150 mg and PQP 750 mg tablets was developed for adults and marketed in India and some countries in Africa for the treatment of P. falciparum malaria. In this combination, AM is a new synthetic trioxolane with proven efficacy and safety and PQP is a proven effective and well-tolerated antimalarial drug [10–12].
This study was conducted to compare the efficacy, safety, and tolerability of FDC of AM 37.5 mg and PQP 187.5 mg dispersible tablets and AL in the treatment of uncomplicated P. falciparum malaria in a larger pediatric population aged 6 months to 12 years.
The cure rate at day 28 was achieved in >95% patients in both treatment arms. The results accord with the high activity reported for other ACTs in children with P. falciparum malaria [13–21]. Also, the efficacy was reported to be consistent with the results of a phase 2 study of FDC of AM and PQP in pediatric patients with P. falciparum malaria and a phase 3 study of FDC of AM and PQP in patients aged 13–65 years with P. falciparum malaria [6, 7].
There was no early treatment failure in this study. The true treatment failures (recrudescence) were reported only in the AL arm at or before day 28. This study reported high rates of reinfections with the AL arm, which are in line with the reported literature [14].
Parasite clearance at day 3 has been considered a landmark for measuring sensitivity of P. falciparum to artemisinins and the efficacy of ACT by WHO [22]. This has been achieved successfully by the AM supported by median PCT of 24 hours and median FCT of 6 hours in this study. The results corroborate with the earlier studies [5, 23].
In this study, no recrudescence was reported at day 28 in AM-PQP arm. This confirms the role of PQP as a favorable partner drug for use in combination with artemisinins [24–26].
The pharmacokinetic parameters values of arterolane and piperaquine observed in this study were similar to those reported in phase 2 trial in pediatric patients, whereas the values for artemether and lumefantrine were in line with the values reported in the literature [6, 26–30].
In both the treatment arms, the overall incidences of AEs were comparable. The incidence of vomiting in this study was reported to be low as compared to earlier phase II study conducted in pediatric patients with P. falciparum malaria [6]. However, this is consistent with occurrence of vomiting reported in the literature [16, 18, 19, 31].
The increase from baseline in QTc interval (Fridericia correction) or QTc prolongation of >60 msec after receiving AM-PQP or AL reported in this study is in line with reported data [32, 33]. Two studies with a dihydroartemisinin–piperaquine arm reported statistically significant lengthening of the mean QTc interval (Bazett correction), 11 (95% CI, 4–18) msec and 15 (95% CI, 5–25) msec. The increase in QTc interval was not associated with any cardiac event, such as Torsade de pointes or signs/symptoms of serious arrhythmia or polymorphic ventricular tachycardia. There were no withdrawals due to QTc prolongation. It has been reported that the normal daily variation of QTc interval is up to 75–100 msec [32, 34].
The results of this trial demonstrated the comparable efficacy and safety of FDC of AM 37.5 mg and PQP 187.5 mg dispersible tablets as compared to artemether-lumefantrine 20 and 120 mg in the treatment of uncomplicated P. falciparum malaria in pediatric patients. This combination offers an easy-to-administer formulation with once-daily dosing, leading to improved patient convenience and compliance with rapid action.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Literature search: A. N., S. K. S. Figures and tables: A. N., S. K. S., P. S., A. R. Study design: R. K. J., S. K. S., A. R. Data collection: O. A. T., V. M., I. S., I. S., O. G., R. T., A. V. M., P. M., N. B., A. K. T., R. R. D., R. K. J., A. N., P. S., S. K. S., A. R. Data analysis: A. R., R. K. J., S. K. S., A. N., P. S. Data interpretation: R. K. J., S. K. S., A. N., A. R. Manuscript writing: A. N., S. K. S.
Acknowledgments. The authors thank Sarfaraz Ahmed, Gaurav Kumar Nigam, Tarun Arora, Manish Kumar Barnwal, Indu Joshi and Partha Banerjee who were in charge of the data collection and monitoring; Bina Srivastava, National Institute of Malaria Research who undertook PCR analysis and reading of the slides.
Financial support. Sun Pharmaceutical Industries Ltd sponsored this trial as part of the clinical development program of AM-PQP.
Potential conflicts of interest. R. K. J., A. N., S. K. S., A. R., P. S., S. A., G. K. N., T. A., M. K. B., I. J., and P. B. are or were employed in Sun Pharmaceutical Industries Ltd while developing the product. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
APPENDIX
The AM-PQP Study Team included the following (in addition to the named authors): Toure N. Beatrice, Ako Ako Aristide Berenger (Anonkoua-koute, Ivory Coast), Kouame N’Goran Valery, Tuo Karim (Ayame General Hospital, Ivory Coast), Landry Tiacoh, Ouattara Kigninma (North Abobo General Hospital, Abidjan, Ivory Coast), Naomi Sibale, Kelvin Kaneka, Mercy Machilika (Queen Elizabeth Central Hospital, Blantyre, Malawi), Sheila Mabote, Juvencio Bonzela, Carlos Lourenceo, (Chókwè Health Research and Training Centre, Mozambique), Bidashimwa Dieudonneée, Paulin Kasonga (Universitée de Kinshasa, Democratic Republic of Congo), Jean-Louis Ndiaye, Modou Diop (Service de Parasitologie, Faculté de Médecine, Université Cheikh Anta Diop, Dakar, Senegal), Bouran Sidibe, Moctar Coulibaly, (Kolle Health Centre, Mali), Bakary Fofana, Sekou Toure (Bougoula-Hameau Health Center, Mali), Ankita Panigrahy, Debasis Patro, Tapan Kumar Biswas, Preetish Kumar Panigrahy (Maharaja Krishna Chandra Gajapati College and Hospital, Berhampur, Odisha, India), Saibal Jana (Shaheed Hospital, Dalli Rajhara, Chhattisgarh, India), Debasish Hota, Bijayini Behera (AIIMS, Bhubaneswar, Odisha, India), Akshaya Mohanty, Goutam Patel (Ispat General Hospital, Rourkela, Odisha, India).
Author notes
Members of the AM-PQP Study Team are listed in the Appendix.




