-
PDF
- Split View
-
Views
-
Cite
Cite
Jordan J Feld, Alnoor Ramji, Stephen D Shafran, Bernard Willems, Paul Marotta, Emmanuelle Huchet, Marie-Louise Vachon, Evguenia S Svarovskaia, K C Huang, Robert H Hyland, Chohee Yun, Benedetta Massetto, Diana M Brainard, John G McHutchison, Edward Tam, Robert Bailey, Curtis Cooper, Eric M Yoshida, Susan Greenbloom, Magdy Elkhashab, Sergio Borgia, Mark G Swain, Ledipasvir-Sofosbuvir Plus Ribavirin in Treatment-Naive Patients With Hepatitis C Virus Genotype 3 Infection: An Open-Label Study, Clinical Infectious Diseases, Volume 65, Issue 1, 1 July 2017, Pages 13–19, https://doi.org/10.1093/cid/cix289
Close - Share Icon Share
Abstract
Patients chronically infected with genotype 3 hepatitis C virus (HCV) have faster disease progression and are less responsive to current direct-acting antiviral regimens than patients infected with other genotypes. We conducted an open-label trial to evaluate the safety, tolerability, and efficacy of ledipasvir and sofosbuvir plus ribavirin in patients with genotype 3 HCV infection.
We enrolled treatment-naive patients with and without compensated cirrhosis at 15 sites in Canada. All patients were treated with ledipasvir-sofosbuvir (90 mg and 400 mg) plus weight-based ribavirin for 12 weeks. The primary endpoint was sustained virologic response 12 weeks after treatment (SVR12). Secondary endpoints included evaluation of baseline and treatment-emergent drug resistance.
Of the 111 patients enrolled, 105 (95%) had subtype 3a HCV and 39 (35%) had compensated cirrhosis. SVR12 was achieved by 99 of 111 patients (89%; 95% confidence interval, 82%–94%). Of the 39 patients with cirrhosis, 31 (79%) achieved SVR12, compared with 68 of 72 (94%) patients without cirrhosis. No treatment-emergent resistance mutations occurred in those who failed treatment. One patient discontinued treatment due to liver cancer and died 22 days after treatment discontinuation. The most common adverse events were fatigue (51%), headache (36%), and nausea (23%).
In this multicenter trial involving treatment-naive patients with genotype 3 HCV, 12 weeks of ledipasvir-sofosbuvir provided a high level of SVR in those without cirrhosis.
NCT02413593.
Genotype 3 hepatitis C virus (HCV) accounts for an estimated 30% of HCV infections, making it the second-most prevalent HCV genotype worldwide, following only genotype 1 [1]. Genotype 3 is the predominant genotype in South Asia, accounting for at least 60% of HCV infections in India and Pakistan, as well as up to 40% of infections in Australia and some European countries [2–4]. Multiple studies have documented that genotype 3 HCV infection is associated with more rapid disease progression than other genotypes, resulting in increased risk of cirrhosis, hepatocellular carcinoma, and all-cause mortality [5–9]. Although remarkable progress in the treatment of HCV has been made with the development of novel direct-acting antivirals (DAAs), genotype 3 has proven more challenging to manage than other genotypes.
Ledipasvir is a potent, well-tolerated NS5A inhibitor with picomolar potency against HCV genotype 1a and 1b [10]. Sofosbuvir is a nucleotide analogue inhibitor of the NS5B RNA-dependent RNA polymerase approved in combination with other DAAs for the treatment of HCV of all genotypes [11, 12]. In combination, ledipasvir-sofosbuvir, with or without ribavirin, has been approved for patients with genotypes 1, 4, 5, and 6 as a 12-week, once-daily treatment [13]. In the ELECTRON-2 study, treatment-naive patients with genotype 3 HCV who were randomized to receive ledipasvir-sofosbuvir with or without ribavirin for 12 weeks achieved rates of SVR of 100% (26 of 26) and 64% (16 of 25), respectively [14]. Of the 4 patients with cirrhosis who received ledipasvir-sofosbuvir alone, one achieved SVR.
At the time the study was undertaken, the only approved interferon-free treatment for genotype 3 was sofosbuvir plus ribavirin, which resulted in very low SVR rates, particularly in those who had been previously treated and those with cirrhosis [15]. Treatments have significantly improved recently with approval of highly effective regimens for genotype 3. However, even the combination of sofosbuvir with the pangenotypic NS5A inhibitor velpatasvir is less effective in patients with genotype 3 and cirrhosis than in those with other genotypes [16]. Access to new regimens for genotype 3 remains a challenge in many regions of the world [17, 18].
In the current study, we further evaluated the efficacy, tolerability, and safety of 12 weeks of ledipasvir and sofosbuvir plus weight-based ribavirin for treatment-naive patients infected with HCV genotype 3, with or without cirrhosis.
METHODS
Study Design and Patients
We conducted this phase 2, open-label, single-cohort study from 1 May 2015 to 23 December 2015 at 15 sites in Canada. Eligible patients were at least 18 years old, chronically infected with genotype 3 HCV (serum HCV RNA >10000 IU/mL), and naive to treatment for HCV. Patients with hepatocellular carcinoma, clinically significant laboratory abnormalities (alanine aminotransferase >10 times the upper limit of normal [ULN], aspartate aminotransferase [AST] >10 × ULN, direct bilirubin >1.5 × ULN, platelet counts <50000 cells/µL, hemoglobin A1c >10%, creatinine clearance by Cockroft-Gault equation <60 mL/minute, hemoglobin <10 g/dL, albumin <3 g/dL, or an international normalized ratio >1.5 × ULN), decompensated liver cirrhosis, or coinfection with hepatitis B virus or human immunodeficiency virus were ineligible for enrollment.
Up to 40% of patients enrolled could have compensated cirrhosis, as demonstrated by liver biopsy (with a Metavir fibrosis score of 4 or Ishak score of at least 5), Fibroscan (showing cirrhosis defined as a score of at least 12.5 kPa), or a Fibrotest score of at least .75 and an AST-to-platelet ratio index of at least 2. Two patients that did not meet the protocol criteria for a cirrhosis determination had Fibrotest scores <0.74 and were categorized as non-cirrhotic for analysis.
All patients received the fixed-dose combination tablet of ledipasvir and sofosbuvir (90 mg/400 mg) once daily plus weight-based ribavirin in a divided dose for 12 weeks. The dosage of ribavirin was based on baseline body weight (1000 mg daily in patients with a body weight of <75 kg, and 1200 mg daily in patients with a body weight of ≥75 kg).
The protocol was reviewed by research ethics boards at all participating sites and all patients provided written informed consent before any study procedures were undertaken. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. All authors had full access to all data in the study reviewed and approved the final article.
Study Procedures
HCV RNA was measured with the COBAS AmpliPrep/COBAS TaqMan HCV Test, version 2.0 for Research Use Only (Roche, Indianapolis, Indiana), with a lower limit of quantification for HCV RNA of 15 IU/mL. HCV genotype and subtype were determined using the Siemens VERSANT HCV Genotype INNO-LiPA 2.0 Assay. Interferon-λ3 (formerly IL28B) genotype was determined by polymerase chain reaction amplification and sequencing of the rs12979860 single-nucleotide polymorphism.
Deep sequencing with a 1% cutoff for the HCV NS5A and NS5B regions was performed at baseline for all patients, and at relapse for patients who experienced virologic failure. The full-length HCV NS5A and NS5B coding regions were amplified and deep sequenced with the Illumina MiSeq deep sequencing platform (Illumina, San Diego, California) by DDL Diagnostic Laboratory (Rijswijk, the Netherlands).
NS5A substitutions considered associated with resistance to NS5A inhibitors were as follows: S24G/N/R, M28A/G/T, A30E/G/H/K/R, L31I/F/M/V, P32L, S38F, P58D/G, E92T, and Y93C/F/H/N/S. The following substitutions were defined as NS5B nucleoside inhibitor resistance-associated substitutions (RASs): S96T, N142T, L159F, E237G, S282any, C289I/L, L320F/I/V, and V321A/I.
All patients were assessed for safety with a physical examination and review of adverse events and clinical laboratory testing of blood samples. The use of hematologic-stimulating agents (eg, erythropoiesis-stimulating agents), granulocyte-colony stimulating factor, and thrombopoietin was prohibited during the screening period and for a minimum of 28 days prior to the baseline/day 1 visit through the end of treatment.
Endpoints
The primary efficacy endpoint was the proportion of patients achieving SVR 12 weeks after the end of therapy (SVR12), defined as serum HCV RNA below the lower limit of quantification (LLOQ, 15 IU/mL), in all patients who received at least 1 dose of study drug. The primary safety endpoint was the proportion of patients who discontinued study treatment due to an adverse event. Secondary endpoints included evaluation of baseline and treatment-emergent drug resistance.
Statistical Analyses
Rates of SVR12 were calculated for the entire population and predefined subgroups (including age [<65 years vs ≥65 years], race [black vs non-black], sex, body mass index [<30 kg/m2 vs ≥30 kg/m2], genotype, and cirrhosis status [present or absent]) with 2-sided 95% exact confidence intervals using the exact binomial distribution based on the Clopper-Pearson method. Efficacy was assessed in all enrolled and treated patients. Safety was assessed in all enrolled patients who received at least 1 dose of the study drug. All analyses were performed with SAS version 9.2 (SAS Institute, Cary, North Carolina). Formal statistical hypothesis testing was not conducted.
RESULTS
Baseline Characteristics
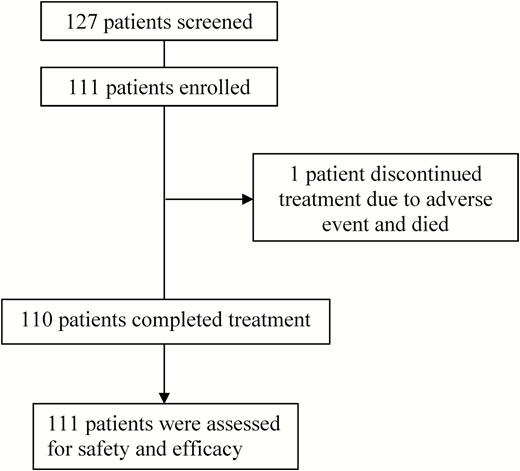
From 1 May 2015 to 17 July 2015, patients were screened for this open-label study at 15 sites in Canada. In total, 127 patients were screened, and 111 patients were enrolled and received at least 1 dose of study treatment (Figure 1 and Supplementary Table 1). Table 1 details the demographic characteristics of the enrolled patients. The mean age of patients was 48 years (range, 26–75 years), with the majority male (61% [n = 68]) and white (70% [n = 78]). Thirty-nine patients (35%) had compensated cirrhosis. For 35 of these 39 patients, cirrhosis determination was based on Fibroscan (median score, 20 [range, 13–59]) and for 4 patients on the combination of Fibrotest (median, 0.9 [range, 0.8–0.9]) and APRI (median, 6 [range, 2.4–6.8]). Of the 111 patients, 105 (95%) had genotype 3a, 3 (3%) had genotype 3b, and 3 patients’ genotype 3 subtypes (3%) could not be confirmed.
| Characteristic . | Ledipasvir-Sofosbuvir Plus Ribavirin for 12 Weeks (n = 111) . |
|---|---|
| Mean age, y (range) | 48 (26–75) |
| Male, No. (%) | 68 (61) |
| Race, No. (%) | |
| American Indian/Alaska Native | 3 (3) |
| Asian | 26 (23) |
| Black/African American | 1 (1) |
| White | 78 (70) |
| Othera | 3 (3) |
| Mean body mass index, kg/m2 (range) | 27.0 (18.1–61.4) |
| Genotype, No. (%) | |
| 3a | 105 (95) |
| 3b | 3 (3) |
| 3 (no confirmed subtype) | 3 (3) |
| Cirrhosis | |
| Yes | 39 (35) |
| No | 72 (65) |
| IL28Bb, No. (%) | |
| CC | 40 (36) |
| CT | 56 (51) |
| TT | 13 (2) |
| Mean HCV RNA, log10 IU/mL (range) | 6.2 (4.4–7.7) |
| Mean ALT, U/L (range) | 98 (18–397) |
| Mean platelets, × 103/μL (range) | 194 (56–420) |
| Characteristic . | Ledipasvir-Sofosbuvir Plus Ribavirin for 12 Weeks (n = 111) . |
|---|---|
| Mean age, y (range) | 48 (26–75) |
| Male, No. (%) | 68 (61) |
| Race, No. (%) | |
| American Indian/Alaska Native | 3 (3) |
| Asian | 26 (23) |
| Black/African American | 1 (1) |
| White | 78 (70) |
| Othera | 3 (3) |
| Mean body mass index, kg/m2 (range) | 27.0 (18.1–61.4) |
| Genotype, No. (%) | |
| 3a | 105 (95) |
| 3b | 3 (3) |
| 3 (no confirmed subtype) | 3 (3) |
| Cirrhosis | |
| Yes | 39 (35) |
| No | 72 (65) |
| IL28Bb, No. (%) | |
| CC | 40 (36) |
| CT | 56 (51) |
| TT | 13 (2) |
| Mean HCV RNA, log10 IU/mL (range) | 6.2 (4.4–7.7) |
| Mean ALT, U/L (range) | 98 (18–397) |
| Mean platelets, × 103/μL (range) | 194 (56–420) |
Abbreviations: ALT, alanine aminotransferase; HCV, hepatitis C virus.
aThree patients specified other races: 1 specified American Indian and white, and 2 specified East Indian.
bIL28B genotype results are not available for 2 patients.
| Characteristic . | Ledipasvir-Sofosbuvir Plus Ribavirin for 12 Weeks (n = 111) . |
|---|---|
| Mean age, y (range) | 48 (26–75) |
| Male, No. (%) | 68 (61) |
| Race, No. (%) | |
| American Indian/Alaska Native | 3 (3) |
| Asian | 26 (23) |
| Black/African American | 1 (1) |
| White | 78 (70) |
| Othera | 3 (3) |
| Mean body mass index, kg/m2 (range) | 27.0 (18.1–61.4) |
| Genotype, No. (%) | |
| 3a | 105 (95) |
| 3b | 3 (3) |
| 3 (no confirmed subtype) | 3 (3) |
| Cirrhosis | |
| Yes | 39 (35) |
| No | 72 (65) |
| IL28Bb, No. (%) | |
| CC | 40 (36) |
| CT | 56 (51) |
| TT | 13 (2) |
| Mean HCV RNA, log10 IU/mL (range) | 6.2 (4.4–7.7) |
| Mean ALT, U/L (range) | 98 (18–397) |
| Mean platelets, × 103/μL (range) | 194 (56–420) |
| Characteristic . | Ledipasvir-Sofosbuvir Plus Ribavirin for 12 Weeks (n = 111) . |
|---|---|
| Mean age, y (range) | 48 (26–75) |
| Male, No. (%) | 68 (61) |
| Race, No. (%) | |
| American Indian/Alaska Native | 3 (3) |
| Asian | 26 (23) |
| Black/African American | 1 (1) |
| White | 78 (70) |
| Othera | 3 (3) |
| Mean body mass index, kg/m2 (range) | 27.0 (18.1–61.4) |
| Genotype, No. (%) | |
| 3a | 105 (95) |
| 3b | 3 (3) |
| 3 (no confirmed subtype) | 3 (3) |
| Cirrhosis | |
| Yes | 39 (35) |
| No | 72 (65) |
| IL28Bb, No. (%) | |
| CC | 40 (36) |
| CT | 56 (51) |
| TT | 13 (2) |
| Mean HCV RNA, log10 IU/mL (range) | 6.2 (4.4–7.7) |
| Mean ALT, U/L (range) | 98 (18–397) |
| Mean platelets, × 103/μL (range) | 194 (56–420) |
Abbreviations: ALT, alanine aminotransferase; HCV, hepatitis C virus.
aThree patients specified other races: 1 specified American Indian and white, and 2 specified East Indian.
bIL28B genotype results are not available for 2 patients.
Virologic Response
By week 4 of treatment, 108 of 111 patients (97%) had HCV RNA below the LLOQ (Table 2 and Supplementary Table 2). By week 8 of treatment, all 111 patients had HCV RNA below the LLOQ. No patients experienced virologic breakthrough or nonresponse during treatment. Ninety-nine of 111 patients (89% [95% confidence interval, 82%–94%]) achieved SVR12. Sixty-eight of 72 patients without cirrhosis (94%) achieved SVR12, as well as 31 of 39 patients with cirrhosis (79%).
| Characteristic . | Ledipasvir-Sofosbuvir Plus Ribavirin for 12 Weeks (n = 111) . |
|---|---|
| HCV RNA <LLOQ (<15 IU/mL) | |
| Treatment week 4 | 108 (97) |
| Treatment week 12 | 109/110 (99) |
| SVR at 4 weeks | 102 (92) |
| SVR at 12 weeks | 99 (89) |
| 95% CI | 82/94 |
| SVR at 12 weeks by cirrhosis status | |
| Cirrhosis | 31/39 (79) |
| No cirrhosis | 68/72 (94) |
| Virologic failure | |
| Relapse | 8 (7) |
| Lost to follow-up | 3 (3) |
| Death | 1 (1) |
| Characteristic . | Ledipasvir-Sofosbuvir Plus Ribavirin for 12 Weeks (n = 111) . |
|---|---|
| HCV RNA <LLOQ (<15 IU/mL) | |
| Treatment week 4 | 108 (97) |
| Treatment week 12 | 109/110 (99) |
| SVR at 4 weeks | 102 (92) |
| SVR at 12 weeks | 99 (89) |
| 95% CI | 82/94 |
| SVR at 12 weeks by cirrhosis status | |
| Cirrhosis | 31/39 (79) |
| No cirrhosis | 68/72 (94) |
| Virologic failure | |
| Relapse | 8 (7) |
| Lost to follow-up | 3 (3) |
| Death | 1 (1) |
Data are presented as No. (%).
Abbreviations: CI, confidence interval; HCV, Hepatitis C Virus; LLOQ, lower limit of quantification; SVR, sustained virologic response.
| Characteristic . | Ledipasvir-Sofosbuvir Plus Ribavirin for 12 Weeks (n = 111) . |
|---|---|
| HCV RNA <LLOQ (<15 IU/mL) | |
| Treatment week 4 | 108 (97) |
| Treatment week 12 | 109/110 (99) |
| SVR at 4 weeks | 102 (92) |
| SVR at 12 weeks | 99 (89) |
| 95% CI | 82/94 |
| SVR at 12 weeks by cirrhosis status | |
| Cirrhosis | 31/39 (79) |
| No cirrhosis | 68/72 (94) |
| Virologic failure | |
| Relapse | 8 (7) |
| Lost to follow-up | 3 (3) |
| Death | 1 (1) |
| Characteristic . | Ledipasvir-Sofosbuvir Plus Ribavirin for 12 Weeks (n = 111) . |
|---|---|
| HCV RNA <LLOQ (<15 IU/mL) | |
| Treatment week 4 | 108 (97) |
| Treatment week 12 | 109/110 (99) |
| SVR at 4 weeks | 102 (92) |
| SVR at 12 weeks | 99 (89) |
| 95% CI | 82/94 |
| SVR at 12 weeks by cirrhosis status | |
| Cirrhosis | 31/39 (79) |
| No cirrhosis | 68/72 (94) |
| Virologic failure | |
| Relapse | 8 (7) |
| Lost to follow-up | 3 (3) |
| Death | 1 (1) |
Data are presented as No. (%).
Abbreviations: CI, confidence interval; HCV, Hepatitis C Virus; LLOQ, lower limit of quantification; SVR, sustained virologic response.
Of the 12 patients who did not achieve SVR12, 8 patients experienced virologic relapse. Among the 8 patients who relapsed, 5 had cirrhosis. Seven patients relapsed by follow-up week 4, and 1 patient by follow-up week 12. Characteristics of the patients who relapsed are provided in Supplementary Table 3. Three patients were lost to follow-up, each with HCV RNA below the LLOQ at their last treatment visits (at week 8, week 12, and follow-up week 4). One patient died of liver cancer after week 8 of treatment, at which time she had HCV RNA below the LLOQ.
Viral Resistance
Known baseline NS5A RASs (deep sequenced with a 1% cutoff) were detected in 15 of the 106 (14%) patients with evaluable sequencing results. Of these 15 patients, 13 (87%) achieved SVR12, and 2 (13%) experienced virologic relapse. Of the 91 patients with no known NS5A RASs at baseline, 85 (93%) achieved SVR12, and 6 (7%) experienced virologic relapse. NS5B nucleoside inhibitor RASs were detected in 10 of the 104 patients (10%) with evaluable NS5B deep sequencing results, all of whom achieved SVR12.
Virus from all 8 patients who relapsed was sequenced for NS5A and NS5B RASs at baseline and at virologic failure. Two of 8 patients had Y93H at baseline (1.0% and 18.1% of viral population), which was no longer detectable at virologic failure. No NS5A or NS5B RASs were detected at baseline or virologic failure for the remaining 6 patients who relapsed.
Safety
Most patients (85%) experienced at least 1 treatment-emergent adverse event. The most common adverse events were fatigue (51%), headache (36%), and nausea (23%) (Table 3). One patient, a 65-year-old Asian woman, discontinued all study drugs on day 79 of treatment due to hepatic cancer, which was unrelated to study treatment. This patient had no medical history of hepatocellular carcinoma or cholangiocarcinoma; and liver imaging by ultrasound during the study screening period did not identify hepatocellular carcinoma and the patient was undiagnosed until the serious adverse event (SAE) occurred. This patient died 22 days later due to either cholangiocarcinoma or hepatocellular carcinoma (an autopsy to ascertain the tumor type was not performed). During her last visit at treatment week 8, she had HCV RNA below the LLOQ.
| Characteristic . | Ledipasvir-Sofosbuvir Plus Ribavirin for 12 Weeks (n = 111) . |
|---|---|
| Any grade AEs | 94 (85) |
| Grade 3 or 4 AEs | 8 (7) |
| Serious AEs | 4 (4) |
| AEs leading to modification or interruption of any study drug | 5 (5) |
| AEs leading to discontinuation from any study drug | 1 (1) |
| Deaths | 1 (1) |
| AEs occurring in ≥10% of patients | |
| Fatigue | 57 (51) |
| Headache | 40 (36) |
| Nausea | 25 (23) |
| Insomnia | 19 (17) |
| Dizziness | 13 (12) |
| Diarrhea | 11 (10) |
| Irritability | 11 (10) |
| Laboratory abnormalities | |
| Hemoglobin <10 g/dL | 9 (8) |
| Hyperglycemia >250 mg/dL | 2 (2) |
| Prothrombin time >1.50 × ULN | 1 (1) |
| AST >10.00 × ULN | 1 (1) |
| Creatine kinase ≥10.0 × ULN | 1 (1) |
| Lipase >5.0 × ULN | 1 (1) |
| Hyponatremia <125 mEq/L | 1 (1) |
| Total bilirubin >2.5 × ULN | 1 (1) |
| Characteristic . | Ledipasvir-Sofosbuvir Plus Ribavirin for 12 Weeks (n = 111) . |
|---|---|
| Any grade AEs | 94 (85) |
| Grade 3 or 4 AEs | 8 (7) |
| Serious AEs | 4 (4) |
| AEs leading to modification or interruption of any study drug | 5 (5) |
| AEs leading to discontinuation from any study drug | 1 (1) |
| Deaths | 1 (1) |
| AEs occurring in ≥10% of patients | |
| Fatigue | 57 (51) |
| Headache | 40 (36) |
| Nausea | 25 (23) |
| Insomnia | 19 (17) |
| Dizziness | 13 (12) |
| Diarrhea | 11 (10) |
| Irritability | 11 (10) |
| Laboratory abnormalities | |
| Hemoglobin <10 g/dL | 9 (8) |
| Hyperglycemia >250 mg/dL | 2 (2) |
| Prothrombin time >1.50 × ULN | 1 (1) |
| AST >10.00 × ULN | 1 (1) |
| Creatine kinase ≥10.0 × ULN | 1 (1) |
| Lipase >5.0 × ULN | 1 (1) |
| Hyponatremia <125 mEq/L | 1 (1) |
| Total bilirubin >2.5 × ULN | 1 (1) |
Data are presented as No. (%).
Abbreviations: AE, adverse event; AST, aspartate aminotransferase; ULN, upper limit of normal.
| Characteristic . | Ledipasvir-Sofosbuvir Plus Ribavirin for 12 Weeks (n = 111) . |
|---|---|
| Any grade AEs | 94 (85) |
| Grade 3 or 4 AEs | 8 (7) |
| Serious AEs | 4 (4) |
| AEs leading to modification or interruption of any study drug | 5 (5) |
| AEs leading to discontinuation from any study drug | 1 (1) |
| Deaths | 1 (1) |
| AEs occurring in ≥10% of patients | |
| Fatigue | 57 (51) |
| Headache | 40 (36) |
| Nausea | 25 (23) |
| Insomnia | 19 (17) |
| Dizziness | 13 (12) |
| Diarrhea | 11 (10) |
| Irritability | 11 (10) |
| Laboratory abnormalities | |
| Hemoglobin <10 g/dL | 9 (8) |
| Hyperglycemia >250 mg/dL | 2 (2) |
| Prothrombin time >1.50 × ULN | 1 (1) |
| AST >10.00 × ULN | 1 (1) |
| Creatine kinase ≥10.0 × ULN | 1 (1) |
| Lipase >5.0 × ULN | 1 (1) |
| Hyponatremia <125 mEq/L | 1 (1) |
| Total bilirubin >2.5 × ULN | 1 (1) |
| Characteristic . | Ledipasvir-Sofosbuvir Plus Ribavirin for 12 Weeks (n = 111) . |
|---|---|
| Any grade AEs | 94 (85) |
| Grade 3 or 4 AEs | 8 (7) |
| Serious AEs | 4 (4) |
| AEs leading to modification or interruption of any study drug | 5 (5) |
| AEs leading to discontinuation from any study drug | 1 (1) |
| Deaths | 1 (1) |
| AEs occurring in ≥10% of patients | |
| Fatigue | 57 (51) |
| Headache | 40 (36) |
| Nausea | 25 (23) |
| Insomnia | 19 (17) |
| Dizziness | 13 (12) |
| Diarrhea | 11 (10) |
| Irritability | 11 (10) |
| Laboratory abnormalities | |
| Hemoglobin <10 g/dL | 9 (8) |
| Hyperglycemia >250 mg/dL | 2 (2) |
| Prothrombin time >1.50 × ULN | 1 (1) |
| AST >10.00 × ULN | 1 (1) |
| Creatine kinase ≥10.0 × ULN | 1 (1) |
| Lipase >5.0 × ULN | 1 (1) |
| Hyponatremia <125 mEq/L | 1 (1) |
| Total bilirubin >2.5 × ULN | 1 (1) |
Data are presented as No. (%).
Abbreviations: AE, adverse event; AST, aspartate aminotransferase; ULN, upper limit of normal.
Six patients experienced 8 grade 3 adverse events: atrial thrombosis, carpal tunnel syndrome, cellulitis, Clostridium, hypertension, hypertensive crisis, intervertebral disc protrusion, and migraine. Two patients experienced 3 grade 4 adverse events: hepatic cancer, homicidal ideation, and suicidal ideation.
Four patients experienced treatment-emergent SAEs (Supplementary Table 4). One of these patients was the female patient described above. A 53-year-old white man was hospitalized for a hypertensive crisis and hyponatremia in his sixth week of treatment, during which study drug was interrupted. Study drug was resumed 3 days later and this patient completed the course of therapy. A 41-year-old Asian woman experienced a worsening spinal disc protrusion in her twelfth week of treatment. Study drug was not interrupted, and this patient completed the course of therapy. Both patients achieved SVR12. Last, a 66-year-old white man disappeared from his home during week 11 of the study and 3 weeks later exhibited SAEs of potential homicidal and suicidal ideation, neither of which was considered treatment related. He was lost to further follow-up. He had HCV RNA below the LLOQ when last measured at treatment week 8.
Three patients reduced or interrupted ribavirin intake due to drug-related adverse events including insomnia, anemia, and decreased hemoglobin. One was the female patient mentioned above who died. The 2 other patients achieved SVR12. The overall mean decline in hemoglobin from baseline was 0.9 g/dL (standard deviation, 0.77). Postbaseline hemoglobin values decreased to <10 g/dL in 9 patients. No patients had postbaseline hemoglobin values <8.5 g/dL.
Several other laboratory abnormalities occurred that were deemed unrelated to study treatment. One patient had an elevated prothrombin time >1.50 × ULN at week 12, which resolved by posttreatment week 4, and 1 patient had total bilirubin ≥2.5 × ULN throughout the study. Two patients with diabetes had isolated events of hyperglycemia >250 mg/dL at the follow-up week 4 visit. One patient had an isolated event of elevated creatine kinase ≥10.0 × ULN at week 2, and 1 patient with a history of hyponatremia experienced decreased serum sodium of 124 mEq/L.
DISCUSSION
Despite remarkable progress in the treatment of chronic HCV infection, genotype 3 infection remains a clinical challenge, especially when cirrhosis is present. In this study, high SVR rates were achieved in treatment-naive patients with genotype 3 infection with the use of coformulated sofosbuvir and ledipasvir combined with weight-based ribavirin for 12 weeks.
Sofosbuvir is a pangenotypic nucleotide polymerase inhibitor with potent activity against all 6 HCV genotypes in both in vitro replicon assays and extensive clinical use. Ledipasvir is a potent and well-tolerated NS5A inhibitor with activity against replicons of genotypes 1a, 1b, 4, 5, and 6, with 50% effective concentration (EC50) values ranging from 0.006 nM (genotype 1b) to 1.1 nM (genotype 6a). However, ledipasvir is much less active against genotype 3a HCV in vitro, with an average EC50 of 168 nM against wild-type virus [19]. To put this in perspective, the 1000-fold shift seen with the signature Y93H resistance-associated substitution in a genotype 1a virus results in an EC50 of approximately 6 nM with a clinically significant reduction in activity [20]. Hence, one might expect that even at baseline the genotype 3 virus is effectively resistant to ledipasvir.
In the ELECTRON-2 study, treatment-naive patients with genotype 3 infection were randomized to receive 12 weeks of ledipasvir and sofosbuvir with or without ribavirin [14]. In the arm without ribavirin, 16 of 25 (64%) achieved SVR12 whereas all 26 patients randomized to receive therapy with ribavirin achieved SVR12, including 6 patients with compensated cirrhosis. These results clearly show that ribavirin is important but also suggest that ledipasvir is more active against genotype 3 HCV than predicted based on the replicon data alone.
In the FISSION trial, treatment-naive patients with genotype 2 or 3 were randomized to sofosbuvir and ribavirin for 12 weeks or peginterferon and ribavirin for 24 weeks [21]. Among patients with genotype 3 infection who received sofosbuvir and ribavirin for 12 weeks, only 61% (89/145) of those without cirrhosis and 34% (13/38) of those with cirrhosis achieved SVR12. It was only with extension of therapy to 24 weeks in the VALENCE trial that higher rates of SVR12 were achieved in patients with genotype 3 infection: 92% (12/13) with and 93% (86/92) without cirrhosis [22]. However, in our study sofosbuvir, ledipasvir, and ribavirin given for 12 weeks was effective in treatment-naive patients with genotype 3 infection.
The overall SVR12 rate was 89% (99/111), with 68 of 72 (94%) patients without cirrhosis and 31 of 39 (79%) patients with compensated cirrhosis achieving SVR12. Although it is challenging to compare across trials, if ledipasvir was as inactive as the replicon data suggest, one would have expected similar results to those seen with sofosbuvir and ribavirin alone for 12 weeks.
Resistance testing showed that no treatment-emergent mutations were identified in the NS5A or NS5B region in patients who relapsed. Interestingly, for 2 patients with minor viral subpopulations of the NS5A RAS Y93H at baseline, Y93H was no longer detectable at virologic failure. The NS5A RASs evaluated were selected based on their known effect on HCV genotypes that are highly susceptible to ledipasvir (genotype 1, 4, and 6), and it is difficult to interpret the significance of the RASs evaluated in the context of a genotype 3 virus. Although they do result in a further shift in the EC50 in a genotype 3 virus, the significance of these shifts is unknown. No other novel substitutions were noted to emerge in the NS5A gene in those who relapsed.
There are several possible explanations for the better than expected activity of ledipasvir in patients with genotype 3 infection. Despite the relatively higher EC50 values for ledipasvir against the genotype 3 replicons than replicons of other genotypes, ledipasvir is highly concentrated in the liver in animal pharmacokinetic studies, and local intrahepatic concentrations may well reach adequate levels to achieve antiviral activity (unpublished data). Alternatively, it may be that the replicon is an inadequate system to measure all antiviral properties of a given agent. This may be particularly relevant for NS5A inhibitors, for which the mechanism of action remains poorly understood. The NS5A protein is involved in forming a scaffold for the replication complex and is also involved in assembly of nascent virions. The replicon accurately recapitulates HCV RNA replication but it does not result in assembly of viral particles; thus, antiviral properties that occur late in the life cycle, after RNA replication, may not be fully represented in the replicon system. If this is an explanation for the better than expected in vivo activity of ledipasvir, this has important implications for reliance on the replicon system for drug discovery and development. Cell culture assays that can assess all stages of the life cycle are limited, particularly for genotype 3 HCV; however, recent advances that allow replication of serum-derived virus may allow for a deeper investigation into the activity of ledipasvir against genotype 3 HCV [22]. It is also possible that ribavirin and/or sofosbuvir increase the sensitivity of genotype 3 HCV to ledipasvir.
Other recommended regimens for treating genotype 3 HCV include daclatasvir, an NS5A inhibitor, in combination with sofosbuvir, with or without ribavirin depending on the presence of cirrhosis [18, 23, 24]. Daclatasvir has a reported EC50 against genotype 3 HCV replicons ranging from of 0.004 nM to 0.52 nM [25]. ALLY-3+ compared 12 or 16 weeks of sofosbuvir and daclatasvir with ribavirin and showed that SVR12 rates were 83% with 12 weeks and 89% with 16 weeks in treatment-naive patients with Metavir F3 and F4 fibrosis [26]. However, the study was not powered to assess the difference between 12 and 16 weeks in cirrhotic patients, with only 17 and 18 patients with cirrhosis included in each arm, respectively.
Since completion of this trial, studies of velpatasvir, a pangenotypic NS5A inhibitor with picomolar activity against genotype 3 HCV, have been completed, leading to its recent approval in a fixed-dose combination with sofosbuvir in patients of all genotypes [27–29]. In particular, the combination of sofosbuvir and velpatasvir in genotype 3 patients led to an SVR12 rate of 98% (160/163) in treatment-naive patients without cirrhosis and 93% (40/43) in those with cirrhosis [16].
The current study has some relevant limitations. It was a single-arm, open-label study with no control group that included only treatment-naive patients; however, >100 patients were included, leading to relatively robust estimates of safety and efficacy.
In this multicenter study, treatment with sofosbuvir, ledipasvir, and ribavirin given for 12 weeks was effective for treatment-naive patients with genotype 3 HCV without cirrhosis, and less so for patients with compensated cirrhosis. Although ledipasvir and sofosbuvir plus ribavirin is not a recommended treatment for genotype 3 HCV infection by international guidelines, these data suggest that it could be an alternative treatment in settings where preferred treatments are not available.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. J. J. F., R. H. H., C. Y., D. M. M., and J. G. M. contributed to the study concept and design. J. J. F., A. R., S. D. S., B. W., P. M., E. H., M.-L. V., E. S. S., E. T., R. B., C. C., E. Y., S. G., M. E., S. B., and M. S. contributed to the acquisition and interpretation of data. K. C. H. conducted the statistical analysis. All authors contributed to the writing and review of the report.
Acknowledgments. We thank Sandra Chen of Gilead Sciences for writing and editorial assistance.
Financial support. This analysis was supported by Gilead Sciences, Foster City, California.
Potential conflicts of interest. The study sponsor oversaw trial management, data collection, statistical analyses, and the writing and review of the report. J. J. F. has received research support from AbbVie, Gilead, Janssen, Merck, and Regulus, and has served on advisory boards for AbbVie, Gilead, Janssen, and Merck. A. R. has received research support from Merck, Gilead, AbbVie, BMS, Janssen, and Novartis; has served on advisory boards for Gilead, AbbVie, Merck, BMS, and Lupin; and has served as a speaker for Gilead, Merck, AbbVie, BMS, Celgene, and Bayer. S. D. S. has received research support from AbbVie, BMS, Gilead, Janssen, and Merck; has served on advisory boards for BMS, Gilead, and Merck; and has served as a speaker for BMS, Gilead, Merck, and Pfizer. B. W. has received research support from Gilead, AbbVie, Boeringher Ingelheim, BMS, and Intercept; has served on advisory boards for Gilead, AbbVie, Intercept, and Boeringher Ingelheim; and has served as a speaker for Gilead Canada. P. M. has received research support from Gilead and BMS, and has served on advisory boards for Gilead, AbbVie, Merck, and BMS. E. H. has received research support from Janssen, Gilead, Merck, AbbVie, and BMS, and has served on advisory boards and as a speaker for Janssen, Gilead, AbbVie, Merck, and BMS. M.-L. V. has received research support from and has served on advisory boards and as a speaker for AbbVie, Gilead, and Merck. E. T. has received research support from AbbVie, BMS, Gilead, Janssen, and Merck; has served on advisory boards for AbbVie, BMS, Gilead, and Merck; and has served as a speaker for AbbVie, Gilead, Merck, and PendoPharm. R. B. has received research support from AbbVie, Merck, Janssen, Cook, Bayer, Intercept, and Gilead. C. C. has received research support from Merck, Gilead, and AbbVie, and has served on advisory boards and as a speaker for Gilead, AbbVie, Merck, and BMS. E. M. Y. has received research support from Gilead, AbbVie, Merck, Janssen, Springbank, and Intercept, and has served as a speaker for Gilead Canada, AbbVie Canada, Merck Canada, and Celgene Canada. M. E. has received research support from Gilead, AbbVie, Janssen, Intercept, Spring Bank, Celgene, Genentech, and Genfit, and has served on advisory boards and as a speaker for Gilead, AbbVie, Merck, and BMS. S. B. has received research support from Merck, Gilead Sciences, and AbbVie, and has served on advisory boards and as a speaker for Gilead, AbbVie, Merck, and BMS. M. G. S. has received research support from Gilead Sciences, BMS Canada, Janssen, Bayer, Merck Canada, Eisai, Duke University, Hologic, Verlyx Pharma, AbbVie, Lumena Pharmaceuticals LLC, CymaBay Therapeutics, Boehringer Ingelheim, Novartis, Roche Canada, Vertex Pharmaceuticals, Intercept Pharmaceuticals, University Health Network, Hyperion Therapeutics, Pendopharm, Tobira Therapeutics, Springbank Pharmaceuticals, Takeda, Transgene, Genfit, Shire, and Novo Nordisk; has served on advisory boards for Gilead Sciences, Intercept, GRI Inc, BMS, and Merck; and has served as a speaker for Gilead Sciences and Merck. E. S. S., K. C. H., R. H. H., C. Y., B. M., D. M. B., and J. G. M. are employees and stock holders of Gilead Sciences. S. G. reports no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Gilead Sciences.
- liver cirrhosis
- fatigue
- headache
- hepatitis c
- antiviral agents
- canada
- genotype
- nausea
- ribavirin
- safety
- infections
- liver cancer
- hepatitis c virus
- surrogate endpoints
- adverse event
- therapy naive
- sofosbuvir
- compensated cirrhosis
- hepatitis c virus genotype 3
- ledipasvir
- sustained virologic response
- ledipasvir/sofosbuvir




