-
PDF
- Split View
-
Views
-
Cite
Cite
Antonio Rivero-Juarez, Mario Frias, Pedro Lopez-Lopez, María de Los Angeles Risalde, Teresa Brieva, Isabel Machuca, Angela Camacho, Antonio Martinez-Peinado, Jose Carlos Gomez-Villamandos, Antonio Rivero, Hepatitis E Virus (HEV) Infection in Anti-HEV Immunoglobulin G-Carrying Patients After Successful Hepatitis C Virus Treatment: Reactivation or Reinfection?, Clinical Infectious Diseases, Volume 64, Issue 7, 1 April 2017, Pages 964–966, https://doi.org/10.1093/cid/cix004
Close - Share Icon Share
Abstract
Although hepatitis E virus (HEV) is regarded as a self-limiting infection and anti-HEV antibodies seem to protect against reinfection, its pathogenesis is not well established. We describe 2 cases of acute symptomatic HEV infection after hepatitis C therapy in patients carrying anti-HEV immunoglobulin G antibodies, raising 2 major questions: reactivation or reinfection?
Hepatitis C virus (HCV) direct-acting antivirals (DAAs) represent a major milestone in HCV treatment [1]. Different combinations have demonstrated very good safety and efficacy in phase 2 and 3 clinical trials. Nevertheless, patients with several comorbidities have been excluded from these trials, so little is known about their safety in this scenario [1]. In this respect, the US Food and Drug Administration has recently warned about the risk of hepatitis B virus (HBV) reactivation in patients with current or previous HBV infections treated with DAAs [2]. Reactivation could lead to serious clinical complications such as liver failure or death [2]. Consequently, the safety of DAAs in real-life settings should be evaluated in HCV-infected patients with other current or past viral infections.
Hepatitis E virus (HEV) infection is an emerging cause of hepatitis in developed countries [3]. It is mainly transmitted by eating contaminated meat (such as pork) or through direct contact with infected animals [4]. While most infections are asymptomatic, HEV can cause acute hepatitis with or without extrahepatic manifestations, and usually resolves itself [5]. Although it is considered a self-limiting infection mediated by the immune response and anti-HEV antibodies appear to protect against reinfection, its pathogenesis has not been well established. We present 2 cases of HEV infection that occurred shortly after successful HCV therapy with DAAs in patients carrying anti-HEV immunoglobulin G (IgG) antibodies.
CASE 1
A 51-year-old woman was diagnosed with human immunodeficiency virus type 1 (HIV-1) and HCV genotype 1a/1b coinfection in 1986. The first HCV treatment program consisted of 12 weeks with ombitasvir/paritaprevir (OMV/PTV) boosted with ritonavir (RTV) in combination with dasabuvir and weight-adjusted ribavirin (RBV). At pretreatment, the patient was receiving atazanavir boosted with RTV in combination with lamivudine as antiretroviral therapy and showed an undetectable HIV RNA load and a total CD4+ T-cell count of 1049 cells/mL. RTV was suspended during HCV therapy. With respect to HCV infection, at pretreatment, the serum viral load was 1726067 IU/mL (Figure 1A), with a liver stiffness value by transient liver elastography (FibroScan, Echosen, Paris, France) of 9.6 kPa, suggestive of advanced liver fibrosis (F3). Baseline HBV status was core antibody positive and surface antibody positive, compatible with a history of past infection. During the first weeks of therapy, the HCV viral load decreased quickly and was undetectable (detection limit set at 15 IU/mL) at week 4 of treatment. The RBV dose was not readjusted during therapy. Two intermittent episodes of HIV detectable viremia were confirmed at week 8 of therapy (29 IU/mL) and week 4 after complete treatment (103 IU/mL). At week 12 after completion of HCV therapy, the patient attended our unit reporting fever (39.5°C) of 5 days’ duration, nausea, jaundice (total bilirubin of 3.9 mg/dL), and anorexia. Analytical tests revealed aspartate aminotransferase (AST), alanine aminotransferase (ALT), and γ-glutamyl transferase (GGT) levels of 96 U/L, 192 U/L, and 214 U/L, respectively (Figure 1C). HCV RNA and HBV DNA tested undetectable, whereas HEV RNA was positive (71200 copies/mL) (Figure 1A), consistent with HEV genotype 3 (Genbank accesion number: KY362760). Hepatitis A virus (HAV) immunoglobulin M (IgM) antibodies, anti-HBV surface antigen, HBV core antigen, Epstein-Barr virus, cytomegalovirus, and HEV IgM antibodies were all negative. IgG anti-HEV was positive at that time and during follow-up (Figure 1A). Anti-HEV IgM seroconversion was not observed during follow-up (Figure 1A). In 2 weeks, the symptoms ceased and HEV RNA was undetectable (detection limit set at 25 copies/mL). Following retrospective determination of anti-HEV IgG antibodies using a frozen serum sample, the patient showed 4.7 World Health Organization (WHO) units per milliliter. At that point, HEV RNA was undetectable. The patient reported no risk factors of interest for HEV infection.
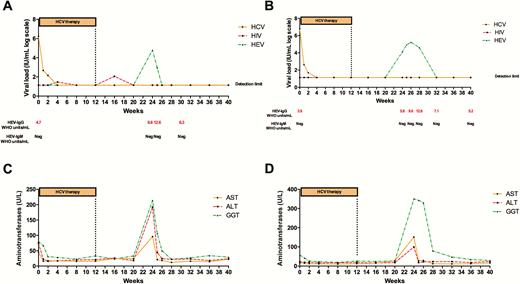
Hepatitis C virus (HCV), human immunodeficiency virus (HIV), and hepatitis E virus (HEV) viral load during HCV therapy and follow-up in case 1 (A) and case 2 (B). The reverse-transcription polymerase chain reaction detection limit for HCV, HIV, and HEV was set at 15 IU/mL, 20 IU/mL, and 25 copies/mL, respectively. Anti-HEV IgG and IgM antibodies were determined using the Wantai Diagnostic enzyme-linked immunosorbent assay kit. Aspartate aminotransferase, alanine aminotransferase, and γ-glutamyl transferase during HCV therapy and follow-up in case 1 (C) and case 2 (D).
CASE 2
A 77-year-old man was diagnosed with HIV-1/HCV genotype 4 coinfection in 1999. The first HCV treatment program consisted of 12 weeks with OMV/PTV boosted with RTV in combination with weight-adjusted RBV. At pretreatment, the patient was receiving darunavir boosted with RTV in combination with abacavir and lamivudine and had an undetectable HIV RNA load and a total CD4+ T-cell count of 644 cells/mL. RTV was suspended during HCV therapy. With respect to the HCV infection, at pretreatment, serum viral load was 60726968 IU/mL with a liver stiffness value by transient liver elastography (FibroScan) of 16.9 kPa, compatible with liver cirrhosis (Figure 1B). Baseline HBV status was core antibody negative and surface antibody positive, compatible with immunization. HCV viral load quickly dropped to undetectable at week 4 of treatment. The RBV dose was adjusted at week 8 of therapy, due to a significant decrease in hemoglobin level (8.6 g/dL). No intermittent episodes of detectable HIV viremia were observed either during HCV therapy or during follow-up. At week 12 after completion of HCV therapy, the patient attended our unit presenting with fever (38.5°C) of 3 days’ duration, vomiting, diarrhea, jaundice (total bilirubin of 4.6 mg/dL), and anorexia. Analytical tests revealed AST, ALT, and GGT levels of 99 U/L, 151 U/L, and 330 U/L, respectively (Figure 1D). HCV RNA and HBV DNA tested undetectable, while HEV RNA was positive (13500 copies/mL) (Figure 1B), consistent with HEV genotype 3 (Genbank accesion number: KY362761). HAV IgM antibodies, anti-HBV surface antigen, HBV core antigen, Epstein-Barr virus, cytomegalovirus, and HEV IgM antibodies were all negative. IgG anti-HEV, but not IgM anti-HEV, was positive at that moment and during the follow-up (Figure 1B). Symptoms were self-limited during the first week of diagnosis, but HEV RNA remained detectable for 4 weeks. Following a retrospective determination of anti-HEV IgG antibodies using a frozen serum sample, the patient showed 3.9 WHO units/mL. The patient did not suffer liver failure or decompensation during the HEV infection. At that point, HEV RNA was undetectable. The patient reported no risk factors of interest for HEV infection.
DISCUSSION
Our study shows 2 cases of acute symptomatic HEV genotype 3 infection in patients carrying anti-HEV IgG antibodies, prompting the major question of whether these were cases of HEV reactivation or reinfection.
Symptomatic HEV infection usually follows the same course as acute self-limiting hepatitis [3, 5]. Nevertheless, its pathogenesis and viral reservoir are not well established. Although serum viral clearance is associated with viral eradication, studies have noted that low viral titers may persist in the liver tissue so that viral reactivation may be possible under specific conditions [6, 7]. In this context, HCV infection inhibits hepatotropic viral replication, as has been described [8]. Consequently, the eradication of HCV following successful treatment could lead to the reactivation of the hepatotropic virus. This phenomenon has been documented in patients coinfected with HCV and HBV, where HCV clearance leads to rapid reactivation of HBV in an interferon-free HCV treatment regimen [2, 9]. It is therefore plausible that residual HEV infection may be reactivated after HCV clearance. This hypothesis could be confirmed in our study, as both patients showed serological markers that suggested past HEV infection, defined as the presence of anti-HEV antibodies. Nevertheless, in contrast to what was observed in the case of HBV infection, the hypothetical HEV reactivation seems to have a different timing, emerging after therapy has ceased. In fact, the 2 patients described here both seemed to have symptoms and detectable HEV RNA at the same time point (during the first 2 months after end of therapy). A possible explanation for this could be the use of drugs employed for HCV therapy. There are no specific target drugs against HEV, although drugs used against HCV infection, such as RBV and sofosbuvir (a viral NS5A inhibitor), have been shown to have antiviral activity against HEV [10]. Hence, a possible HEV reactivation could be contained temporarily due to the administration of HCV DAAs, with HEV infection emerging after completion of treatment. This point requires further investigation.
Another explanation for this observation could be HEV reinfection, which is a controversial topic. Studies conducted with both humans and primate models have shown that IgG anti-HEV antibodies are protective and that a new infection could not establish itself [5]. Although the minimum protective antibody concentration has not been determined as it has for other viral infections, a large randomized clinical trial testing an HEV vaccine suggested that an antibody concentration of 2.5 WHO units/mL might be enough to protect against reinfection by HEV [11]. Nevertheless, in other settings, such as immunocompromised patients, the minimum protective antibody concentration could rise to 7 WHO units/mL [12]. Our patients, although coinfected with HIV, showed normal CD4+ cell counts and sustained virological suppression, carrying an anti-HEV IgG titer higher than the suggested cutoff that could theoretically circumvent a new infection.
Our study has 2 limitations that should be noted. First, we cannot confirm reinfection or reactivation because the viral strain that caused the appearance of anti-HEV IgG antibodies was not available for analysis. Second, there is no consensus or specific seroassay approved for the detection of anti-HEV IgM antibodies, so the enzyme immunoassay employed in our study may have failed to identify IgM seroconversion.
In conclusion, our study shows 2 cases of HEV infection after successful completion of HCV therapy using DAAs in patients previously carrying IgG anti-HEV antibodies. This finding justifies the investigation and reconsideration of HEV pathogenesis.
Notes
Acknowledgments. The authors thank Ismael Zafra-Soto and Laura Ruiz Torres for their technical support.
Author contributions. A. R.-J. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: A. R.-J. and A. R. Analysis and interpretation of the data: A. R.-J., M. F., A. C., A. R. Drafting of the manuscript: A. R.-J. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: A. R.-J. Obtained funding: A. R.-J. Administrative, technical, or material support: A. R.-J., P. L.-L., T. B., M. F., A. M.-P., A. C. Study supervision: A. R.
Financial support. This work was supported by the Ministerio de Sanidad (RD12/0017/0012), integrated into the Plan Nacional de I+D+I and co-financed by the ISCIII-Subdirección General de Evaluación, the Fondo Europeo de Desarrollo Regional, and the Fundación para la Investigación en Salud del Instituto Carlos III (PI16/01297).
Potential conflicts of interest. All authors: No potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Correspondence: A. Rivero-Juarez, Avenida Menendez Pidal s/n, Edificio IMIBIC, Segunda Planta. Laboratorio de Investigación en Enfermedades Infecciosas (GC-03), Despacho 134, 14004, Córdoba, Spain ([email protected]).




