-
PDF
- Split View
-
Views
-
Cite
Cite
Patrick Ingiliz, Stefan Christensen, Torben Kimhofer, Dietrich Hueppe, Thomas Lutz, Knud Schewe, Heiner Busch, Günther Schmutz, Malte H. Wehmeyer, Christoph Boesecke, Karl-Georg Simon, Florian Berger, Jürgen K. Rockstroh, Julian Schulze zur Wiesch, Axel Baumgarten, Stefan Mauss, Sofosbuvir and Ledipasvir for 8 Weeks for the Treatment of Chronic Hepatitis C Virus (HCV) Infection in HCV-Monoinfected and HIV-HCV–Coinfected Individuals: Results From the German Hepatitis C Cohort (GECCO-01), Clinical Infectious Diseases, Volume 63, Issue 10, 15 November 2016, Pages 1320–1324, https://doi.org/10.1093/cid/ciw567
Close - Share Icon Share
Abstract
Background. Shortening the duration of treatment with HCV direct-acting antivirals (DAAs) leads to substantial cost reductions. According to the label, sofosbuvir and ledipasvir can be prescribed for 8 weeks (SL8) in noncirrhotic women or men with HCV genotype 1 and low viral loads. However, real-world data about the efficacy and safety of SL8 are largely missing.
Methods. Interim results from an ongoing prospective, multicenter cohort of 9 treatment centers in Germany (GECCO). All patients started on treatment with HCV DAAs since January 2014 were included. This report describes safety and efficacy outcomes in 210 patients with HCV monoinfection and 35 with human immunodeficiency virus (HIV)–HCV coinfection given SL8 in a real-world setting.
Results. Of 1353 patients included into the GECCO cohort until December 2015, a total of 1287 had complete data sets for this analysis; 337 (26.2%) fulfilled the criteria for SL8 according to the package insert, but only 193 (57.2%) were eventually treated for 8 weeks. Another 52 patients did not fulfill the criteria but were treated for 8 weeks. SL8 was generally well tolerated. The overall sustained virologic response rate 12 weeks after the end of treatment was 93.5% (186 of 199). The on-treatment response rate was 99.4% (159 of 160) in HCV-monoinfected and 96.4% (27 of 28) in HIV-HCV–coinfected patients. Ten patients were lost to follow-up.
Conclusions. SL8 seems highly effective and safe in well-selected HCV-monoinfected and HIV-HCV–coinfected patients in a real-world setting.
In the last decade, the management of patients chronically infected with the hepatitis C virus (HCV) has substantially changed due to the outstanding success of the clinical development of direct-acting antiviral agents (DAAs) [1]. These drugs are characterized by rapid viral decline within days [2–4] owing to inhibition of the viral replication cycle and a favorable tolerability profile compared with interferon alfa. To date, 4 classes of HCV DAAs have made their way into clinical practice, which target 3 viral enzymes: the NS5B polymerase, NS3/4A protease, and the NS5A protein as part of the viral replicase [5, 6]. Combined treatment with ≥2 HCV-DAA classes, with or without ribavirin, has demonstrated higher sustained virologic response (SVR) rates than classic treatment strategies with pegylated interferon alfa and ribavirin, with much shorter treatment durations (8–12 vs 24–48 weeks) and substantially better tolerability. Depending on the subgroup studied, virologic cure rates of >90% have been achieved in most cases [7]. However, earlier real-world treatment data with combination therapy with first-generation protease inhibitors revealed much lower SVR rates than the respective phase 3 trials [8].
Factors associated with virologic treatment failure in HCV-DAA combination trials were HCV genotype, the presence of liver cirrhosis, male sex, the interleukin 28B (IL28B) genotype, or the presence of resistance-associated variants to HCV DAAs [9]. Moreover, most trials with treatment durations <12 weeks led to lower SVR rates [10–12].
In a phase 3 trial, the ION-3 trial, the all-oral fixed-dose combination of ledipasvir (90 mg) plus sofosbuvir (400 mg), given once daily for 8 weeks in treatment-naive noncirrhotic patients with HCV genotype 1, led to overall cure rates of 94% [13]. A post hoc analysis suggested that patients with an HCV viral load <6 million IU/mL, female patients, and patients with a favorable IL28B CC genotype responded as well to the 8-week as to the recommended 12-week treatment course [14, 15]. This finding has led to European and US guidelines implementing this strategy for treatment-naive, noncirrhotic women or men with HCV genotype 1 and low viral loads [16, 17]. To study the efficacy of sofosbuvir plus ledipasvir for 8 weeks (SL8) in a real-world setting in HCV-monoinfected and human immunodeficiency virus (HIV)–HCV–coinfected subjects, we extracted treatment data from a large German real-world cohort (GECCO).
METHODS
The prospective, multicenter German hepatitis C cohort (GECCO) was set up in February 2014 shortly after the European approval of the first NS5B polymerase inhibitor, sofosbuvir, to obtain real-world data for HCV-DAA–based treatments. Nine German treatment centers have been included in the cohort so far: the University Hospital in Bonn, the Gastroenterology Practice in Leverkusen, the Gastroenterology Practice in Herne, the Center for HIV and Hepatogastroenterology in Duesseldorf, the Center for Interdisciplinary Medicine in Muenster, the Infectiology Center in Hamburg, the Infektiologikum in Frankfurt, the Center for Infectiology in Berlin, and the Viral Hepatitis Center of the University Medical Center Hamburg-Eppendorf.
All patients started on HCV-DAA–based regimens were included in the database. Along with HCV-specific variables, such as viral genotype, viral load levels, and treatment information, extensive metadata were recorded for every patient, including demographic characteristics, presence and degree of fibrosis or other comorbid conditions, comedication, and clinical laboratory information from measurements at baseline up to 24 weeks after treatment. The present analysis evaluated interim data from the ongoing database on the treatment effectiveness of SL8, using data from 210 HCV-monoinfected and 35 HIV-HCV–coinfected individuals in a real-world setting.
All laboratory analyses were performed locally. To measure HCV RNA, 5 centers (in Duesseldorf, Herne, Frankfurt and Hamburg) used the Roche COBAS AmpliPrep/COBAS TaqMan HCV Test, version 2.0, with a lower limit of quantification of 15 IU/mL (1.17 log IU/mL); 3 centers (Berlin, Muenster, and Bonn) used the Abbott RealTime HCV assay, with a lower limit of quantification of 12 IU/mL (1.08 log IU/mL); and 1 center (Leverkusen) used both methods in 2 different laboratories. An SVR was defined as an undetectable HCV RNA level 12 weeks after the end of treatment, and relapse was defined as reemergence of a detectable HCV RNA after the end of treatment.
For comparative analyses, normally distributed variables are expressed as means (with standard deviations) and nonnormally distributed variables as medians (with ranges). Pairwise group comparisons were performed with χ² test for categorical variables (eg, sex and cirrhosis status) and with 2-sided t test (or Wilcoxon rank sum test) for normally (or nonnormally) distributed numerical variables (eg, age and serum HCV RNA levels). Group comparisons that resulted in a P value equal to or less than α = .05 were deemed statistically significant. All data analyses were performed using the statistical computing language R (version 3.2.3).
RESULTS
At the time of analysis, 1353 patients with chronic HCV infection were included in the GECCO cohort. To analyze the number of patients who were theoretically eligible for a short-course treatment with ledipasvir-sofosbuvir we excluded 5 patients with double entry due to retreatment and 61 patients from 1 center (Leverkusen) where the method of polymerase chain reaction measurement was not documented and could not be retrieved retrospectively.
Of the remaining 1287 patients, 337 HCV genotype 1 patients (26.2%) fulfilled the criteria for shortening sofosbuvir-ledipasvir treatment to 8 weeks, including 152 women (45.1%), 185 men (54.9%); 42 (12.5%) were HIV coinfected. However, only 193 (57.2%) were eventually treated for 8 weeks (Figure 1). Another 52 patients did not strictly fulfill the criteria but were still given SL8 because they were interferon alfa experienced (n = 38), had HCV genotype 4 (n = 4), were cirrhotic (n = 3), or were men with an HCV RNA level above the threshold of 6 million IU/mL (Roche COBAS TaqMan) or the comparable threshold of 2 million IU/mL (Abbott RealTime) (n = 19)[18].
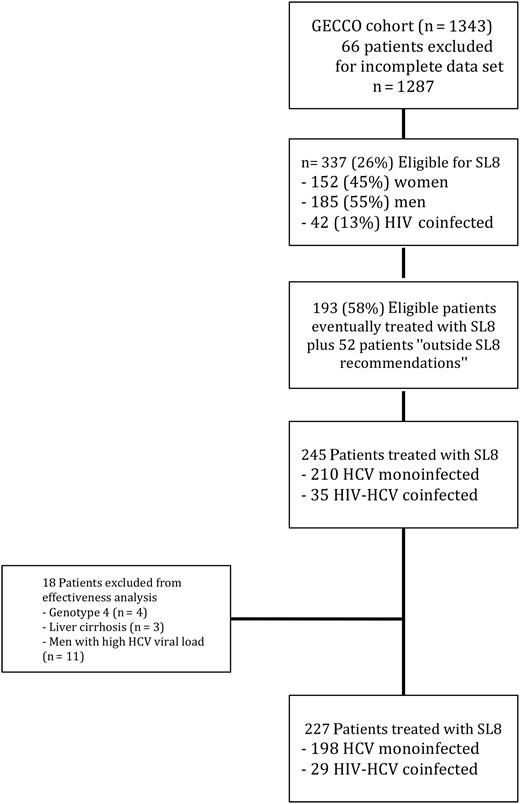
Patients treated with sofosbuvir plus ledipasvir for 8 weeks (SL8) within the German hepatitis C cohort (GECCO). Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus.
The baseline characteristics of the 245 patients receiving SL8 are shown in Table 1, stratified by their HIV status. Overall, 124 patients (51%) were female, and the mean age was 50 years (standard deviation, 12.4 years). The median baseline alanine aminotransferase level was 54 IU/mL (range, 16–649 IU/mL), and the median HCV viral load was 8.3 × 105 IU/mL (12–1.4 × 107 IU/mL).
Baseline Characteristics of the 245 Patients Treated With Sofosbuvir-Ledipasvir for 8 Weeks
| Characteristic . | HCV Monoinfected (n = 210) . | HIV-HCV Coinfected (n = 35) . | P Value . |
|---|---|---|---|
| Male sex, No. (%) | 90 (43) | 31 (89) | <.001 |
| Age, median (range), y | 52 (17–81) | 47 (31–65) | .08 |
| ALT, median (range), U/L | 52 (16–382) | 71 (31–649) | <.001 |
| HCV genotype, No. (%) | |||
| Genotype 1 | 209 (99.5) | 32 (91) | <.01 |
| Genotype 4 | 1 (0.5) | 3 (9) | |
| HCV viral load, median (range), IU/mL | 880 000(1842–14 100 917) | 564 547(12–10 638 832) | .13 |
| HCV transmission, No. (%) | |||
| IDU | 59 (28) | 7 (20) | <.001 |
| Blood products | 54 (26) | … | |
| MSM | … | 15 (43) | |
| Unknown/other | 97 (46) | 13 (37) | |
| Fibroscan value, median (range), kPa | 5.6 (3.1–19.4) | 6.01 (3.7–10.2) | .59 |
| HCV treatment-experienced No. (%) | 33 (15.7) | 4 (11.4) | .68 |
| Met recommended criteria for 8-wk treatment, No. (%)a | 168 (80) | 25 (71) | .58 |
| CD4 cell count, median (range), cells/μL | NA | 601 (152–1446) | NA |
| Opiate substitution treatment, No. (%) | 43 (21) | 2 (6) | .06 |
| Characteristic . | HCV Monoinfected (n = 210) . | HIV-HCV Coinfected (n = 35) . | P Value . |
|---|---|---|---|
| Male sex, No. (%) | 90 (43) | 31 (89) | <.001 |
| Age, median (range), y | 52 (17–81) | 47 (31–65) | .08 |
| ALT, median (range), U/L | 52 (16–382) | 71 (31–649) | <.001 |
| HCV genotype, No. (%) | |||
| Genotype 1 | 209 (99.5) | 32 (91) | <.01 |
| Genotype 4 | 1 (0.5) | 3 (9) | |
| HCV viral load, median (range), IU/mL | 880 000(1842–14 100 917) | 564 547(12–10 638 832) | .13 |
| HCV transmission, No. (%) | |||
| IDU | 59 (28) | 7 (20) | <.001 |
| Blood products | 54 (26) | … | |
| MSM | … | 15 (43) | |
| Unknown/other | 97 (46) | 13 (37) | |
| Fibroscan value, median (range), kPa | 5.6 (3.1–19.4) | 6.01 (3.7–10.2) | .59 |
| HCV treatment-experienced No. (%) | 33 (15.7) | 4 (11.4) | .68 |
| Met recommended criteria for 8-wk treatment, No. (%)a | 168 (80) | 25 (71) | .58 |
| CD4 cell count, median (range), cells/μL | NA | 601 (152–1446) | NA |
| Opiate substitution treatment, No. (%) | 43 (21) | 2 (6) | .06 |
Abbreviations: ALT, alanine aminotransferase; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, injection drug use; MSM, men who have sex with men; NA, not applicable.
a Treatment-naive, noncirrhotic women or men with HCV genotype 1 and low viral loads.
Baseline Characteristics of the 245 Patients Treated With Sofosbuvir-Ledipasvir for 8 Weeks
| Characteristic . | HCV Monoinfected (n = 210) . | HIV-HCV Coinfected (n = 35) . | P Value . |
|---|---|---|---|
| Male sex, No. (%) | 90 (43) | 31 (89) | <.001 |
| Age, median (range), y | 52 (17–81) | 47 (31–65) | .08 |
| ALT, median (range), U/L | 52 (16–382) | 71 (31–649) | <.001 |
| HCV genotype, No. (%) | |||
| Genotype 1 | 209 (99.5) | 32 (91) | <.01 |
| Genotype 4 | 1 (0.5) | 3 (9) | |
| HCV viral load, median (range), IU/mL | 880 000(1842–14 100 917) | 564 547(12–10 638 832) | .13 |
| HCV transmission, No. (%) | |||
| IDU | 59 (28) | 7 (20) | <.001 |
| Blood products | 54 (26) | … | |
| MSM | … | 15 (43) | |
| Unknown/other | 97 (46) | 13 (37) | |
| Fibroscan value, median (range), kPa | 5.6 (3.1–19.4) | 6.01 (3.7–10.2) | .59 |
| HCV treatment-experienced No. (%) | 33 (15.7) | 4 (11.4) | .68 |
| Met recommended criteria for 8-wk treatment, No. (%)a | 168 (80) | 25 (71) | .58 |
| CD4 cell count, median (range), cells/μL | NA | 601 (152–1446) | NA |
| Opiate substitution treatment, No. (%) | 43 (21) | 2 (6) | .06 |
| Characteristic . | HCV Monoinfected (n = 210) . | HIV-HCV Coinfected (n = 35) . | P Value . |
|---|---|---|---|
| Male sex, No. (%) | 90 (43) | 31 (89) | <.001 |
| Age, median (range), y | 52 (17–81) | 47 (31–65) | .08 |
| ALT, median (range), U/L | 52 (16–382) | 71 (31–649) | <.001 |
| HCV genotype, No. (%) | |||
| Genotype 1 | 209 (99.5) | 32 (91) | <.01 |
| Genotype 4 | 1 (0.5) | 3 (9) | |
| HCV viral load, median (range), IU/mL | 880 000(1842–14 100 917) | 564 547(12–10 638 832) | .13 |
| HCV transmission, No. (%) | |||
| IDU | 59 (28) | 7 (20) | <.001 |
| Blood products | 54 (26) | … | |
| MSM | … | 15 (43) | |
| Unknown/other | 97 (46) | 13 (37) | |
| Fibroscan value, median (range), kPa | 5.6 (3.1–19.4) | 6.01 (3.7–10.2) | .59 |
| HCV treatment-experienced No. (%) | 33 (15.7) | 4 (11.4) | .68 |
| Met recommended criteria for 8-wk treatment, No. (%)a | 168 (80) | 25 (71) | .58 |
| CD4 cell count, median (range), cells/μL | NA | 601 (152–1446) | NA |
| Opiate substitution treatment, No. (%) | 43 (21) | 2 (6) | .06 |
Abbreviations: ALT, alanine aminotransferase; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, injection drug use; MSM, men who have sex with men; NA, not applicable.
a Treatment-naive, noncirrhotic women or men with HCV genotype 1 and low viral loads.
Thirty-five patients (15%) were HIV-HCV coinfected; these patients were significantly more often male (P < .001) and had higher alanine aminotransferase, aspartate aminotransferase, and γ-glutamyltransferase levels (P < .01). All HIV-infected patients received antiretroviral treatment, and their median CD4 cell count was 601/μL (range 152–1446/μL).
SL8 was generally well tolerated. The most common documented adverse effects were headache (10%), fatigue (7%), nausea (3%), and arthralgia (2%). One patient stopped treatment after 1 week owing to an allergic reaction. For the effectiveness analysis, we excluded 18 patients who were treated outside the 8-week recommendations owing to a small sample size. Only 1 relapse occurred in this subgroup, in an HIV-coinfected male patient with a high viral load. The 34 interferon-experienced patients remained within the analysis. However, only 4 of these experienced patients were prior nonresponders, and 11 patients were prior relapsers; 14 had stopped interferon-based treatments prematurely for tolerability reasons, and in 5 patients the type of response was unknown.
The proportion of patients with an undetectable HCV RNA level 12 weeks after the end of treatment (SVR12) was 93.5% overall (186 of 199 patients). The SVR12 rate in HCV-monoinfected patients was 93% (159 of 171 patients), compared with 96.4% (27 of 28) in HIV-HCV-coinfected patients. The SVR12 rate in treatment-naive patients was 94% (155 of 166) versus 91% (30 of 33) in treatment-experienced individuals. Only 2 patients (1%) had a virologic failure (relapse), but 10 (5%) were lost to follow-up (Figure 2), and 1 patient stopped treatment prematurely. The on-treatment SVR rate was 99.4% (159 of 160) in HCV-monoinfected and 96.4% (27 of 28) in HIV-HCV–coinfected patients (P = .19).
![Rates of sustained virologic response (undetectable hepatitis C virus [HCV] RNA level) at 12 weeks (SVR12) in the German hepatitis C cohort (GECCO) for the overall cohort and subgroups. The 13 patients without SVR12 included only 2 relapsers; 10 were lost to follow-up, and 1 stopped treatment prematurely. Abbreviations: HIV, human immunodeficiency virus; ITT, intent to treat.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/63/10/10.1093_cid_ciw567/3/m_ciw56702.jpeg?Expires=1750242204&Signature=19aTK3QMV-spCP9IYbkfUF38b1wMiwv5a2gVMEt0ckqL4LL3E6uFLmzubRByZEtF6MmLWY6ZhEhJloqRMdnOxtyl4zYBf9h9a0Gev5ny0j61OCf1shNbFIj4MZIao3zJ7MEs4KumA2DGtMTnTWzuAs7-l1xmCGkXnmpcMUYptAHmKqUHJua~1uC8Le2wLy1A~OIZe761X1p01q5Ky899uN0Nxbs0MTaE7GePQiNIk8xCD2ylTWGko36YHzdKlY26KyF1CG66Wd7OG2a79Vh~x1pDKabaD5QGAx0pdgiLm-sBrmqM-kmTu5iDlhHlA2OxEgj8Zw-7KxgPIDd9sYWX2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Rates of sustained virologic response (undetectable hepatitis C virus [HCV] RNA level) at 12 weeks (SVR12) in the German hepatitis C cohort (GECCO) for the overall cohort and subgroups. The 13 patients without SVR12 included only 2 relapsers; 10 were lost to follow-up, and 1 stopped treatment prematurely. Abbreviations: HIV, human immunodeficiency virus; ITT, intent to treat.
DISCUSSION
These interim results of the German multicenter GECCO cohort show that high SVR rates obtained in clinical trials with 8 weeks of oral sofosbuvir and ledipasvir are reproducible in a real-word setting. We observed an intent-to-treat SVR rate of 93.5% (186 of 199 patients) and an on-treatment SVR rate of 99% (186 of 188) in patients chronically infected with HCV. A small number of HIV-HCV–coinfected patients were also subjected to this treatment regimen, leading to a promising SVR12 rate of 96% (27 of 28 patients).
Moreover, 34 patients receiving SL8 who were not treatment naive but had been treated with interferon-based treatments in the past showed an on-treatment SVR of 97% (30 of 31). However, only 4 of those patients were prior nonresponders, 9 were relapsers, and most patients had interrupted interferon-based treatment owing to toxicity and therefore cannot be considered to have had true prior treatment failure. Prior nonresponders to interferon-based therapies have historically been among the hardest-to-treat patient groups, whereas prior relapsers tend to respond better [19], but in the sofosbuvir-ledipasvir trials for pretreated patients, shortening of treatment was generally not allowed [20].
In a mainly white population, the interim results of GECCO-01 confirm the results of the ION-3 phase 3 trial and are indeed encouraging, because a shorter treatment duration might reduce costs and therefore enable more patients to have access to treatment. Several phase 2 trials investigating treatment duration of 8 weeks have been conducted in HCV genotype 1 patients in the last couple of years. The C-WORTHY trial studied the combination of grazoprevir and elbasvir in HCV-monoinfected and HIV-HCV–coinfected patients for 8–12 weeks, and the 8-week-arm led to an SVR rate of 80% [12]. In the Aviator trial, the 8-week arm of paritaprevir/ritonavir/ombitasvir/dasabuvir led to a cure rate of 88% [21]. More recently, the combination of velpatasvir and sofosbuvir for 8 weeks in treatment-naive genotype 1 patients led to response rates between 81% and 87%m, depending on the velpatasvir dosage [22]. In the ALLY-2 trial, an 8-week treatment course of daclatasvir and sofosbuvir in HIV-HCV–coinfected patients led to a disappointing response rate of 76% [10]. A baseline HCV viral load >2 million IU/mL (COBAS TaqMan HCV Test 2.0) was associated with relapse in that trial.
Notably, HIV-HCV–coinfected patients showed response rates similar to those of HCV-monoinfected patients in HCV-DAA combination trials [23, 24]. In the GECCO cohort, a small number of HIV-HCV–coinfected patients were treated with SL8, with an excellent response rate. The HIV-infected patients in the GECCO cohort were all receiving antiretroviral treatment and had a stable immunological situation and a comparatively low HCV viral load. We speculate that the good response rate in our HIV-infected patients is related partly to the fact that SL8 has a low potential for drug-drug interactions with HIV drugs [25] and partly to the fact that our patients were favorably preselected by their treating physicians, especially with regard to HCV viral load. Consequently, we conclude that SL8 might be safe and effective in selected HIV-HCV–coinfected patients. However, it will be important to confirm these first results in larger trials with HIV/HCV–coinfected patients.
The GECCO cohort has several other limitations. First, the population consists mainly of German patients of European ancestry origin. Conclusions can therefore not be drawn for other ethnic groups, such as African Americans, who have been shown to respond less well to this regimen [26]. Moreover, other factors related to treatment response, such as IL28B genotype and HCV resistance tests, have not been systematically documented.
In conclusion, data from the real-world GECCO cohort confirm that SL8 is highly effective in well-selected patients with chronic HCV infection. The recommended HCV RNA thresholds seem applicable to clinical practice. HIV-HCV–coinfected patients also respond well to this regimen and might be considered for it if baseline factors are favorable. Future guidelines should carefully take these findings into account.
Notes
Financial support. J. S. z. W. is supported by the German Research Agency (DFG) and the German Center for Infection research (DZIF).
Potential conflicts of interest. P. I. has served on speakers bureaus for Abbvie, Bristol-Myers Squibb (BMS), Gilead, Janssen, and Merck Sharp & Dohme (MSD) and advisory boards for Gilead, Abbvie, and ViiV. S. C. has served on speakers bureaus for Abbvie, BMS, Gilead, Janssen and advisory boards for Abbvie, BMS, Gilead, Janssen, MSD, and ViiV. D. H. has served on speakers bureaus for Abbvie, BMS, Gilead, and Janssen and advisory boards for Abbvie, BMS, and Gilead. K. S. has served on speakers bureaus and advisory boards for Abbvie, BMS, Gilead, Janssen, MSD, and Hexal. M. H. W, has served as a speaker for MSD. C. B. has served as a consultant and on speakers bureaus for Abbvie, ViiV, BMS, MSD, Gilead, and Janssen. K. G. S. has served on speakers bureaus for Abbvie, BMS, Gilead, Janssen, and Norgine and on advisory boards for Abbvie, BMS, Gilead, Janssen, and MSD. J. K. R. has served on speakers bureaus and advisory boards for Abbvie, BMS, Gilead, Janssen, and MSD. J. S. z. W. has served as a consultant for Gilead. A. B. has served on speakers bureaus for Abbvie, BMS, Gilead, and Janssen and advisory boards for Abbvie, BMS, Gilead, and MSD. S. M. has served on speakers bureaus for Abbvie, BMS, Gilead, Janssen and advisory boards for Abbvie, BMS, Gilead, Janssen, MSD, and ViiV. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
Author notes
P. I. and S. C. contributed equally to this work.




