-
PDF
- Split View
-
Views
-
Cite
Cite
Samuel P. Costello, Emily C. Tucker, Justin La Brooy, Mark N. Schoeman, Jane M. Andrews, Establishing a Fecal Microbiota Transplant Service for the Treatment of Clostridium difficile Infection, Clinical Infectious Diseases, Volume 62, Issue 7, 1 April 2016, Pages 908–914, https://doi.org/10.1093/cid/civ994
Close - Share Icon Share
Abstract
Recurrent or refractory Clostridium difficile infection (CDI) has become an increasing problem in the past decade. Fecal microbiota transplant (FMT) is a highly efficacious treatment for recurrent CDI; however, a number of technical, logistical, and regulatory issues have hampered the development of an FMT capability at many hospitals. The development of a frozen stool bank of screened donor stool is an important step in the standardization of the procedure. This gives clinicians rapid access to thoroughly screened donor stool when needed, without the ethical and logistical problems associated with patient-selected donors. We describe the practicalities of establishing such a service using a stool bank of prescreened donor stool including detail regarding donor recruitment and screening, stool preparation, and delivery of the FMT.
Clostridium difficile is the most common cause of healthcare associated diarrhea, and its incidence has been increasing in the last decade [1]. The recent emergence of hypervirulent strains has also led to increased morbidity and mortality associated with the infection [2]. A major impediment to the successful treatment of patients with C. difficile infection (CDI) has been the 25%–30% rate of relapse following antibiotic therapy [3]. In those patients who do relapse, further antibiotic treatments give diminishing rates of cure in that, after a second recurrence, the chance of further recurrence increases to 60%, and it is even greater for subsequent recurrences [3].
The problem of recurrent CDI (rCDI) is thought to result from an underlying deficiency of the microbiome as well as, in some patients, defective antibody mediated immunity [4]. Patients with CDI usually have a depleted commensal flora with reduced diversity of organisms [5]. This is commonly caused by previous exposure to antibiotics or is a manifestation of other conditions such as ulcerative colitis [6]. Traditional antibiotic therapy for CDI can perpetuate this dysbiosis, thus leaving an ecological void into which C. difficile emerges and proliferates [7]. Fecal microbiota transplant (FMT) is thought to be effective as it replenishes the colonic microbial diversity by providing a new and diverse microbiome that occupies the niche into which C. difficile would otherwise multiply [5].
FMT is by far the most successful treatment for rCDI, with primary cure rates between 81% and 94% [8–12]. A randomized control trial of FMT delivered via duodenal infusion for rCDI demonstrated symptom resolution in 81% of patients receiving a single FMT compared to only 31% receiving vancomycin and 23% receiving vancomycin with bowel lavage. This study was stopped early after an interim analysis revealed the clear superiority of FMT over vancomycin therapy [10]. It is therefore now incumbent upon hospitals to establish FMT services so that rCDI can be effectively managed with this therapy. However, from a large institutional viewpoint, there are technical and logistical issues in establishing such a nonstandardized, nondrug therapy with appropriate safety and governance.
ESTABLISHING AN FMT SERVICE
An FMT service should be run in a standardized and efficient manner that allows safe and effective treatment to be delivered in a timely fashion. This is important because patients with rCDI are often unwell, elderly patients with comorbidities that make prompt treatment critical. The development of a frozen stool bank is the optimal way to standardize the FMT process and allow stool to be available on demand [11]. This is particularly important in cases where delayed treatment could result in colectomy [13]. A frozen stool bank also allows fecal donors to be recruited and thoroughly screened ahead of time, in a methodical manner, without time pressure. Donor stool banks can be established at individual treatment centers or stool can be shipped from a centralized stool bank such as the not for profit organization “open-biome” in the United States [14].
Historically, patients undergoing FMT would often select their own stool donor from family or friends, and this known donor would then undergo screening prior to giving their stool donation. This creates delays with therapy; moreover, there are a number of potential problems with this approach. These include the possibility of coercion of donors and also ethical and confidentiality concerns regarding screening known donors in the event that disease is found in a donor or transmitted to the recipient. Stringent exclusion criteria can be more easily and dispassionately applied to volunteer donors from the community than recipient-directed donors as there are a greater number of potential candidates and no perceived personal obligation between recipients and donors. There is also evidence from blood transfusion safety analyses that recipient-directed donors are more likely to test positive for infectious disease than unrelated volunteer donors [15]. Depending on stool weight, up to 8 treatments can be produced from each stool donation, and donors to the frozen stool bank can give multiple samples over a short period of time, making the process more economical [16]. Lastly, stool contains viable bacteria after 6 months of frozen storage at −80°C [11], and in case series, frozen stool appears to be as effective as fresh stool for treating patients with rCDI [11, 16, 17]. For these reasons, units such as ours have moved to only using prescreened, unrelated donors who are anonymous to the recipient.
COMMON PROBLEMS ASSOCIATED WITH ESTABLISHING AN FMT SERVICE
A major difficulty in establishing FMT services around the world has been regulatory restriction and uncertainties surrounding the practice [18]. This has, in part, occurred due to concern regarding potential risk to patients and the nonstandardized nature of donor feces. To date the short-term risk of FMT for rCDI is documented to be very low, and there have not been any directly attributable long-term adverse effects reported although there are currently little long-term data [9, 19]. Despite randomized control trial efficacy data, the US Food and Drug Administration (FDA) have implemented a policy of “enforcement discretion” regarding FMT only for rCDI after initially imposing a moratorium on the procedure [20]. There has also been difficulty classifying FMT, whether as a therapeutic “drug” or tissue [21]. In the authors' opinion, feces for FMT would be better classified as a bodily tissue donation in a similar way that blood and blood donation are regarded. Stool is derived from human donors and is not a “standard product” as are manufactured drugs. In Australia [22] and many European countries [23], the development of FMT services has been left to local health administrations rather than national organizations such as the FDA in the United States. Therefore, there remains a need for consensus guidelines to achieve greater standardization of the procedure.
Funding for FMT services can also be problematic with most third-party payers not rebating or covering the costs of individual FMT treatments or service establishment and delivery. Our FMT service has been established as a linked benefit from a clinical trial examining FMT in ulcerative colitis from research funds. However, for long-term sustainability, clinical funding from the health service will be needed.
RECRUITMENT AND SCREENING OF DONORS
Donor screening is expensive and time consuming, and therefore recruiting donors who are more likely to pass screening is advantageous. We found young, healthy donors by advertising at a nearby university. There is currently no evidence that donor characteristics or “enterotypes” predict the success of FMT treatment for CDI, and so donor screening focuses on risk reduction rather than increasing the therapeutic effect.
Donor screening has a high exclusion rate with rates of donor eligibility as low as 10% in an Australian study where donors were sought by advertisement to the general community [24]. We have found that even when targeting young, apparently healthy university students only 14 (31%) of 44 respondents were eligible donors after completing screening (Figure 1). When screening potential stool donors, it is advisable to take the medical history and physical examination prior to stool and then blood screening, because the majority of candidates are excluded on history and stool testing, thus avoiding the additional costs of blood testing (Table 1).
| Medical interview (exclusions) |
| Age: <18 or >65 |
| Antimicrobial therapy or probiotics in the past 3 mo |
| Active medical illness or symptoms |
| Any medications |
| International travel in last 6 mo to areas at high risk of travelers' diarrhea |
| High risk sexual activity (unprotected sex in last 3 mo outside of a monogamous relationship, men who have sex with men, sex for drugs or money) |
| Illicit drug use |
| Tattoo or body piercing within 6 mo |
| Known HIV or viral hepatitis exposure in the last 12 mo |
| Incarceration or a history of incarceration. |
| Family history of colorectal carcinoma involving 2 or more first degree relatives |
| Household members with active GI infection |
| Medical history and Examination (exclusions) |
| Any gastrointestinal disorder |
| Obesity (BMI > 30),hypertension, type 2 diabetes and dyslipidaemia |
| Malnutrition (BMI < 18) |
| Autoimmune disease |
| Atopic disease |
| Depression |
| Infection with HIV, Syphilis, Hepatitis B or C |
| Malignancy |
| Chronic pain syndromes, neurologic or neurodevelopmental disorders |
| Blood screening |
| Full blood count |
| Electrolytes, Urea and Creatinine |
| Liver function tests |
| Human T-cell lymphotropic virus 1 and 2 serology |
| Epstein Barr Virus IgM and IgG |
| Cytomegalovirus IgM and IgG |
| Syphilis (Rapid plasma reagin) |
| Strongyloides stercoralis, Entamoeba histolytica, Helicobacter pylori serology |
| Hepatitis A virus IgM |
| Hepatitis B surface Antigen, Hepatitis B core Antibody, Hepatitis C virus Antibody |
| HIV type 1 and 2 Antibody and p24 antigen |
| Antinuclear antibody |
| Fasting lipids and Blood sugar level |
| C-Reactive Protein and Erythrocyte Sedimentation Rate |
| Stool screening |
| Microscopy and Culture |
| Rotavirus, Norovirus, and Adenovirus PCR |
| Clostridium difficle toxin PCR |
| Egg, cysts and parasites (including Cryptosporidium spp., Giardia spp., Dientamoeba fragilis and Entamoeba histolytica PCR) |
| Vancomycin resistant enterococcus screen |
| Medical interview (exclusions) |
| Age: <18 or >65 |
| Antimicrobial therapy or probiotics in the past 3 mo |
| Active medical illness or symptoms |
| Any medications |
| International travel in last 6 mo to areas at high risk of travelers' diarrhea |
| High risk sexual activity (unprotected sex in last 3 mo outside of a monogamous relationship, men who have sex with men, sex for drugs or money) |
| Illicit drug use |
| Tattoo or body piercing within 6 mo |
| Known HIV or viral hepatitis exposure in the last 12 mo |
| Incarceration or a history of incarceration. |
| Family history of colorectal carcinoma involving 2 or more first degree relatives |
| Household members with active GI infection |
| Medical history and Examination (exclusions) |
| Any gastrointestinal disorder |
| Obesity (BMI > 30),hypertension, type 2 diabetes and dyslipidaemia |
| Malnutrition (BMI < 18) |
| Autoimmune disease |
| Atopic disease |
| Depression |
| Infection with HIV, Syphilis, Hepatitis B or C |
| Malignancy |
| Chronic pain syndromes, neurologic or neurodevelopmental disorders |
| Blood screening |
| Full blood count |
| Electrolytes, Urea and Creatinine |
| Liver function tests |
| Human T-cell lymphotropic virus 1 and 2 serology |
| Epstein Barr Virus IgM and IgG |
| Cytomegalovirus IgM and IgG |
| Syphilis (Rapid plasma reagin) |
| Strongyloides stercoralis, Entamoeba histolytica, Helicobacter pylori serology |
| Hepatitis A virus IgM |
| Hepatitis B surface Antigen, Hepatitis B core Antibody, Hepatitis C virus Antibody |
| HIV type 1 and 2 Antibody and p24 antigen |
| Antinuclear antibody |
| Fasting lipids and Blood sugar level |
| C-Reactive Protein and Erythrocyte Sedimentation Rate |
| Stool screening |
| Microscopy and Culture |
| Rotavirus, Norovirus, and Adenovirus PCR |
| Clostridium difficle toxin PCR |
| Egg, cysts and parasites (including Cryptosporidium spp., Giardia spp., Dientamoeba fragilis and Entamoeba histolytica PCR) |
| Vancomycin resistant enterococcus screen |
Abbreviations: BMI, body mass index; GI, gastrointestinal; HIV, human immunodeficiency virus; IgG, immunoglobulin G; IgM, immunoglobulin M; PCR, polymerase chain reaction.
| Medical interview (exclusions) |
| Age: <18 or >65 |
| Antimicrobial therapy or probiotics in the past 3 mo |
| Active medical illness or symptoms |
| Any medications |
| International travel in last 6 mo to areas at high risk of travelers' diarrhea |
| High risk sexual activity (unprotected sex in last 3 mo outside of a monogamous relationship, men who have sex with men, sex for drugs or money) |
| Illicit drug use |
| Tattoo or body piercing within 6 mo |
| Known HIV or viral hepatitis exposure in the last 12 mo |
| Incarceration or a history of incarceration. |
| Family history of colorectal carcinoma involving 2 or more first degree relatives |
| Household members with active GI infection |
| Medical history and Examination (exclusions) |
| Any gastrointestinal disorder |
| Obesity (BMI > 30),hypertension, type 2 diabetes and dyslipidaemia |
| Malnutrition (BMI < 18) |
| Autoimmune disease |
| Atopic disease |
| Depression |
| Infection with HIV, Syphilis, Hepatitis B or C |
| Malignancy |
| Chronic pain syndromes, neurologic or neurodevelopmental disorders |
| Blood screening |
| Full blood count |
| Electrolytes, Urea and Creatinine |
| Liver function tests |
| Human T-cell lymphotropic virus 1 and 2 serology |
| Epstein Barr Virus IgM and IgG |
| Cytomegalovirus IgM and IgG |
| Syphilis (Rapid plasma reagin) |
| Strongyloides stercoralis, Entamoeba histolytica, Helicobacter pylori serology |
| Hepatitis A virus IgM |
| Hepatitis B surface Antigen, Hepatitis B core Antibody, Hepatitis C virus Antibody |
| HIV type 1 and 2 Antibody and p24 antigen |
| Antinuclear antibody |
| Fasting lipids and Blood sugar level |
| C-Reactive Protein and Erythrocyte Sedimentation Rate |
| Stool screening |
| Microscopy and Culture |
| Rotavirus, Norovirus, and Adenovirus PCR |
| Clostridium difficle toxin PCR |
| Egg, cysts and parasites (including Cryptosporidium spp., Giardia spp., Dientamoeba fragilis and Entamoeba histolytica PCR) |
| Vancomycin resistant enterococcus screen |
| Medical interview (exclusions) |
| Age: <18 or >65 |
| Antimicrobial therapy or probiotics in the past 3 mo |
| Active medical illness or symptoms |
| Any medications |
| International travel in last 6 mo to areas at high risk of travelers' diarrhea |
| High risk sexual activity (unprotected sex in last 3 mo outside of a monogamous relationship, men who have sex with men, sex for drugs or money) |
| Illicit drug use |
| Tattoo or body piercing within 6 mo |
| Known HIV or viral hepatitis exposure in the last 12 mo |
| Incarceration or a history of incarceration. |
| Family history of colorectal carcinoma involving 2 or more first degree relatives |
| Household members with active GI infection |
| Medical history and Examination (exclusions) |
| Any gastrointestinal disorder |
| Obesity (BMI > 30),hypertension, type 2 diabetes and dyslipidaemia |
| Malnutrition (BMI < 18) |
| Autoimmune disease |
| Atopic disease |
| Depression |
| Infection with HIV, Syphilis, Hepatitis B or C |
| Malignancy |
| Chronic pain syndromes, neurologic or neurodevelopmental disorders |
| Blood screening |
| Full blood count |
| Electrolytes, Urea and Creatinine |
| Liver function tests |
| Human T-cell lymphotropic virus 1 and 2 serology |
| Epstein Barr Virus IgM and IgG |
| Cytomegalovirus IgM and IgG |
| Syphilis (Rapid plasma reagin) |
| Strongyloides stercoralis, Entamoeba histolytica, Helicobacter pylori serology |
| Hepatitis A virus IgM |
| Hepatitis B surface Antigen, Hepatitis B core Antibody, Hepatitis C virus Antibody |
| HIV type 1 and 2 Antibody and p24 antigen |
| Antinuclear antibody |
| Fasting lipids and Blood sugar level |
| C-Reactive Protein and Erythrocyte Sedimentation Rate |
| Stool screening |
| Microscopy and Culture |
| Rotavirus, Norovirus, and Adenovirus PCR |
| Clostridium difficle toxin PCR |
| Egg, cysts and parasites (including Cryptosporidium spp., Giardia spp., Dientamoeba fragilis and Entamoeba histolytica PCR) |
| Vancomycin resistant enterococcus screen |
Abbreviations: BMI, body mass index; GI, gastrointestinal; HIV, human immunodeficiency virus; IgG, immunoglobulin G; IgM, immunoglobulin M; PCR, polymerase chain reaction.
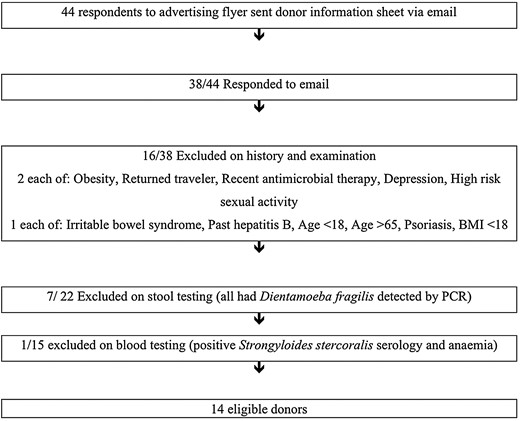
Donor recruitment. Abbreviations: BMI, body mass index; PCR, polymerase chain reaction.
The risk of infection transmission is minimized with a thorough history for known exposures or risk factors as well as stool and blood screening. A number of atopic, autoimmune, psychiatric, malignant, and neurological diseases are associated with gut dysbiosis, and so donors with these conditions are excluded [7]. Transmission of an obese phenotype has been demonstrated in animal studies [25], and the possible transmission of obesity has been reported in a single human case report [26]. Also, increased insulin sensitivity has been demonstrated in obese subjects following duodenal infusion of feces from lean donors [27]. Given these findings, elements of the metabolic syndrome should also be donor exclusions. Our screening protocol has evolved and is adapted from previous guidelines [10, 12, 28] (Table 1). Screening guidelines should reflect the risk of diseases applicable to the local population as this may vary depending on the geographical location of donor recruitment.
PROCESSING STOOL
Once screening is completed, stool should be collected from an individual donor within 1 month [28] (Figure 2). Alternatively, screening can be undertaken both before and after a period of donation to ensure that all stool collected and frozen between the 2 dates is safe [17]. We give donors a clean opaque plastic bag that can be opened over a toilet to collect the stool and then sealed with a cable tie and placed in a larger ziplock bag. Donors have the option of donating on site or taking the bag home with a cooler box and an ice pack so it can be delivered within 1 hour of defecation. Stool can be stored for up to 8 hours at 4°C without significant impact on bacterial survival, but viability declines at room temperature or at 4°C for more than 8 hours [29].
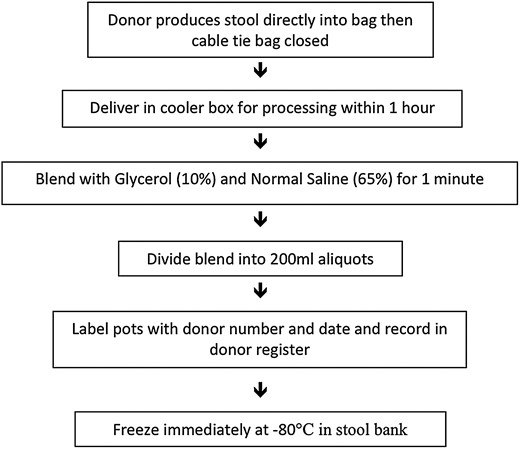
Approximately 50 g of stool is required for each treatment [8]. Fresh stool (25%) should be blended with normal saline (65%) and pharmaceutical grade glycerol (10%). This ratio maximizes the amount of stool in suspension without being too viscous to deliver via the biopsy channel of a colonoscope or naso-duodenal tube. Glycerol is used to retain bacterial viability in the frozen stool preparations [11]. The entire donor stool can be placed in the blender to make as many 200 mL aliquots as possible with a minimum of 50 g stool content. Once blending is complete, the stool mixture should be aliquoted into individual cryotolerant pots and immediately frozen at −80°C. We use 250 mL pots filled with 200 mL of stool suspension as the liquid expands on freezing. In our experience, the number of aliquots obtained from a single stool can vary from insufficient for a single preparation to 8 aliquots. After 57 individual stool donations from 14 donors, we have found a wide range in donor stool size from 7 g to 436 g with a median 105 g (IQR 51–220 g). The mean stool weight was significantly greater for male donors (172 g, CI, 122–213 g) than female donors (92 g, CI, 67–118 g) P = .006.
We conduct the blending in an anaerobic chamber to reduce operator exposure, and it has the theoretical advantage of preventing oxygen exposure to obligate anaerobic bacteria during the blending process. However, there is evidence that processing and freezing stool under aerobic conditions is also clinically effective [17, 30] and can be conducted without a fume hood as exposure risk is probably less than for colonoscopy (given the rigorous stool screening) [7, 30]. Blending for 1 minute produces a suspension with sufficiently small particle size for it to be easily drawn into a catheter tip syringe and flushed down a biopsy channel of a colonoscope with the cap removed. There is no need to strain the blended suspension for colonoscopic or enema delivery. We have conducted more than 80 FMT procedures using colonoscopic delivery of blended stool without filtration with no instances of colonoscope channel or syringe blockage. Many butyrate producing colonic bacteria require fiber as a substrate [31] and so there are also theoretical reasons for not removing fibrous material from the suspension. However, if delivery is via a naso-duodenal or naso-gastric tube, filtering is required to prevent tube blockage.
If a blender or an autoclave are not available, the FMT suspension can be prepared by combining stool, saline, and glycerol in the collection bag and manually agitating the contents. Alternatively, stool can be mixed directly in the plastic storage container with a spatula [30] or shaken in a bottle of normal saline [32]. Although these methods are simple, they can result in a suspension with large unsuspended particles that can block the syringe, and so filtering the suspension is often required.
Stool donors should be given an identification number that is marked onto each pot of stool suspension with the date the sample was produced. This identification number should be recorded in a secure donor document along with contact details and screening results so that the donor is de-identified to the recipient but can be traced in the event of illness developing in the recipient.
CLEANING EQUIPMENT
Cleaning the equipment between donor stool processing is important to minimize the risk of cross contamination. We use a blender that has a stainless steel container (Waring SS515) with Teflon seals and a stainless steel lid both of which can be sterilized with an autoclave as this is the best infection control practice [33]. The blender container and spoon must be cleaned to remove all residue prior to autoclaving. We use an enzymatic wash and then detergent wash followed by a water rinse and then autoclave both at 121°C for 20 minutes. The container and spoon are then autoclaved again immediately prior to next use. Given donor stool is screened for potential pathogens, it is likely to be safer than stool encountered during routine colonoscopy. Therefore, we believe endoscope cleaning following FMT should follow standard protocols.
The equipment required to establish such a service with a frozen stool bank is listed in Table 2.
| Equipment Required . |
|---|
| Opaque plastic bags and cable ties |
| Cooler boxes and ice packs |
| Blender with autoclaveable container |
| Stainless steel spoon |
| Normal saline |
| Pharmaceutical grade glycerol |
| Safe work bench on which to blend stool (fume hood or anaerobic chamber ideally) |
| −80°C Freezer |
| Cryotolerant screw top containers (250 mL) |
| Catheter tip syringes (60 mL) |
| Personal protective equipment–gloves, gown, face shield |
| Autoclave |
| Equipment Required . |
|---|
| Opaque plastic bags and cable ties |
| Cooler boxes and ice packs |
| Blender with autoclaveable container |
| Stainless steel spoon |
| Normal saline |
| Pharmaceutical grade glycerol |
| Safe work bench on which to blend stool (fume hood or anaerobic chamber ideally) |
| −80°C Freezer |
| Cryotolerant screw top containers (250 mL) |
| Catheter tip syringes (60 mL) |
| Personal protective equipment–gloves, gown, face shield |
| Autoclave |
| Equipment Required . |
|---|
| Opaque plastic bags and cable ties |
| Cooler boxes and ice packs |
| Blender with autoclaveable container |
| Stainless steel spoon |
| Normal saline |
| Pharmaceutical grade glycerol |
| Safe work bench on which to blend stool (fume hood or anaerobic chamber ideally) |
| −80°C Freezer |
| Cryotolerant screw top containers (250 mL) |
| Catheter tip syringes (60 mL) |
| Personal protective equipment–gloves, gown, face shield |
| Autoclave |
| Equipment Required . |
|---|
| Opaque plastic bags and cable ties |
| Cooler boxes and ice packs |
| Blender with autoclaveable container |
| Stainless steel spoon |
| Normal saline |
| Pharmaceutical grade glycerol |
| Safe work bench on which to blend stool (fume hood or anaerobic chamber ideally) |
| −80°C Freezer |
| Cryotolerant screw top containers (250 mL) |
| Catheter tip syringes (60 mL) |
| Personal protective equipment–gloves, gown, face shield |
| Autoclave |
PATIENT SELECTION
The decision to proceed with FMT should be made on an individual patient basis; however, there are 3 main factors that influence the decision: the number of CDI recurrences, the severity of the episode, and whether the disease is refractory to antimicrobial therapy [28]. More than 2 relapses of CDI following antimicrobial therapy gives <35% chance that subsequent antimicrobial therapy alone will be successful. In these patients FMT offers a much higher chance of success [9, 10]. A severe infection with CDI resulting in shock or requiring supportive care in hospital in which recurrence of CDI could be life threatening is another indication, as is moderate disease not responding to antimicrobial therapy for at least 1 week [28]. Gastrointestinal perforation is an absolute contraindication and anaphylactic food allergy a relative contraindication. Severe immunosuppression had previously been regarded as a contraindication; however, FMT in patients with at least moderate immunosuppression appears safe [34]. Patients with toxic megacolon should be offered subtotal colectomy in the first instance, and FMT via colonoscopy is contraindicated in these patients. In those refusing surgery FMT via the upper gastrointestinal (GI) route can be cautiously considered and has resulted in cure [13].
CONSENT
There have been 2 deaths directly attributable to FMT with both of these patients developing aspiration pneumonia. The first patient aspirated during sedated endoscopic FMT delivery to the duodenum [35], and the other aspirated during the anesthetic for colonoscopic FMT [34]. One other death following FMT occurred due to toxic megacolon and sepsis [36]; however, this may have been attributable to the recurrence of the underlying CDI or a gastrostomy tube leak and not the FMT. There have been no other directly attributable long-term adverse effects of FMT in over 600 cases in the literature [19]. There is a paucity of long-term data [37], and so the possibility of as yet unknown long-term risk needs to be factored into any screening protocol and discussed with patients when consenting patients for FMT. A cohort study of patients who had received FMT for rCDI found 4 of 77 patients developed new autoimmune disease during the follow-up period of 3–68 months [37]. This study had no control group and thus no association between FMT and the development of autoimmune disease could be made.
ROUTE OF DELIVERY OF FMT
FMT can be delivered directly to the colon via colonoscopy or retention enema or alternatively into the upper GI tract, via nasogastric tube, nasoduodenal tube, or duodenoscope. Another potential delivery method of FMT is the use of enteric-coated or lyophilized capsules that contain stool [38] or synthetic stool made of multiple different bacterial strains. These have shown success in small case series and phase I/II trials, and similar preparations, although not widely available, are under commercial development [39].
The only randomized control trial comparing methods of delivery had 10 patients receive FMT via nasogastric tube and 10 patients receive FMT via colonoscopy for rCDI [17]. Resolution of diarrhea was achieved in 6/10 in the nasogastric tube delivery group compared with 8/10 in the colonoscopic delivery group. There was no significant difference between groups although the numbers in this study were small. In a systematic review of case series, cure rates varied depending on the site of infusion; when FMT was infused into the stomach, duodenum/jejunum, caecum/ascending colon, and rectum the rates of cure rate were 81%, 86%, 93%, and 84%, respectively [40]. Colonoscopy appears to have a higher cure rate than other methods; however, it is difficult to compare case series with heterogenous populations. Colonoscopy does have the advantage of assessing the degree of inflammation and assessing other pathology that may be present; however, it is resource intensive and costly, and for this reason enema delivery is a reasonable alternative [18]. The upper GI route carries the risk of fever and abdominal cramping [8, 10], whereas colonoscopic delivery carries the theoretical but unreported risk of colonic perforation. The FMT is delivered down the biopsy channel of the colonoscope into the caecum by removing the cap from the biopsy channel and opposing the catheter tip syringe.
PATIENT PREPARATION AND FOLLOW-UP
The standard approach has been to give 5–10 days of oral vancomycin 250 mg QID and then ceasing 36–72 hours prior to the procedure [12]; however, there are no data comparing no antimicrobial preparation or various different antimicrobial preparations (Figure 3). FMT via the duodenal route does not require bowel lavage, whereas colonoscopic delivery usually does [17]. We give the patient 4 mg of loperamide 3 hours prior to the procedure and have them lie on their right side at the point of delivery and for 1 hour following the procedure to aid with retention of the FMT. Symptoms of diarrhea and cramping often improve quite rapidly, and thus there is no routine need for further stool testing; however, if diarrhea persists for longer than 1 week, then repeat C. difficile toxin polymerase chain reaction (PCR) should be done and FMT repeated if positive.
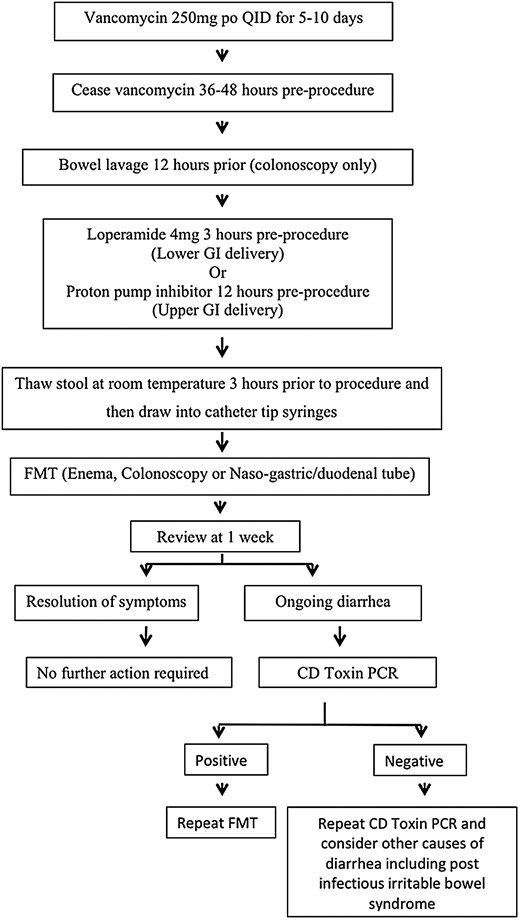
Patient preparation and follow-up. Abbreviations: CD, Clostridium difficile; FMT, fecal microbiota transplant; GI, gastrointestinal; PCR, polymerase chain reaction; po, per oral; QID, four times daily.
CONCLUSION
FMT is the most efficacious treatment available for the increasing problem of recurrent or refractory CDI. This necessitates that healthcare services develop the capability to deliver FMT safely and reliably. A stool bank of prescreened frozen aliquots from healthy volunteers is the most practical, ethical, and cost-effective approach. The practical steps outlined here should assist other facilities to establish an FMT capability.
Notes
Financial support. This work was supported by funding from the Gutsy group and National Health and Medical Research Council for the “FIRST-UC” study that provided infrastructure that assisted in establishing the fecal microbiota transplant (FMT) for Clostridium dif!cile infection service. Material support for the development of the FMT service was provided by Commonwealth Scientific and Industrial Research Organisation and the departments of Gastroenterology at the Royal Adelaide and Queen Elizabeth Hospitals.
Potential conflicts of interest. J. M. A. has been on Advisory Boards, Educational meetings, Steering Committees and on Speakers panels for AbbVie and Janssen. She has also received research support and speakers fees from each company. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




