-
PDF
- Split View
-
Views
-
Cite
Cite
Christian Theilacker, Katrin Ludewig, Annerose Serr, Julia Schimpf, Jürgen Held, Martin Bögelein, Viola Bahr, Stephan Rusch, Annette Pohl, Klaus Kogelmann, Sigrun Frieseke, Ralph Bogdanski, Frank M. Brunkhorst, Winfried V. Kern, for the Splenectomy, Pneumococcus, and Fulminant Infection (SPLEEN OFF) Study Group, Overwhelming Postsplenectomy Infection: A Prospective Multicenter Cohort Study, Clinical Infectious Diseases, Volume 62, Issue 7, 1 April 2016, Pages 871–878, https://doi.org/10.1093/cid/civ1195
Close - Share Icon Share
Abstract
Background. Recent population-based cohort studies have questioned the role of pneumococci as the most frequent pathogen causing severe infection in patients after splenectomy. The aim of the study was to define the causative pathogens and clinical presentation of patients with overwhelming postsplenectomy infection (OPSI).
Methods. In a prospective cohort study in 173 German intensive care units, we searched for patients with and without asplenia and community-acquired severe sepsis/septic shock. Clinical and laboratory variables and survival of patients were assessed.
Results. Fifty-two patients with severe sepsis or septic shock with asplenia and 52 without asplenia were included. OPSI patients more often had a history of malignancy (38% vs 17%; P = .016) and had a lower body mass index (24 kg/m2 vs 28 kg/m2; P = .004). Streptococcus pneumoniae was detected more frequently in OPSI patients (42% vs 12% without asplenia; P < .001) and more frequently manifested as bloodstream infection (31% vs 6%; P = .002). Gram-negative infection was similar in both groups (12% vs 19%; P = .157). Pneumococcal vaccine coverage of OPSI patients was low overall (42% vs 8% among patients without asplenia; P < .001). Purpura fulminans was a frequent complication, developing in 19% of OPSI patients vs 5% of patients without asplenia (P = .038). The interval between splenectomy and OPSI was 6 years (range, 1 month–50 years). On multivariable Poisson regression, asplenia was the only predictive variable independently associated with pneumococcal sepsis (adjusted relative risk, 2.53 [95% confidence interval, 1.06–6.08]).
Conclusions. Pneumococcal infections remain the most important cause of severe sepsis and septic shock following splenectomy.
Approximately 25 000 and 8000 splenectomies are performed annually in the United States and Germany, respectively [1, 2]. The total number of asplenic persons living in the United States is estimated at 1 million, a prevalence that is comparable to that of human immunodeficiency virus [1]. Following splenectomy, asplenic patients carry an immunodeficiency that predisposes them to life-threatening bacterial infections, also known as overwhelming postsplenectomy infection (OPSI). In a recent survey, the risk for hospitalization due to sepsis was 5–6 times higher in splenectomized patients than in other patients [3]. Earlier systematic reviews reported that >50% of OPSI cases are caused by Streptococcus pneumoniae [4, 5], and pneumococcal vaccination has therefore been recommended as a standard of care for all patients undergoing splenectomy [1, 6, 7]. Until 2014, German guidelines recommended the vaccination of adults patients with splenectomy with the 23-valent pneumococcal polysaccharide vaccine and revaccination with the same vaccine every 5 years [8]. Antibiotic prophylaxis, on the other hand, is not routinely recommended in Germany for adult splenectomized patients [9].
Recent population-based studies have found very few pneumococcal infections in patients following splenectomy, suggesting a shifting microbial epidemiology that is possibly associated with increasing pneumococcal vaccination coverage [10, 11]. However, most previous epidemiological information on the OPSI syndrome is based on single-center cohorts or case series [4, 5]. Furthermore, the definitions used for severity of infection and sepsis were not standardized, and individual vaccination status was never assessed. Population-based cohort studies [3, 10–12] have relied on hospital discharge codes, which might be affected by confounding due to variation in coding quality [13].
We therefore conducted a prospective, multicenter cohort study including adult patients admitted with community-acquired severe sepsis or septic shock with or without asplenia to the intensive care unit (ICU) to assess the current clinical epidemiology, microbiology, and outcome in a region with low pneumococcal vaccination rates and before the widespread use of pneumococcal conjugate vaccines in adults.
METHODS
Study Design and Patient Selection
The Splenectomy, Pneumococcus, and Fulminant Infection Study (SPLEEN OFF) was a prospective cohort study performed between November 2011 and October 2013 at 173 German ICUs. The characteristics of participating ICUs are summarized in Supplementary Table 1. The trial was approved by the ethics committee of each participating institution and is registered at the German Clinical Trials Register (identifier DRKS00000417).
Patients were eligible for the study when they were aged ≥18 years, and were admitted due to community-acquired severe sepsis or septic shock to one of the participating ICUs during the study period. Severe sepsis and septic shock were defined according to international consensus criteria and infection was considered community-acquired if symptoms occurred <48 hours after hospital admission [14, 15]. Patients with a missing spleen documented either in medical records or by imaging studies or patients with a rudimentary spleen in imaging studies and Howell–Jolly bodies in peripheral blood smears as a biomarker of splenic dysfunction were also included in the study [6]. As a comparison cohort, we identified patients without history of splenectomy/spleen dysfunction who were matched for the same age decade, sex, and type of ICU, otherwise fulfilling the same inclusion criteria. Patients presenting with healthcare-associated infection according to the definition of the US Centers for Disease Control and Prevention were excluded from the study population [15]. At least 2 independent sets of blood cultures on admission and after study inclusion were required. In addition, 2 ethylenediaminetetraacetic acid blood samples (drawn at different sites) and a serum and urine sample were obtained from each patient and sent within 48 hours to a central laboratory. Blood cultures were processed locally. Due to logistic constraints, we were unable to retrieve the information on pneumococcal serotypes of study patients.
Written informed consent was obtained from all patients or their legal representative. For patients from whom consent could not be obtained because of critical illness or the use of sedative or anesthetic drugs, the ethics committees approved a provision for delayed consent. In such cases, a surrogate decision maker was fully informed as soon as possible. Either consent was then obtained, or the patient was removed from the study and all study-related procedures were terminated and collected data deleted.
Data Collection
The clinical and laboratory parameters collected and their definitions are described in the Supplementary Material. Survival and subsequent hospital admissions due to infection were recorded for study patients by telephone interviews up to 12 months after study entry. Completeness and consistency of the clinical data were validated through programmed electronic checks as well as by means of manual checks of plausibility and correctness by the primary study investigator (C. T.). Microbiology results and clinical data were reviewed for plausibility by 2 experienced infectious diseases specialists (W. V. K. and C. T.) blinded to patient allocation and independent of each others assessments and verdict.
Outcome Measures
The primary endpoint of the study was the proportion of patients with and without asplenia with severe sepsis/septic shock due to S. pneumoniae. Secondary endpoints were sepsis severity, frequency of purpura fulminans, days of mechanical ventilation, days on ICU and in-hospital, and 28-day and 1-year survival.
Sample Size and Statistical Analysis
The study was designed to detect a significant difference between patients with asplenia and patients without asplenia in the proportion of pneumococcal infection with a significance level of .05 and a power of 80%. Assuming a 50% pathogen identification rate, a proportion of 60% pneumococcal disease among pathogen-defined OPSI patients, and a 30% proportion among patients without asplenia, a sample size of 100 patients per group was required. No formal interim analyses were planned.
Continuous variables are reported as median values with interquartile ranges. The χ2 test or the Fisher exact test was used to compare categorical data, and the Mann–Whitney U test was used for comparison of 2 groups of continuous data. As the incidence of the outcome event was >10%, relative risks (RRs) were calculated to avoid an overestimation of associations in contrast to odds ratios [16]. RRs for failure were estimated by Poisson regression with robust error variance [17]. All variables found to be significant in univariable analysis were included in the multivariable regression model. Differences were considered statistically significant at a P value of <.05. Survival analysis was by Kaplan–Meier statistics. Statistical analyses were performed with SPSS for Mac version 21.
RESULTS
In total, 173 ICUs participated in the study. The overall characteristics of participating ICUs were similar to a previously described representative sample of German ICUs (Supplementary Table 1) [18]. Fifty-four patients with asplenia and the same number of patients without asplenia were recruited (Figure 1). Due to slow recruitment and limited funding, the study was terminated after 24 months, before the recruitment goal was reached. Two patients with asplenia were ineligible and were removed from analysis along with the corresponding patients without asplenia (Figure 1).
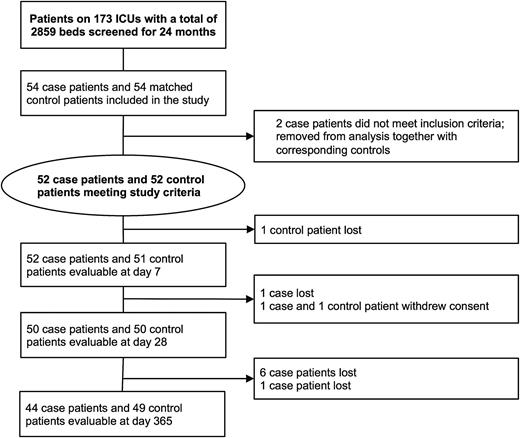
Screening and inclusion process for patients in the study. Of the 9 patients who were lost to follow-up, 7 patients did not return phone calls and 2 patients moved without leaving further contact information. Abbreviation: ICU, intensive care unit.
Baseline Characteristics
OPSI patients had a significantly lower body mass index (BMI than patients with severe sepsis/septic shock without asplenia and were slightly younger without reaching a level of significance (Table 1). Other demographic variables and comorbidity were similar between patients with and without asplenia, with the exception of malignancy as underlying disease, which was more frequent among OPSI patients (Table 1). The frequency of uncontrolled malignancy, however, was low in both patient groups (Table 1). A minority of the patients in boof the patients in both groupsth groups had received current or recent immunosuppressive medication or antineoplastic chemotherapy. OPSI patients tended to receive immunosuppressive therapy and chemotherapy slightly more frequently, without reaching significance levels.
Baseline Characteristics Including Vaccination History in Patients With or Without Asplenia
| Characteristic . | Asplenia (n = 52) . | No Asplenia (n = 52) . | P Value . |
|---|---|---|---|
| Demographics and general risk factors | |||
| Agea | 55 (44–66) | 61 (45–69) | .258 |
| Male sex | 29 (56) | 29 (56) | 1.000 |
| Body mass index, kg/m2a | 24 (22–27) | 28 (23–34) | .004 |
| Current smokingb | 14 (27) | 18 (35) | .524 |
| Alcohol usec | 6 (12) | 11 (21) | .289 |
| Comorbidity | |||
| Charlson comorbidity indexa | 2 (0–4) | 2 (0–3) | .510 |
| History of malignancy | 20 (38) | 9 (17) | .016 |
| Active neoplastic disease | 6 (12) | 3 (6) | .488 |
| Immunosuppressive therapyd | 5 (10) | 2 (4) | .437 |
| Antineoplastic chemotherapye | 4 (8) | 1 (2) | .363 |
| Previous hospitalization due to infectionf | 17 (33) | 14 (27) | .669 |
| Vaccination status | |||
| Pneumococcal vaccine | |||
| Any timeg | 22 (42) | 4 (8) | <.001 |
| Past 5 y | 11 (21) | 4 (8) | .045 |
| Meningococcal vaccine (any time)h | 3 (6) | 0 (0) | .243 |
| Haemophilus influenzae type B vaccine (any time) | 6 (12) | 0 (0) | <.001 |
| Influenza vaccine (previous season) | 6 (12) | 12 (23) | .194 |
| Unknown | 5 (10) | 5 (10) | 1.000 |
| Antibiotic prophylaxis | 3 (6) | 4 (8) | 1.000 |
| Characteristic . | Asplenia (n = 52) . | No Asplenia (n = 52) . | P Value . |
|---|---|---|---|
| Demographics and general risk factors | |||
| Agea | 55 (44–66) | 61 (45–69) | .258 |
| Male sex | 29 (56) | 29 (56) | 1.000 |
| Body mass index, kg/m2a | 24 (22–27) | 28 (23–34) | .004 |
| Current smokingb | 14 (27) | 18 (35) | .524 |
| Alcohol usec | 6 (12) | 11 (21) | .289 |
| Comorbidity | |||
| Charlson comorbidity indexa | 2 (0–4) | 2 (0–3) | .510 |
| History of malignancy | 20 (38) | 9 (17) | .016 |
| Active neoplastic disease | 6 (12) | 3 (6) | .488 |
| Immunosuppressive therapyd | 5 (10) | 2 (4) | .437 |
| Antineoplastic chemotherapye | 4 (8) | 1 (2) | .363 |
| Previous hospitalization due to infectionf | 17 (33) | 14 (27) | .669 |
| Vaccination status | |||
| Pneumococcal vaccine | |||
| Any timeg | 22 (42) | 4 (8) | <.001 |
| Past 5 y | 11 (21) | 4 (8) | .045 |
| Meningococcal vaccine (any time)h | 3 (6) | 0 (0) | .243 |
| Haemophilus influenzae type B vaccine (any time) | 6 (12) | 0 (0) | <.001 |
| Influenza vaccine (previous season) | 6 (12) | 12 (23) | .194 |
| Unknown | 5 (10) | 5 (10) | 1.000 |
| Antibiotic prophylaxis | 3 (6) | 4 (8) | 1.000 |
Data are presented as No. (%) of patients unless otherwise indicated. P values indicating significant differences in boldface.
a Median (interquartile range).
b Current smokers and former smokers who quit ≤12 months before study.
c Alcohol consumption >16 standard drinks (women) or >20 standard drinks (men).
d Including current or recent (≤3 months) treatment with corticosteroids (≥20 mg of prednisolone or equivalent for ≥3 weeks), calcineurin inhibitors, antagonists against tumor necrosis factor α, interleukin-1, interleukin-6, T- or B-cell–depleting antibodies, azathioprine, mycophenolate, methotrexate, cyclophosphamide, thalidomide, leflunomide.
e Current or recent (≤3 months) chemotherapy.
f Since splenectomy or matched interval in patients without splenectomy.
g All patients with asplenia had received the 23-valent pneumococcal polysaccharide (PPV-23) vaccine. One without asplenia had received the pneumococcal conjugate vaccine, and the remaining 3 had been vaccinated with PPV-23.
h One patient was vaccinated with the monovalent meningococcal conjugate vaccine, and 2 patients were vaccinated with the 4-valent meningococcal polysaccharide vaccine.
Baseline Characteristics Including Vaccination History in Patients With or Without Asplenia
| Characteristic . | Asplenia (n = 52) . | No Asplenia (n = 52) . | P Value . |
|---|---|---|---|
| Demographics and general risk factors | |||
| Agea | 55 (44–66) | 61 (45–69) | .258 |
| Male sex | 29 (56) | 29 (56) | 1.000 |
| Body mass index, kg/m2a | 24 (22–27) | 28 (23–34) | .004 |
| Current smokingb | 14 (27) | 18 (35) | .524 |
| Alcohol usec | 6 (12) | 11 (21) | .289 |
| Comorbidity | |||
| Charlson comorbidity indexa | 2 (0–4) | 2 (0–3) | .510 |
| History of malignancy | 20 (38) | 9 (17) | .016 |
| Active neoplastic disease | 6 (12) | 3 (6) | .488 |
| Immunosuppressive therapyd | 5 (10) | 2 (4) | .437 |
| Antineoplastic chemotherapye | 4 (8) | 1 (2) | .363 |
| Previous hospitalization due to infectionf | 17 (33) | 14 (27) | .669 |
| Vaccination status | |||
| Pneumococcal vaccine | |||
| Any timeg | 22 (42) | 4 (8) | <.001 |
| Past 5 y | 11 (21) | 4 (8) | .045 |
| Meningococcal vaccine (any time)h | 3 (6) | 0 (0) | .243 |
| Haemophilus influenzae type B vaccine (any time) | 6 (12) | 0 (0) | <.001 |
| Influenza vaccine (previous season) | 6 (12) | 12 (23) | .194 |
| Unknown | 5 (10) | 5 (10) | 1.000 |
| Antibiotic prophylaxis | 3 (6) | 4 (8) | 1.000 |
| Characteristic . | Asplenia (n = 52) . | No Asplenia (n = 52) . | P Value . |
|---|---|---|---|
| Demographics and general risk factors | |||
| Agea | 55 (44–66) | 61 (45–69) | .258 |
| Male sex | 29 (56) | 29 (56) | 1.000 |
| Body mass index, kg/m2a | 24 (22–27) | 28 (23–34) | .004 |
| Current smokingb | 14 (27) | 18 (35) | .524 |
| Alcohol usec | 6 (12) | 11 (21) | .289 |
| Comorbidity | |||
| Charlson comorbidity indexa | 2 (0–4) | 2 (0–3) | .510 |
| History of malignancy | 20 (38) | 9 (17) | .016 |
| Active neoplastic disease | 6 (12) | 3 (6) | .488 |
| Immunosuppressive therapyd | 5 (10) | 2 (4) | .437 |
| Antineoplastic chemotherapye | 4 (8) | 1 (2) | .363 |
| Previous hospitalization due to infectionf | 17 (33) | 14 (27) | .669 |
| Vaccination status | |||
| Pneumococcal vaccine | |||
| Any timeg | 22 (42) | 4 (8) | <.001 |
| Past 5 y | 11 (21) | 4 (8) | .045 |
| Meningococcal vaccine (any time)h | 3 (6) | 0 (0) | .243 |
| Haemophilus influenzae type B vaccine (any time) | 6 (12) | 0 (0) | <.001 |
| Influenza vaccine (previous season) | 6 (12) | 12 (23) | .194 |
| Unknown | 5 (10) | 5 (10) | 1.000 |
| Antibiotic prophylaxis | 3 (6) | 4 (8) | 1.000 |
Data are presented as No. (%) of patients unless otherwise indicated. P values indicating significant differences in boldface.
a Median (interquartile range).
b Current smokers and former smokers who quit ≤12 months before study.
c Alcohol consumption >16 standard drinks (women) or >20 standard drinks (men).
d Including current or recent (≤3 months) treatment with corticosteroids (≥20 mg of prednisolone or equivalent for ≥3 weeks), calcineurin inhibitors, antagonists against tumor necrosis factor α, interleukin-1, interleukin-6, T- or B-cell–depleting antibodies, azathioprine, mycophenolate, methotrexate, cyclophosphamide, thalidomide, leflunomide.
e Current or recent (≤3 months) chemotherapy.
f Since splenectomy or matched interval in patients without splenectomy.
g All patients with asplenia had received the 23-valent pneumococcal polysaccharide (PPV-23) vaccine. One without asplenia had received the pneumococcal conjugate vaccine, and the remaining 3 had been vaccinated with PPV-23.
h One patient was vaccinated with the monovalent meningococcal conjugate vaccine, and 2 patients were vaccinated with the 4-valent meningococcal polysaccharide vaccine.
The median interval between splenectomy and OPSI onset was 5.75 years (interquartile range, 1–18.25 years), with a range between 1 month and 50 years. Previous episodes of infections requiring hospitalization in the period between splenectomy and study entry or in the matched time period for patients without splenectomy were similar in both groups (Table 1).
Indication for Splenectomy
The most common reason for splenectomy was surgery because of malignancy (11 patients [21%]), followed by trauma (9 patients [17%]), therapeutic splenectomy for hematologic disorders (8 patients [15%]), and surgery for other benign pancreatic processes (6 patients [12%]) or other benign conditions (12 patients [23%]). In 5 patients, OPSI occurred in the context of splenic atrophy. Taken together, in 79% of OPSI patients the indication for splenectomy was unrelated to malignant underlying conditions.
Vaccination Status and Antibiotic Prophylaxis
Although higher than in patients without asplenia, the pneumococcal vaccination rate in patients with splenectomy and functional asplenia was only 42% (Table 1). Vaccine coverage was 47% if only splenectomized patients were considered. According to German vaccine recommendations valid during the study recruitment period, 30 of the 52 OPSI patients should have received a pneumococcal booster vaccination. However, only 3 of the eligible patients (10%) had actually received the vaccine booster.
Vaccination against meningococci, Haemophilus influenzae type B, and seasonal influenza was even more infrequent among OPSI patients. Few OPSI patients took daily prophylactic antibiotics prior to OPSI onset (Table 1).
Causative Pathogens, Focus of Infection, and Initial Antibiotic Treatment
At study enrollment, blood cultures were obtained from 96% of patients with asplenia and 90% without asplenia. Pneumococcal urinary antigen test was performed in 87% of OPSI patients and 79% patients without asplenia, and 16S ribosomal RNA broad-range polymerase chain reaction in 77% of patients with asplenia and 90% without asplenia. Based on the aggregated microbiologic investigations, sepsis was pathogen-defined in 73% of patients with asplenia and 63% without asplenia. In patients with bloodstream infection, a causative pathogen could be defined in 26 patients with asplenia vs 19 patients without asplenia, respectively (50% vs 37%; P = .307).
The most commonly identified pathogen in OPSI was S. pneumoniae, causing 42% of infections among patients with asplenia (vs 12% among patients without asplenia; P < .001) including 16 (vs 3) bloodstream infections (31% vs 6%; P = .002) (Table 2). Details of microbiologic diagnostics of pneumococcal infection are described in the Supplementary Material.
Microorganisms Causing Infection With Severe Sepsis/Septic Shock in Patients With and Without Asplenia
| Characteristic . | Bloodstream Infection . | All Infections . | ||||
|---|---|---|---|---|---|---|
| Asplenia (n = 52) . | No Asplenia (n = 52) . | P Value . | Asplenia (n = 52) . | No Asplenia (n = 52) . | P Value . | |
| Gram-positive pathogen | 19 (37) | 11 (21) | .098 | 26 (50) | 18 (35) | .157 |
| Streptococcus pneumoniae | 16 (31) | 3 (6) | .002 | 22 (42) | 6 (12) | <.001 |
| Staphylococcus aureus | … | 3 (6) | .243 | 1 (2) | 7 (14) | .060 |
| Streptococcus pyogenes | … | 3 (6) | .243 | … | 3 (6) | .243 |
| Other Streptococcus species | 2 (4) | … | .495 | 2 (4) | … | .495 |
| Other gram-positive pathogens | 1 (2) | 2 (4) | 1.000 | 1 (2) | 2 (4) | 1.000 |
| Gram-negative pathogen | 4 (8) | 8 (15) | .098 | 6 (12) | 10 (19) | .157 |
| Escherichia coli | … | 7 (14) | .118 | 3 (6) | 7 (14) | .319 |
| Klebsiella species | 2 (4) | … | .495 | 2 (4) | 1 (2) | 1.000 |
| Pseudomonas aeruginosa | 1 (2) | 1 (2) | 1.000 | 1 (2) | 1 (2) | 1.000 |
| Other gram-negative pathogens | 1 (2) | … | 1.000 | … | 1 (2) | 1.000 |
| Polymicrobiala | 3 (6) | … | .243 | 6 (12) | 2 (4) | .269 |
| Virus | … | … | … | … | 3 (6) | .286 |
| No pathogen identified | 26 (50) | 33 (64) | .235 | 14 (27) | 19 (37) | .400 |
| Characteristic . | Bloodstream Infection . | All Infections . | ||||
|---|---|---|---|---|---|---|
| Asplenia (n = 52) . | No Asplenia (n = 52) . | P Value . | Asplenia (n = 52) . | No Asplenia (n = 52) . | P Value . | |
| Gram-positive pathogen | 19 (37) | 11 (21) | .098 | 26 (50) | 18 (35) | .157 |
| Streptococcus pneumoniae | 16 (31) | 3 (6) | .002 | 22 (42) | 6 (12) | <.001 |
| Staphylococcus aureus | … | 3 (6) | .243 | 1 (2) | 7 (14) | .060 |
| Streptococcus pyogenes | … | 3 (6) | .243 | … | 3 (6) | .243 |
| Other Streptococcus species | 2 (4) | … | .495 | 2 (4) | … | .495 |
| Other gram-positive pathogens | 1 (2) | 2 (4) | 1.000 | 1 (2) | 2 (4) | 1.000 |
| Gram-negative pathogen | 4 (8) | 8 (15) | .098 | 6 (12) | 10 (19) | .157 |
| Escherichia coli | … | 7 (14) | .118 | 3 (6) | 7 (14) | .319 |
| Klebsiella species | 2 (4) | … | .495 | 2 (4) | 1 (2) | 1.000 |
| Pseudomonas aeruginosa | 1 (2) | 1 (2) | 1.000 | 1 (2) | 1 (2) | 1.000 |
| Other gram-negative pathogens | 1 (2) | … | 1.000 | … | 1 (2) | 1.000 |
| Polymicrobiala | 3 (6) | … | .243 | 6 (12) | 2 (4) | .269 |
| Virus | … | … | … | … | 3 (6) | .286 |
| No pathogen identified | 26 (50) | 33 (64) | .235 | 14 (27) | 19 (37) | .400 |
Data are presented as No. (%) of patients. P values indicating significant differences in boldface.
a None of the polymicrobial infections included pneumococci.
Microorganisms Causing Infection With Severe Sepsis/Septic Shock in Patients With and Without Asplenia
| Characteristic . | Bloodstream Infection . | All Infections . | ||||
|---|---|---|---|---|---|---|
| Asplenia (n = 52) . | No Asplenia (n = 52) . | P Value . | Asplenia (n = 52) . | No Asplenia (n = 52) . | P Value . | |
| Gram-positive pathogen | 19 (37) | 11 (21) | .098 | 26 (50) | 18 (35) | .157 |
| Streptococcus pneumoniae | 16 (31) | 3 (6) | .002 | 22 (42) | 6 (12) | <.001 |
| Staphylococcus aureus | … | 3 (6) | .243 | 1 (2) | 7 (14) | .060 |
| Streptococcus pyogenes | … | 3 (6) | .243 | … | 3 (6) | .243 |
| Other Streptococcus species | 2 (4) | … | .495 | 2 (4) | … | .495 |
| Other gram-positive pathogens | 1 (2) | 2 (4) | 1.000 | 1 (2) | 2 (4) | 1.000 |
| Gram-negative pathogen | 4 (8) | 8 (15) | .098 | 6 (12) | 10 (19) | .157 |
| Escherichia coli | … | 7 (14) | .118 | 3 (6) | 7 (14) | .319 |
| Klebsiella species | 2 (4) | … | .495 | 2 (4) | 1 (2) | 1.000 |
| Pseudomonas aeruginosa | 1 (2) | 1 (2) | 1.000 | 1 (2) | 1 (2) | 1.000 |
| Other gram-negative pathogens | 1 (2) | … | 1.000 | … | 1 (2) | 1.000 |
| Polymicrobiala | 3 (6) | … | .243 | 6 (12) | 2 (4) | .269 |
| Virus | … | … | … | … | 3 (6) | .286 |
| No pathogen identified | 26 (50) | 33 (64) | .235 | 14 (27) | 19 (37) | .400 |
| Characteristic . | Bloodstream Infection . | All Infections . | ||||
|---|---|---|---|---|---|---|
| Asplenia (n = 52) . | No Asplenia (n = 52) . | P Value . | Asplenia (n = 52) . | No Asplenia (n = 52) . | P Value . | |
| Gram-positive pathogen | 19 (37) | 11 (21) | .098 | 26 (50) | 18 (35) | .157 |
| Streptococcus pneumoniae | 16 (31) | 3 (6) | .002 | 22 (42) | 6 (12) | <.001 |
| Staphylococcus aureus | … | 3 (6) | .243 | 1 (2) | 7 (14) | .060 |
| Streptococcus pyogenes | … | 3 (6) | .243 | … | 3 (6) | .243 |
| Other Streptococcus species | 2 (4) | … | .495 | 2 (4) | … | .495 |
| Other gram-positive pathogens | 1 (2) | 2 (4) | 1.000 | 1 (2) | 2 (4) | 1.000 |
| Gram-negative pathogen | 4 (8) | 8 (15) | .098 | 6 (12) | 10 (19) | .157 |
| Escherichia coli | … | 7 (14) | .118 | 3 (6) | 7 (14) | .319 |
| Klebsiella species | 2 (4) | … | .495 | 2 (4) | 1 (2) | 1.000 |
| Pseudomonas aeruginosa | 1 (2) | 1 (2) | 1.000 | 1 (2) | 1 (2) | 1.000 |
| Other gram-negative pathogens | 1 (2) | … | 1.000 | … | 1 (2) | 1.000 |
| Polymicrobiala | 3 (6) | … | .243 | 6 (12) | 2 (4) | .269 |
| Virus | … | … | … | … | 3 (6) | .286 |
| No pathogen identified | 26 (50) | 33 (64) | .235 | 14 (27) | 19 (37) | .400 |
Data are presented as No. (%) of patients. P values indicating significant differences in boldface.
a None of the polymicrobial infections included pneumococci.
The RR for pneumococcal disease and pneumococcal bacteremia in patients with asplenia vs patients without asplenia was 1.99 (95% confidence interval [CI], 1.42–2.79 and 1.45–2.73, respectively). Fourteen of the 16 patients with asplenia with proven invasive pneumococcal disease had not been vaccinated against pneumococci in the last 5 years. Gram-negative infection was similar in patients with OPSI and patients without asplenia (Table 2). Of note, no OPSI caused by Neisseria meningitidis or H. influenzae was identified. Supplementary Table 3 illustrates the clinical features of patients with pneumococcal OPSI.
The most frequent focus of infection in both OPSI patients and patients without asplenia was the respiratory tract, followed by intra-abdominal infection (Table 3). Primary bacteremia without focus was more common and skin and soft tissue infections less frequent in OPSI than in patients without asplenia (Table 3). All patients received broad-spectrum β-lactams or vancomycin as part of the empiric antibiotic therapy and none of the pneumococcal isolates were penicillin-resistant (Supplementary Table 2).
| Parameter . | Asplenia (n = 52) . | No Asplenia (n = 52) . | P Value . |
|---|---|---|---|
| Infection focusa | |||
| Primary bacteremia (no site) | 6 (12) | 0 (0) | .012 |
| Respiratory tract | 21 (40) | 27 (52) | .330 |
| Intra-abdominal | 13 (25) | 8 (15) | .225 |
| Urinary tract | 1 (2) | 5 (10) | .205 |
| Central nervous system | 4 (8) | 3 (6) | .713 |
| Skin/soft tissue | 0 (0) | 6 (12) | .012 |
| Other/not specified | 7 (13) | 3 (6) | .483 |
| Sepsis severity on admission | |||
| APACHE II scoreb,c | 23 (16–29) | 25 (19–33) | .069 |
| SOFA scoreb,d | 10 (8–13) | 11 (8–14) | .047 |
| Metabolic acidosise | 34 (65) | 37 (71) | .826 |
| Procalcitonin level, ng/mLb | 34 (3–98) | 15 (2–54) | .236 |
| Lactate, mmol/Lb | 4.9 (1.9–10.1) | 2.8 (1.6–6.4) | .106 |
| Purpura fulminans | 10 (19) | 3 (5) | .038 |
| Outcomes | |||
| Days on ICUf | 13 (3–23) | 18 (11–25) | .106 |
| Days in hospitalf | 29 (26–32) | 31 (26–36) | .327 |
| Days on ventilatorf | 6 (1–11) | 12 (7–16) | .030 |
| 7-day mortalityg | 11 (21) | 6 (12) | .289 |
| 28-day mortalityh | 18 (35) | 18 (35) | 1.000 |
| Parameter . | Asplenia (n = 52) . | No Asplenia (n = 52) . | P Value . |
|---|---|---|---|
| Infection focusa | |||
| Primary bacteremia (no site) | 6 (12) | 0 (0) | .012 |
| Respiratory tract | 21 (40) | 27 (52) | .330 |
| Intra-abdominal | 13 (25) | 8 (15) | .225 |
| Urinary tract | 1 (2) | 5 (10) | .205 |
| Central nervous system | 4 (8) | 3 (6) | .713 |
| Skin/soft tissue | 0 (0) | 6 (12) | .012 |
| Other/not specified | 7 (13) | 3 (6) | .483 |
| Sepsis severity on admission | |||
| APACHE II scoreb,c | 23 (16–29) | 25 (19–33) | .069 |
| SOFA scoreb,d | 10 (8–13) | 11 (8–14) | .047 |
| Metabolic acidosise | 34 (65) | 37 (71) | .826 |
| Procalcitonin level, ng/mLb | 34 (3–98) | 15 (2–54) | .236 |
| Lactate, mmol/Lb | 4.9 (1.9–10.1) | 2.8 (1.6–6.4) | .106 |
| Purpura fulminans | 10 (19) | 3 (5) | .038 |
| Outcomes | |||
| Days on ICUf | 13 (3–23) | 18 (11–25) | .106 |
| Days in hospitalf | 29 (26–32) | 31 (26–36) | .327 |
| Days on ventilatorf | 6 (1–11) | 12 (7–16) | .030 |
| 7-day mortalityg | 11 (21) | 6 (12) | .289 |
| 28-day mortalityh | 18 (35) | 18 (35) | 1.000 |
Data are presented as No. (%) of patients unless otherwise indicated. P values indicating significant differences in boldface.
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ICU, intensive care unit; SOFA, Sequential Organ Failure Assessment.
a As reported by study physician.
b Median (interquartile range).
c A total of 16 subscores for patients with asplenia and 4 subscores for patients without asplenia were missing and substituted with a subscore of 0.
d A total of 5 subscores for patients with asplenia and 3 subscores for patients without asplenia were missing. One imputation for patients with asplenia and 4 imputations for patients without asplenia were made as described in the “Methods” section.
e Metabolic acidosis: pH ≤7.30 or base deficit ≥5.0 mmol/L or plasma lactate concentration ≥1.5 times the upper normal limit used in the local laboratory.
f Median of Kaplan–Meier estimate (95% confidence interval).
g Missing data for 1 patient without asplenia.
h Missing data for 2 patients with asplenia and 2 patients without asplenia.
| Parameter . | Asplenia (n = 52) . | No Asplenia (n = 52) . | P Value . |
|---|---|---|---|
| Infection focusa | |||
| Primary bacteremia (no site) | 6 (12) | 0 (0) | .012 |
| Respiratory tract | 21 (40) | 27 (52) | .330 |
| Intra-abdominal | 13 (25) | 8 (15) | .225 |
| Urinary tract | 1 (2) | 5 (10) | .205 |
| Central nervous system | 4 (8) | 3 (6) | .713 |
| Skin/soft tissue | 0 (0) | 6 (12) | .012 |
| Other/not specified | 7 (13) | 3 (6) | .483 |
| Sepsis severity on admission | |||
| APACHE II scoreb,c | 23 (16–29) | 25 (19–33) | .069 |
| SOFA scoreb,d | 10 (8–13) | 11 (8–14) | .047 |
| Metabolic acidosise | 34 (65) | 37 (71) | .826 |
| Procalcitonin level, ng/mLb | 34 (3–98) | 15 (2–54) | .236 |
| Lactate, mmol/Lb | 4.9 (1.9–10.1) | 2.8 (1.6–6.4) | .106 |
| Purpura fulminans | 10 (19) | 3 (5) | .038 |
| Outcomes | |||
| Days on ICUf | 13 (3–23) | 18 (11–25) | .106 |
| Days in hospitalf | 29 (26–32) | 31 (26–36) | .327 |
| Days on ventilatorf | 6 (1–11) | 12 (7–16) | .030 |
| 7-day mortalityg | 11 (21) | 6 (12) | .289 |
| 28-day mortalityh | 18 (35) | 18 (35) | 1.000 |
| Parameter . | Asplenia (n = 52) . | No Asplenia (n = 52) . | P Value . |
|---|---|---|---|
| Infection focusa | |||
| Primary bacteremia (no site) | 6 (12) | 0 (0) | .012 |
| Respiratory tract | 21 (40) | 27 (52) | .330 |
| Intra-abdominal | 13 (25) | 8 (15) | .225 |
| Urinary tract | 1 (2) | 5 (10) | .205 |
| Central nervous system | 4 (8) | 3 (6) | .713 |
| Skin/soft tissue | 0 (0) | 6 (12) | .012 |
| Other/not specified | 7 (13) | 3 (6) | .483 |
| Sepsis severity on admission | |||
| APACHE II scoreb,c | 23 (16–29) | 25 (19–33) | .069 |
| SOFA scoreb,d | 10 (8–13) | 11 (8–14) | .047 |
| Metabolic acidosise | 34 (65) | 37 (71) | .826 |
| Procalcitonin level, ng/mLb | 34 (3–98) | 15 (2–54) | .236 |
| Lactate, mmol/Lb | 4.9 (1.9–10.1) | 2.8 (1.6–6.4) | .106 |
| Purpura fulminans | 10 (19) | 3 (5) | .038 |
| Outcomes | |||
| Days on ICUf | 13 (3–23) | 18 (11–25) | .106 |
| Days in hospitalf | 29 (26–32) | 31 (26–36) | .327 |
| Days on ventilatorf | 6 (1–11) | 12 (7–16) | .030 |
| 7-day mortalityg | 11 (21) | 6 (12) | .289 |
| 28-day mortalityh | 18 (35) | 18 (35) | 1.000 |
Data are presented as No. (%) of patients unless otherwise indicated. P values indicating significant differences in boldface.
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ICU, intensive care unit; SOFA, Sequential Organ Failure Assessment.
a As reported by study physician.
b Median (interquartile range).
c A total of 16 subscores for patients with asplenia and 4 subscores for patients without asplenia were missing and substituted with a subscore of 0.
d A total of 5 subscores for patients with asplenia and 3 subscores for patients without asplenia were missing. One imputation for patients with asplenia and 4 imputations for patients without asplenia were made as described in the “Methods” section.
e Metabolic acidosis: pH ≤7.30 or base deficit ≥5.0 mmol/L or plasma lactate concentration ≥1.5 times the upper normal limit used in the local laboratory.
f Median of Kaplan–Meier estimate (95% confidence interval).
g Missing data for 1 patient without asplenia.
h Missing data for 2 patients with asplenia and 2 patients without asplenia.
Sepsis Severity and Length of Stay
The APACHE II score was 23 in OPSI patients and 25 in patients without asplenia (P = .069). Both OPSI patients and patients without asplenia had high Sequential Organ Failure Assessment scores at baseline, and the decline over time was similar in the 2 groups (Supplementary Figure 1 and Table 3). The initial rate of metabolic acidosis and levels of procalcitonin and lactate did not differ significantly between both groups of patients. The 2 groups were also similar regarding total length of stay and duration of ICU admission (Table 3). Patients without asplenia, however, were ventilated longer than OPSI patients (Table 3).
Ten OPSI patients developed purpura fulminans, a rate that was significantly higher than among patients without asplenia (19% vs 5%; RR, 1.74 [95% CI, 1.19–2.54]). In all OPSI patients with purpura fulminans, S. pneumoniae was isolated as a pathogen, making this complication highly prevalent among this group (45%).
Survival and Infections During Follow-up
Among both OPSI patients and patients without asplenia, 28-day mortality was 35% (Table 3). The mortality at 12 months was 44% for OPSI patients and 46% for patients without asplenia (Supplementary Figure 2).
Risk Factors for Pneumococcal Infection
In univariate analysis, asplenia was associated with an increased risk for pneumococcal infection (RR, 3.67 [95% CI, 1.62–8.30]), whereas age and BMI were protective factors (Table 4). Other baseline variables had no impact on this outcome (Table 4). In a multivariable Poisson regression analysis, asplenia was the only independent variable predictive of severe pneumococcal sepsis or septic shock (adjusted RR, 2.53 [95% CI, 1.06–6.08]).
Variables Associated With Pneumococcal Infection in Patients With Severe Sepsis or Septic Shock
| Variable . | Patients Without Pneumococcal Sepsis (n = 76) . | Patients With Pneumococcal Sepsis (n = 28) . | Univariate Analysis . | Multivariate Analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| RR . | 95% CI . | P Value . | Adjusted RR . | 95% CI . | P Value . | |||
| Age ≥70 years | 18 (24) | 5 (18) | 0.97a | (.95–1.00) | .028 | 0.99a | (.96–1.01) | .198 |
| Male sex | 44 (58) | 14 (50) | 0.79 | (.42–1.49) | .510 | |||
| Body mass index ≤20 kg/m2 | 8 (13) | 2 (8) | 0.94a | (.89–.99) | .017 | 0.96a | (.91–1.02) | .173 |
| Current smoking | 22 (29) | 10 (36) | 1.25 | (.65–2.40) | .632 | |||
| Alcohol use | 14 (18) | 3 (11) | 0.61 | (.21–1.81) | .550 | |||
| Asplenia | 30 (39) | 22 (79) | 3.67 | (1.62–8.30) | .001 | 2.50 | (1.07–5.84) | .034 |
| Previous hospitalization due to infectionb | 24 (32) | 7 (25) | 0.79 | (.37–1.65) | .632 | |||
| Charlson comorbidity index ≥5 | 17 (22) | 4 (14) | 0.89a | (.75–1.06) | .177 | |||
| History of malignancy | 22 (29) | 8 (29) | 0.99 | (.49–1.99) | 1.000 | |||
| Active neoplastic disease | 7 (10) | 2 (7) | 0.81 | (.23–2.87) | 1.000 | |||
| Immunosuppressive therapy | 7 (10) | 0 (0) | 0.21 | (.01–3.20) | .118 | |||
| Antineoplastic chemotherapy | 5 (7) | 0 (0) | 0.29 | (.02–4.23) | .367 | |||
| Pneumococcal vaccination (any time) | 18 (24) | 8 (29) | 1.20 | (.60–2.39) | .617 | |||
| Variable . | Patients Without Pneumococcal Sepsis (n = 76) . | Patients With Pneumococcal Sepsis (n = 28) . | Univariate Analysis . | Multivariate Analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| RR . | 95% CI . | P Value . | Adjusted RR . | 95% CI . | P Value . | |||
| Age ≥70 years | 18 (24) | 5 (18) | 0.97a | (.95–1.00) | .028 | 0.99a | (.96–1.01) | .198 |
| Male sex | 44 (58) | 14 (50) | 0.79 | (.42–1.49) | .510 | |||
| Body mass index ≤20 kg/m2 | 8 (13) | 2 (8) | 0.94a | (.89–.99) | .017 | 0.96a | (.91–1.02) | .173 |
| Current smoking | 22 (29) | 10 (36) | 1.25 | (.65–2.40) | .632 | |||
| Alcohol use | 14 (18) | 3 (11) | 0.61 | (.21–1.81) | .550 | |||
| Asplenia | 30 (39) | 22 (79) | 3.67 | (1.62–8.30) | .001 | 2.50 | (1.07–5.84) | .034 |
| Previous hospitalization due to infectionb | 24 (32) | 7 (25) | 0.79 | (.37–1.65) | .632 | |||
| Charlson comorbidity index ≥5 | 17 (22) | 4 (14) | 0.89a | (.75–1.06) | .177 | |||
| History of malignancy | 22 (29) | 8 (29) | 0.99 | (.49–1.99) | 1.000 | |||
| Active neoplastic disease | 7 (10) | 2 (7) | 0.81 | (.23–2.87) | 1.000 | |||
| Immunosuppressive therapy | 7 (10) | 0 (0) | 0.21 | (.01–3.20) | .118 | |||
| Antineoplastic chemotherapy | 5 (7) | 0 (0) | 0.29 | (.02–4.23) | .367 | |||
| Pneumococcal vaccination (any time) | 18 (24) | 8 (29) | 1.20 | (.60–2.39) | .617 | |||
Data are presented as No. (%) of patients. For definitions of variables also see Table 1. Values indicating significant differences in boldface.
Abbreviations: CI, confidence interval; RR, relative risk.
a Per-unit increase.
b Since splenectomy or matched interval in patients without splenectomy.
Variables Associated With Pneumococcal Infection in Patients With Severe Sepsis or Septic Shock
| Variable . | Patients Without Pneumococcal Sepsis (n = 76) . | Patients With Pneumococcal Sepsis (n = 28) . | Univariate Analysis . | Multivariate Analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| RR . | 95% CI . | P Value . | Adjusted RR . | 95% CI . | P Value . | |||
| Age ≥70 years | 18 (24) | 5 (18) | 0.97a | (.95–1.00) | .028 | 0.99a | (.96–1.01) | .198 |
| Male sex | 44 (58) | 14 (50) | 0.79 | (.42–1.49) | .510 | |||
| Body mass index ≤20 kg/m2 | 8 (13) | 2 (8) | 0.94a | (.89–.99) | .017 | 0.96a | (.91–1.02) | .173 |
| Current smoking | 22 (29) | 10 (36) | 1.25 | (.65–2.40) | .632 | |||
| Alcohol use | 14 (18) | 3 (11) | 0.61 | (.21–1.81) | .550 | |||
| Asplenia | 30 (39) | 22 (79) | 3.67 | (1.62–8.30) | .001 | 2.50 | (1.07–5.84) | .034 |
| Previous hospitalization due to infectionb | 24 (32) | 7 (25) | 0.79 | (.37–1.65) | .632 | |||
| Charlson comorbidity index ≥5 | 17 (22) | 4 (14) | 0.89a | (.75–1.06) | .177 | |||
| History of malignancy | 22 (29) | 8 (29) | 0.99 | (.49–1.99) | 1.000 | |||
| Active neoplastic disease | 7 (10) | 2 (7) | 0.81 | (.23–2.87) | 1.000 | |||
| Immunosuppressive therapy | 7 (10) | 0 (0) | 0.21 | (.01–3.20) | .118 | |||
| Antineoplastic chemotherapy | 5 (7) | 0 (0) | 0.29 | (.02–4.23) | .367 | |||
| Pneumococcal vaccination (any time) | 18 (24) | 8 (29) | 1.20 | (.60–2.39) | .617 | |||
| Variable . | Patients Without Pneumococcal Sepsis (n = 76) . | Patients With Pneumococcal Sepsis (n = 28) . | Univariate Analysis . | Multivariate Analysis . | ||||
|---|---|---|---|---|---|---|---|---|
| RR . | 95% CI . | P Value . | Adjusted RR . | 95% CI . | P Value . | |||
| Age ≥70 years | 18 (24) | 5 (18) | 0.97a | (.95–1.00) | .028 | 0.99a | (.96–1.01) | .198 |
| Male sex | 44 (58) | 14 (50) | 0.79 | (.42–1.49) | .510 | |||
| Body mass index ≤20 kg/m2 | 8 (13) | 2 (8) | 0.94a | (.89–.99) | .017 | 0.96a | (.91–1.02) | .173 |
| Current smoking | 22 (29) | 10 (36) | 1.25 | (.65–2.40) | .632 | |||
| Alcohol use | 14 (18) | 3 (11) | 0.61 | (.21–1.81) | .550 | |||
| Asplenia | 30 (39) | 22 (79) | 3.67 | (1.62–8.30) | .001 | 2.50 | (1.07–5.84) | .034 |
| Previous hospitalization due to infectionb | 24 (32) | 7 (25) | 0.79 | (.37–1.65) | .632 | |||
| Charlson comorbidity index ≥5 | 17 (22) | 4 (14) | 0.89a | (.75–1.06) | .177 | |||
| History of malignancy | 22 (29) | 8 (29) | 0.99 | (.49–1.99) | 1.000 | |||
| Active neoplastic disease | 7 (10) | 2 (7) | 0.81 | (.23–2.87) | 1.000 | |||
| Immunosuppressive therapy | 7 (10) | 0 (0) | 0.21 | (.01–3.20) | .118 | |||
| Antineoplastic chemotherapy | 5 (7) | 0 (0) | 0.29 | (.02–4.23) | .367 | |||
| Pneumococcal vaccination (any time) | 18 (24) | 8 (29) | 1.20 | (.60–2.39) | .617 | |||
Data are presented as No. (%) of patients. For definitions of variables also see Table 1. Values indicating significant differences in boldface.
Abbreviations: CI, confidence interval; RR, relative risk.
a Per-unit increase.
b Since splenectomy or matched interval in patients without splenectomy.
DISCUSSION
The main finding of this prospective study was that S. pneumoniae remains the most frequent pathogen in the OPSI syndrome in a setting where pneumococcal vaccination rates among adult patients are low overall. Streptococcus pneumoniae was identified in 42% of patients with asplenia, most frequently manifesting as invasive disease. Asplenia was a strong and independent risk factor for pneumococcal infection in patients with community-acquired severe sepsis/septic shock. As in other cohorts of severe sepsis patients, the most frequent pathogens documented in patients without asplenia were Escherichia coli, S. pneumoniae, and Staphylococcus aureus [13, 19].
Our findings confirm reviews of earlier studies published between 1952 and 1996 but are in contrast to results of recent population-based retrospective cohort studies from Denmark that reported S. pneumoniae in only 3% of positive blood cultures in hospitalized patients after splenectomy [4, 5, 10, 11]. These discrepancies may be explained in part by differences in definitions and inclusion criteria. Unlike the present study, the investigators from Denmark did not restrict their analysis to patients with community-acquired severe sepsis or septic shock but analyzed all hospitalized patients with bacteremia, including those with hospital-acquired infection. Accordingly, bacteremic infection within 90 days after splenectomy accounted for 62% of all bloodstream infections documented in the Danish cohort and to some extent may reflect postoperative complications [10]. In the current study, which excluded hospital-acquired infections, only 2 of 37 culture-positive OPSI patients fell within this period. Other factors, such as differences in the rate of pneumococcal vaccination between Denmark and Germany, may have had an additional impact on the different findings.
Unlike previous studies published before 1990, when H. influenzae was the second most common pathogen in OPSI patients [4], no such patients were found in the present cohort. Most likely this reflects the sharp decline in invasive H. influenzae serotype B infections in the general population since the introduction of the conjugate vaccine in Germany in 1990 [20]. Interestingly, we also did not identify meningococcal disease among the OPSI patients. Nevertheless, these results should be interpreted with caution, as our study was not designed and powered to analyze the frequency of H. influenzae or meningococci in OPSI patients.
Another key finding in the present study was that less than half of the OPSI patients had received prior pneumococcal vaccination despite guidelines that uniformly recommend this vaccine after splenectomy [7, 8]. Vaccination rates among splenectomized patients of 59%–88% have been reported from Scotland, the Netherlands, Denmark, and Switzerland [11, 21–23]. No specific data on the overall uptake of pneumococcal vaccine among splenectomized patients in Germany are available, but vaccine coverage for patients with high-risk conditions for invasive pneumococcal disease was only 52% in a recent survey [24]. The high proportion of pneumococcal OPSI in our cohort may be a result of poor compliance with vaccine guidelines, or possibly reflect earlier observations of an overall increased risk of nonvaccinated asplenic individuals for invasive pneumococcal disease [25]. With the limitation of small sample size, vaccination with the pneumococcal polysaccharide vaccine, however, was not a protective factor against pneumococcal sepsis in our cohort.
A noteworthy observation in our study was that OPSI occurred later than previously estimated. OPSI developed after a median of almost 6 years after splenectomy, and 70% of the infections occurred >2 years after splenectomy. Previous studies have reported mean intervals between splenectomy and first severe sepsis of only 1 or 2 years [4, 5, 10, 12]. Due to the limited length of follow-up, however, these cohort studies may have underestimated late-occurring events. Also, many studies did not exclude postoperative infections after splenectomy, potentially leading to increased incidence of infections early after splenectomy [4, 5, 10, 12].
In the present trial, 19% of OPSI patients developed purpura fulminans, a substantially higher rate than among patients without asplenia. All cases of purpura fulminans occurred in pneumococcal OPSI, affecting 45% of patients with OPSI due to S. pneumoniae. Our findings confirm anecdotal evidence from case reports and case series [26–28]. In a retrospective analysis of 4360 patients from clinical trials of drotrecogin alfa, the incidence of purpura fulminans in severe sepsis/septic shock was only 2% [29]. In comparison, our findings suggest that in patients with invasive pneumococcal disease with purpura fulminans, diagnostic tests for hyposplenia or asplenia should be performed.
Our study has limitations. Due to slow recruitment and limited funding, the study was terminated before the recruitment goal of 200 patients was reached. However, our initial sample size calculation turned out to be too conservative, and the number of patients enrolled was eventually sufficient to analyze the primary endpoint with adequate power. Because OPSI is such a rare condition, neither a formal assessment of patient screening nor measurement of the incidence of OPSI among ICU patients was feasible. Also, the intensity and quality of screening for patients meeting the inclusion criteria was not monitored, which is relevant to the representativeness of the patients included. In this regard, study physicians may have screened for a typical presentation of OPSI and may have overmatched patients without asplenia, leading to selection bias. Because our study screened only ICU patients, the trial also does not provide data on severe infections treated outside the ICU. Only a few pneumococcal isolates were sent for serotyping and further characterization. Therefore, we cannot provide data on the seroepidemiology of pneumococcal infection in vaccinated or nonvaccinated study participants. Finally, uneven losses to follow-up and different access to healthcare may constitute additional sources of bias.
CONCLUSIONS
Our study highlights the fact that OPSI remains a critical illness, and most cases are still caused by a potentially vaccine-preventable infection. Furthermore, the increased risk for infection may persist long after splenectomy. In light of these findings, more efforts should be made to improve the uptake of pneumococcal vaccination after splenectomy, enhance its efficacy, and develop vaccine strategies for lifelong protection. Growing evidence supports the use of pneumococcal conjugate vaccines, which offer improved immunogenicity and protection compared with polysaccharide vaccines [30, 31].
Notes
Acknowledgments. The authors thank the following persons for their support. Statistical support in trial design: Jan Beyersmann (Institute of Medical Biometry and Medical Informatics, University Medical Center Freiburg) and Sarah Tschudin-Sutter (Division of Infectious Diseases and Hospital Epidemiology, University Hospital Basel, Switzerland). Clinical data collection and management: Christine Winterhalter (Clinical Research Unit, Center for Chronic Immunodeficiency [CCI] Freiburg). Study Nurses: Petra Bloos, Almut Noack, Daniela Fergen, Ulrike Redlich, and Anke Braune (Department of Anesthesiology and Intensive Care Medicine, Jena). Central Sample Biobank: Ilka Fuchs (CCI Freiburg). Study administration, case record form design and trial management: Ralf Tostmann (Clinical Trials Unit, University Medical Center Freiburg), Christine Winterhalter, Annette Uhlmann (both Clinical Research Unit, CCI Freiburg). Microbiologic diagnostics: Heike Wölk (Institute of Medical Microbiology and Hygiene, University Medical Center Freiburg), and Martina Vavra (Center for Infectious Diseases and Travel Medicine, University Medical Center Freiburg). The full list of the Spleen Off Study investigators is shown in the Supplementary Materials.
Financial support. This study was supported by the Federal Ministry of Education and Research (grant number BMBF 01 EO 0803).
Potential conflicts of interest. C. T. has received personal fees and nonfinancial support from Pfizer. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Presented in part: European Congress of Medical Microbiology and Infectious Diseases (ECCMID), Barcelona, Spain, 11 May 2014.
Current affiliation: Division of Infectious Diseases and Hospital Epidemiology, University Hospital Basel, Switzerland.
F. M. B. and W. V. K. contributed equally to this work.
Members of the SPLEEN OFF Study Group are listed in the Supplementary Appendix.
- sepsis
- body mass index procedure
- septic shock
- cancer
- intensive care unit
- pneumococcal infections
- pneumococcal vaccine
- prospective studies
- splenectomy
- streptococcus pneumoniae
- infections
- pathogenic organism
- purpura fulminans
- sepsis, severe
- congenital absence of spleen
- post-splenectomy septicemia
- bloodstream infections
- community




