-
PDF
- Split View
-
Views
-
Cite
Cite
Vincent C. C. Cheng, Jonathan H. K. Chen, Sally C. Y. Wong, Sally S. M. Leung, Simon Y. C. So, David C. Lung, Wan-Mui Lee, Nigel J. Trendell-Smith, Wai-Ming Chan, Desmond Ng, Liza To, Albert K. W. Lie, Kwok-Yung Yuen, Hospital Outbreak of Pulmonary and Cutaneous Zygomycosis due to Contaminated Linen Items From Substandard Laundry, Clinical Infectious Diseases, Volume 62, Issue 6, 15 March 2016, Pages 714–721, https://doi.org/10.1093/cid/civ1006
Close - Share Icon Share
Abstract
Background. Healthcare laundry-related infection is rare, and pulmonary zygomycosis due to contaminated hospital linens has never been reported.
Methods. We reported an outbreak investigation of zygomycosis in a university-affiliated teaching hospital. Air samplers, sponge swabs and Replicate Organism Detection and Counting (RODAC) contact plates were used for environmental sampling. The fungal isolates from clinical and environmental samples were identified by morphology, MALDI-TOF MS, and ITS1-5.8S-ITS2 rRNA gene cluster sequencing.
Results. From 2 June 2015 to 18 July 2015, 6 immunosuppressed patients developed pulmonary (n = 4) and/or cutaneous (n = 3) infection by a spore-forming mold, Rhizopus microsporus, through direct inhalation and skin contact of contaminated linen items supplied by a designated laundry. Seventy (27.8%) of 252 freshly laundered clothing and 15 (3.4%) of 443 nonclothing laundered linen items (pillow case, bed sheet, draw sheet) were contaminated by R. microsporus, which was significantly higher than those from other hospital laundries (0%, n = 451; P < .001) supplying linen to hospitals with no cases of zygomycosis reported during the same period. The fungal isolates from patients and linens were phylogenetically related. In sum, 61% of environmental samples and 100% of air samples at the designated laundry were also positive for zygomycetes, suggesting heavy environmental contamination. RODAC contact plates revealed mean total viable bacteria counts of freshly laundered items (1028 ± 611 CFU/100 cm2) far exceeded the “hygienically clean” standard of 20 CFU/100 cm2 set by the US healthcare textile certification requirement.
Conclusions. Suboptimal conditions of washing, drying, and storage contributed to the massive linen contamination and the outbreak of zygomycosis.
Invasive zygomycosis is an emerging infection that is increasingly reported in immunosuppressed hosts [1] and hospital outbreaks of gastrointestinal, sinopulmonary, or cutaneous zygomycosis [2–4]. The source of such outbreaks has been traced to adhesive bandages, wooden tongue depressors, ostomy bags, damaged water circuitry, and adjacent building construction activity [5, 6]. An outbreak of gastrointestinal zygomycosis in our Hemopoietic Stem Cell Transplant Center (HSCT) due to contaminated allopurinol tablets was previously reported [2], whereas an outbreak of cutaneous zygomycosis due to contaminated linen items by Rhizopus species was recently reported in Louisiana. Here we report a cluster of pulmonary and cutaneous zygomycosis due to R. microsporus within 2 months among immunosuppressed patients due to the use of contaminated linen items supplied by a designated laundry. Because only 12 hospital outbreaks have been related to laundered linen items in the past 43 years, there is currently no public health consensus on the standard of hygienically clean linen items, and its importance has mostly been overlooked in most hospitals [7]. Our findings suggested that such standards should be adopted to prevent similar outbreaks due to the inadvertent exposure of laundered linen items to environmental contamination such as dust in storage areas or process failure during laundering.
METHODS
Outbreak Investigation
An outbreak of pulmonary and cutaneous zygomycosis occurred over a period of 2 months (between 2 June and 18 July 2015) in Queen Mary Hospital (QMH), a 1700-bed university-affiliated tertiary referral center with acute emergency and comprehensive service for refractory illnesses including major organ failures requiring organ support and solid organ transplantation, and hematological malignancies requiring hemopoietic stem cell transplantation in Hong Kong. A case was defined as patient with zygomycetes isolated from their clinical specimens during hospitalization between 1 January and 31 July 2015.
Microbiological laboratory data were retrieved from the Laboratory Information System to identify any unrecognized cases and to establish the background rate of this rare infection. The medical records and clinical data in the Clinical Management System of the case patients were reviewed by clinical microbiologists and the infection control team, as described in our previous outbreak investigations [2, 8–10]. Nursing staff were asked and inspected for any changes in their patient care practice. Patients and their relatives, if available, were interviewed in detail for their hygienic measures and any potential source of infection acquired from the community. Air samples and environmental samples were collected. Because of the cutaneous nature of infection in the first 2 cases, diagnosed in two different wards, environmental agents such as linen items, dressing, and adhesive tapes directly applied onto the patients' skin were further investigated by fungal culture on 16 July 2015 (Figure 1). Notably, a high proportion of laundered linen items, but not air and other environmental samples, in ward storage were positive for zygomycetes; thus a site visit and environmental sampling at the central linen storage rooms was conducted on 18 July 2015. Laundered linen items just arriving from the designated laundry (SWL) to QMH were also sampled. Again a high proportion of these freshly laundered linen items were positive for zygomycetes. Consequently, linen supply by SWL was suspended immediately, and a site visit to SWL was conducted on 20 July 2015 to ascertain the source of contamination.
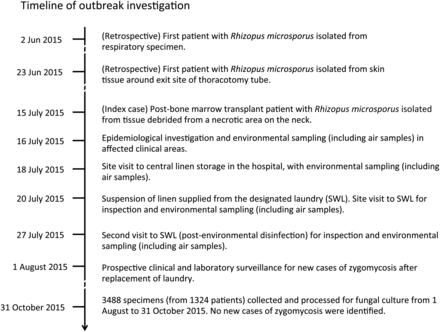
Case-control study was performed to identify risk factors of zygomycosis among the immunosuppressed patients. Clinical and laboratory surveillance of immunosuppressed patients for any new cases of zygomycosis continued after replacement of service provider from 1 August to 31 October 2015. Incidence of zygomycosis and linen contamination rate with the implicated zygomycetes in case hospital supplied by SWL and control hospitals supplied by other laundries were analyzed.
This study was approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Hospital Cluster.
Screening of Linen, Environmental, and Air Samples
Linen and environmental samples were collected using Polywipe sponge swabs (Medical Wire & Equipment, UK). These swabs are sterile premoistened thin flexible sponges tailor-made for sampling environmental surfaces. For linen samples, a surface area of 50 cm × 30 cm (visual reference size of a small pillow case) was swabbed by each sponge swab. We also performed quantitative culture using Replicate Organism Detection and Counting (RODAC) plates, which were pressed onto the fabric's surface with a contact time of 10 seconds [11]. For environmental samples, a surface area of 50 cm × 30 cm was swabbed, except for the conveyor belts and the machine wheel adjacent to the conveyor belts of the calendering machines, which was swabbed according to the available surface areas at different locations in SWL. The sampled sponge swabs were put into sealed sterile plastic bags individually and were properly labeled before further processing in the laboratory.
An air sampler, SAS Super ISO 180 model 86834 (VWR International PBI S.r.l., Milan, Italy) was used to collect 1000 liters of air at a rate of 180 liters of air per minute for each fungal air sampling. The air collected directly passed onto the Sabouraud dextrose agar plates during the 6-minute process. The number of colony forming units of fungi was counted and the fungi were further speciated.
Specimen Processing in Laboratory
The RODAC plates and air sample dextrose agar plates were incubated directly after sample collection at 37°C for 2 days and at 42°C for 5 days, respectively, whereas all other environmental samples required initial processing in Class II biosafety cabinets. For each sponge swab, 2 mL sterile normal saline (0.85% saline) was added into the plastic bag and was absorbed by the sponge swab. The sponge swab was then squeezed repeatedly for proper mixing. Then, 1 mL suspension from the bag was transferred to 8 mL Sabouraud dextrose broth (Oxoid, UK) supplemented with vancomycin (10 µg/mL), meropenem (10 µg/mL), and anidulafungin (10 µg/mL). The broth was then incubated at 42°C for 5 days and examined daily for visible fungal ball growth. Broth with suspected fungal growth was subcultured onto similarly supplemented Sabouraud dextrose agar for mature single colonies for morphological identification.
Laboratory Identification of Mold Isolates
Fungal colonies were primarily examined under direct microscopy for morphological characteristic of zygomycetes [12]. Identification was further confirmed by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) or fungal internal transcribed spacer (ITS) gene sequencing.
MALDI-TOF MS Identification
Hyphae of mature fungal colonies on the supplemented Sabouraud dextrose agar were picked for MALDI-TOF MS identification. Proteins were extracted from fungal hyphae using ethanol-formic acid extraction method. Protein spectra of the samples were measured by Microflex LT (Bruker Daltonik, Germany) and matched against the Bruker Biotyper filamentous fungi database version 1.0 and HKU in-house spectra library including an extra 18 Mucorales reference isolates (8 R. microsporus, 3 R. oryzae, 3 Lichitheimia species, 2 Cunninghamella species, and 2 Syncephalastrum species) collected in Hong Kong. Samples with confidence score >2.0 in the Biotyper software were accepted as species level identification.
ITS Sequencing and Phylogenetic Analysis
Fungal DNA extraction was performed by the EZ1 Advanced automated nucleic acid extraction system (Qiagen, Hilden, Germany). Polymerase chain reaction (PCR) amplification and DNA sequencing of the ITS were performed according to published protocols [13]. The sequences of the PCR products were compared with sequences of closely related species in the GenBank by multiple sequence alignment using BioEdit version 7.0.9. Phylogenetic tree construction was performed using the neighbor joining method with 1000 bootstrap replicates by MEGA 6 [14], and 648 bp nucleotide positions of ITS were included in the analysis.
Statistical Analysis
The χ2 or Fisher exact test was used to compare independent categorical variables between groups. All reported P values were 2-sided. A P value of <.05 was considered to be statistically significant. SPSS version 19 was used to perform the statistical analyses.
RESULTS
Outbreak Investigation
Between 2 June and 18 July 2015, 6 adult immunosuppressed patients presented with pulmonary (3 cases) or cutaneous (2 cases) or both pulmonary and cutaneous (1 case) infection by R. microsporus (Table 1 and Supplementary Figure 1A–C). The female to male ratio was 2:1 with a median age of 49.5 years (range 42–74 years). The mean time interval from admission to the diagnosis of fungal infection was 69 ± 59 days. Case-control analysis with age- and sex-matched immunosuppressed patients revealed that the duration of hospitalization was the most significant risk factor associated with nosocomial zygomycosis (Table 2). Upon interview with 3 cases (case 1, 5, and 6), no known risk factors for community acquisition of zygomycosis such as renovation of household or neighborhood was identified. All 10 air samples collected from the patient levels and ventilation levels of the affected units were negative for the implicated zygomycetes (R. microsporus and Lichtheimia species). Because of the cutaneous nature of zygomycosis in 2 case patients and the diverse locations of the outbreak, agents that were directly applied onto patients' skin or linen items were suspected to be the common source of this outbreak. Of 34 nonlinen environmental samples taken in the clinical areas, including surgical dressing, gauze dressing, adhesive tapes, none were positive for zygomycetes. Eighteen (26.9%) of 67 laundered linen items collected in clinical areas and central linen storage at QMH and 13 (65.0%) of 20 freshly arrived linen items from SWL were positive for the implicated zygomycetes (Supplementary Table 1). The mean total viable count of 15 freshly laundered linens items just delivered from SWL was 1028 ± 611 CFU/100 cm2, which were mostly Bacillus species on the RODAC plates.
Clinical and Epidemiological Characteristics of Patients With Isolation of Zygomycetes During the Outbreak
| Case (Status) [Clinical Unit/Ward] . | Sex/Age . | Underlying Diseases and (Immunosuppressive Therapy) [Date of Admission] . | Clinical Manifestation . | Laboratory Diagnosis of Zygomycetes (Date of Culture) . | Sequential Antifungal Therapy Before and (After) Diagnosis . | Outcome . |
|---|---|---|---|---|---|---|
| 1 (index)a [HSCT/J8] | M/49 | Follicular lymphoma, MDS, BMT on 30 June 2015 (cyclosporin A and MMF) [15 June 2015] | Cutaneous: carbuncle at the back of neck on 10 July 2015; incision & drainage on 15 July 2015; radiological evidence of lung involvement on 21 July 2015 | Culture of neck tissue: R. microsporus and Lichtheimia spp. (15 July 2015); Fine needle aspirate of pulmonary lesion fungal elements seen, culture negative (26 August 2015) | Itraconaozle, micafungin, anidulafungin, (AmBisome, posaconazole) | Survived |
| 2 (retrospective) [AICU/E2] | M/74 | HBV, HCC, post-liver transplantation [24 April 2015] | Cutaneous: necrotic skin around chest drain insertion site on 22 June 2015 | Culture of necrotic tissue: R. microsporus (23 June 2015) | Anidulafungin | Died (1 d after diagnosis) |
| 3 (retrospective) [AICU/E2] | F/42 | Interstitial lung disease (methylprednisolone) [29 April 2015] | Pulmonary: progressive worsening of pneumonia | Culture of BAL: R. microsporus (2 June 2015) | Voriconazole, (AmBisome, posaconazole, terbinafine) | Died (42 d after diagnosis) |
| 4 (retrospective) [medical/K21] | F/50 | Palliative ALL, relapse post-BMT (dexamethasone, MMF) [13 March 2015] | Pulmonary: nosocomial pneumonia of left lower lobe | Culture of sputum: R. microsporus (4 July 2015) | Voriconazole, (AmBisome) | Died (47 d after diagnosis) |
| 5 (retrospective) [CTSU/D5] | F/49 | Post-double lung transplantation (prednisolone, everolimus, MMF, tacrolimus) [9 January 2015] | Pulmonary: Blood stained sputum; CXR showed right lower zone infiltrates | Culture of BAL: R. microsporus (24 June 2015) | (Posaconazole) | Survived |
| 6 (prospective)b [RDC/K18S] | F/72 | MDS, ESRF on regular HD [8 July 2015] | Cutaneous: purulent discharge from HD exit site on 18 July 2015 | Culture of exit site of HD: R. microsporus (18 July 2015) | (Posaconazole) | Survived |
| Case (Status) [Clinical Unit/Ward] . | Sex/Age . | Underlying Diseases and (Immunosuppressive Therapy) [Date of Admission] . | Clinical Manifestation . | Laboratory Diagnosis of Zygomycetes (Date of Culture) . | Sequential Antifungal Therapy Before and (After) Diagnosis . | Outcome . |
|---|---|---|---|---|---|---|
| 1 (index)a [HSCT/J8] | M/49 | Follicular lymphoma, MDS, BMT on 30 June 2015 (cyclosporin A and MMF) [15 June 2015] | Cutaneous: carbuncle at the back of neck on 10 July 2015; incision & drainage on 15 July 2015; radiological evidence of lung involvement on 21 July 2015 | Culture of neck tissue: R. microsporus and Lichtheimia spp. (15 July 2015); Fine needle aspirate of pulmonary lesion fungal elements seen, culture negative (26 August 2015) | Itraconaozle, micafungin, anidulafungin, (AmBisome, posaconazole) | Survived |
| 2 (retrospective) [AICU/E2] | M/74 | HBV, HCC, post-liver transplantation [24 April 2015] | Cutaneous: necrotic skin around chest drain insertion site on 22 June 2015 | Culture of necrotic tissue: R. microsporus (23 June 2015) | Anidulafungin | Died (1 d after diagnosis) |
| 3 (retrospective) [AICU/E2] | F/42 | Interstitial lung disease (methylprednisolone) [29 April 2015] | Pulmonary: progressive worsening of pneumonia | Culture of BAL: R. microsporus (2 June 2015) | Voriconazole, (AmBisome, posaconazole, terbinafine) | Died (42 d after diagnosis) |
| 4 (retrospective) [medical/K21] | F/50 | Palliative ALL, relapse post-BMT (dexamethasone, MMF) [13 March 2015] | Pulmonary: nosocomial pneumonia of left lower lobe | Culture of sputum: R. microsporus (4 July 2015) | Voriconazole, (AmBisome) | Died (47 d after diagnosis) |
| 5 (retrospective) [CTSU/D5] | F/49 | Post-double lung transplantation (prednisolone, everolimus, MMF, tacrolimus) [9 January 2015] | Pulmonary: Blood stained sputum; CXR showed right lower zone infiltrates | Culture of BAL: R. microsporus (24 June 2015) | (Posaconazole) | Survived |
| 6 (prospective)b [RDC/K18S] | F/72 | MDS, ESRF on regular HD [8 July 2015] | Cutaneous: purulent discharge from HD exit site on 18 July 2015 | Culture of exit site of HD: R. microsporus (18 July 2015) | (Posaconazole) | Survived |
Abbreviations: AICU, adult intensive care unit; ALL, acute lymphocytic leukemia; BMT, blood and marrow transplantation; CTSU, cardiothoracic surgical unit; CXR, Chest X-Ray; ESRF, end-stage renal failure; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HD, hemodialysis; HSCT, hemopoietic stem cell transplant center; MDS, myelodysplastic syndrome; MMF, mycophenolate mofetil; RDC, renal dialysis center.
a Subsequent development of pulmonary coin-size lesion suggestive of pulmonary zygomycosis.
b This patient was diagnosed immediately before the suspension of linen supply by the designated laundry (SWL).
Clinical and Epidemiological Characteristics of Patients With Isolation of Zygomycetes During the Outbreak
| Case (Status) [Clinical Unit/Ward] . | Sex/Age . | Underlying Diseases and (Immunosuppressive Therapy) [Date of Admission] . | Clinical Manifestation . | Laboratory Diagnosis of Zygomycetes (Date of Culture) . | Sequential Antifungal Therapy Before and (After) Diagnosis . | Outcome . |
|---|---|---|---|---|---|---|
| 1 (index)a [HSCT/J8] | M/49 | Follicular lymphoma, MDS, BMT on 30 June 2015 (cyclosporin A and MMF) [15 June 2015] | Cutaneous: carbuncle at the back of neck on 10 July 2015; incision & drainage on 15 July 2015; radiological evidence of lung involvement on 21 July 2015 | Culture of neck tissue: R. microsporus and Lichtheimia spp. (15 July 2015); Fine needle aspirate of pulmonary lesion fungal elements seen, culture negative (26 August 2015) | Itraconaozle, micafungin, anidulafungin, (AmBisome, posaconazole) | Survived |
| 2 (retrospective) [AICU/E2] | M/74 | HBV, HCC, post-liver transplantation [24 April 2015] | Cutaneous: necrotic skin around chest drain insertion site on 22 June 2015 | Culture of necrotic tissue: R. microsporus (23 June 2015) | Anidulafungin | Died (1 d after diagnosis) |
| 3 (retrospective) [AICU/E2] | F/42 | Interstitial lung disease (methylprednisolone) [29 April 2015] | Pulmonary: progressive worsening of pneumonia | Culture of BAL: R. microsporus (2 June 2015) | Voriconazole, (AmBisome, posaconazole, terbinafine) | Died (42 d after diagnosis) |
| 4 (retrospective) [medical/K21] | F/50 | Palliative ALL, relapse post-BMT (dexamethasone, MMF) [13 March 2015] | Pulmonary: nosocomial pneumonia of left lower lobe | Culture of sputum: R. microsporus (4 July 2015) | Voriconazole, (AmBisome) | Died (47 d after diagnosis) |
| 5 (retrospective) [CTSU/D5] | F/49 | Post-double lung transplantation (prednisolone, everolimus, MMF, tacrolimus) [9 January 2015] | Pulmonary: Blood stained sputum; CXR showed right lower zone infiltrates | Culture of BAL: R. microsporus (24 June 2015) | (Posaconazole) | Survived |
| 6 (prospective)b [RDC/K18S] | F/72 | MDS, ESRF on regular HD [8 July 2015] | Cutaneous: purulent discharge from HD exit site on 18 July 2015 | Culture of exit site of HD: R. microsporus (18 July 2015) | (Posaconazole) | Survived |
| Case (Status) [Clinical Unit/Ward] . | Sex/Age . | Underlying Diseases and (Immunosuppressive Therapy) [Date of Admission] . | Clinical Manifestation . | Laboratory Diagnosis of Zygomycetes (Date of Culture) . | Sequential Antifungal Therapy Before and (After) Diagnosis . | Outcome . |
|---|---|---|---|---|---|---|
| 1 (index)a [HSCT/J8] | M/49 | Follicular lymphoma, MDS, BMT on 30 June 2015 (cyclosporin A and MMF) [15 June 2015] | Cutaneous: carbuncle at the back of neck on 10 July 2015; incision & drainage on 15 July 2015; radiological evidence of lung involvement on 21 July 2015 | Culture of neck tissue: R. microsporus and Lichtheimia spp. (15 July 2015); Fine needle aspirate of pulmonary lesion fungal elements seen, culture negative (26 August 2015) | Itraconaozle, micafungin, anidulafungin, (AmBisome, posaconazole) | Survived |
| 2 (retrospective) [AICU/E2] | M/74 | HBV, HCC, post-liver transplantation [24 April 2015] | Cutaneous: necrotic skin around chest drain insertion site on 22 June 2015 | Culture of necrotic tissue: R. microsporus (23 June 2015) | Anidulafungin | Died (1 d after diagnosis) |
| 3 (retrospective) [AICU/E2] | F/42 | Interstitial lung disease (methylprednisolone) [29 April 2015] | Pulmonary: progressive worsening of pneumonia | Culture of BAL: R. microsporus (2 June 2015) | Voriconazole, (AmBisome, posaconazole, terbinafine) | Died (42 d after diagnosis) |
| 4 (retrospective) [medical/K21] | F/50 | Palliative ALL, relapse post-BMT (dexamethasone, MMF) [13 March 2015] | Pulmonary: nosocomial pneumonia of left lower lobe | Culture of sputum: R. microsporus (4 July 2015) | Voriconazole, (AmBisome) | Died (47 d after diagnosis) |
| 5 (retrospective) [CTSU/D5] | F/49 | Post-double lung transplantation (prednisolone, everolimus, MMF, tacrolimus) [9 January 2015] | Pulmonary: Blood stained sputum; CXR showed right lower zone infiltrates | Culture of BAL: R. microsporus (24 June 2015) | (Posaconazole) | Survived |
| 6 (prospective)b [RDC/K18S] | F/72 | MDS, ESRF on regular HD [8 July 2015] | Cutaneous: purulent discharge from HD exit site on 18 July 2015 | Culture of exit site of HD: R. microsporus (18 July 2015) | (Posaconazole) | Survived |
Abbreviations: AICU, adult intensive care unit; ALL, acute lymphocytic leukemia; BMT, blood and marrow transplantation; CTSU, cardiothoracic surgical unit; CXR, Chest X-Ray; ESRF, end-stage renal failure; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HD, hemodialysis; HSCT, hemopoietic stem cell transplant center; MDS, myelodysplastic syndrome; MMF, mycophenolate mofetil; RDC, renal dialysis center.
a Subsequent development of pulmonary coin-size lesion suggestive of pulmonary zygomycosis.
b This patient was diagnosed immediately before the suspension of linen supply by the designated laundry (SWL).
Characteristics of Age- and Sex-Matched Hospitalized Immunosuppressed Patients at Queen Mary Hospital With or Without Culture Documented Zygomycosisa
| . | Case (n = 6) . | Control (n = 24) . | P Value . |
|---|---|---|---|
| Age (mean ± SD) | 56 ± 13 | 55 ± 11 | .875 |
| Female sex | 4 (66.6%) | 14 (58.3%) | .709 |
| Predominant underlying diseaseb | |||
| Post-solid organ transplantation | 2 (33.3%) | 1 (4.2%) | .171 |
| Hematological malignancy | 3 (50%)c | 5 (20.8%)d | .353 |
| Solid organ malignancy with metastasis | 0 | 7 (29.2%) | .331 |
| Solid organ malignancy without metastasis | 0 | 9 (37.5%) | .195 |
| Autoimmune disease | 1 (16.7%) | 2 (8.3%) | .543 |
| Hematological parameterse | |||
| Total white cell count (mean ± SD) x109/L | 1.86 ± 1.04 | 4.37 ± 3.15 | .067 |
| Duration of leucopenia (mean day ± SD)f | 36.6 ± 15.0 | 7.7 ± 1.6 | .005 |
| Absolute neutrophil count (mean ± SD) ×109/L | 0.97 ± 0.86 | 2.88 ± 2.32 | .061 |
| Absolute lymphocyte count (mean ± SD) ×109/L | 0.20 ± 0.18 | 0.94 ± 0.74 | .024 |
| Hemoglobin (mean ± SD) g/dL | 6.7 ± 0.5 | 11.2 ± 2.6 | <.001g |
| Platelet count (mean ± SD) ×109/L | 79 ± 155 | 160 ± 114 | .163 |
| Use of immunosuppressive therapyh | |||
| Corticosteroids | 3 (50%) | 7 (29.2%) | .628 |
| Cyclosporin A/Mycophenolate/Sirolimus/Tacrolimus | 3 (50%) | 4 (16.7%) | .235 |
| Presence of percutaneous medical devicesi | 5 (83.3%) | 7 (29.2%) | .050 |
| Presence of skin lesions (ulcer or wound) | 3 (50%) | 0 | .004 |
| Duration of hospitalization (mean day ± SD) | 68.8 ± 59.4 | 8.8 ± 9.9 | <.001 |
| . | Case (n = 6) . | Control (n = 24) . | P Value . |
|---|---|---|---|
| Age (mean ± SD) | 56 ± 13 | 55 ± 11 | .875 |
| Female sex | 4 (66.6%) | 14 (58.3%) | .709 |
| Predominant underlying diseaseb | |||
| Post-solid organ transplantation | 2 (33.3%) | 1 (4.2%) | .171 |
| Hematological malignancy | 3 (50%)c | 5 (20.8%)d | .353 |
| Solid organ malignancy with metastasis | 0 | 7 (29.2%) | .331 |
| Solid organ malignancy without metastasis | 0 | 9 (37.5%) | .195 |
| Autoimmune disease | 1 (16.7%) | 2 (8.3%) | .543 |
| Hematological parameterse | |||
| Total white cell count (mean ± SD) x109/L | 1.86 ± 1.04 | 4.37 ± 3.15 | .067 |
| Duration of leucopenia (mean day ± SD)f | 36.6 ± 15.0 | 7.7 ± 1.6 | .005 |
| Absolute neutrophil count (mean ± SD) ×109/L | 0.97 ± 0.86 | 2.88 ± 2.32 | .061 |
| Absolute lymphocyte count (mean ± SD) ×109/L | 0.20 ± 0.18 | 0.94 ± 0.74 | .024 |
| Hemoglobin (mean ± SD) g/dL | 6.7 ± 0.5 | 11.2 ± 2.6 | <.001g |
| Platelet count (mean ± SD) ×109/L | 79 ± 155 | 160 ± 114 | .163 |
| Use of immunosuppressive therapyh | |||
| Corticosteroids | 3 (50%) | 7 (29.2%) | .628 |
| Cyclosporin A/Mycophenolate/Sirolimus/Tacrolimus | 3 (50%) | 4 (16.7%) | .235 |
| Presence of percutaneous medical devicesi | 5 (83.3%) | 7 (29.2%) | .050 |
| Presence of skin lesions (ulcer or wound) | 3 (50%) | 0 | .004 |
| Duration of hospitalization (mean day ± SD) | 68.8 ± 59.4 | 8.8 ± 9.9 | <.001 |
Abbreviation: SD, standard deviation.
a Of 413 hospitalized immunosuppressed patients who were admitted to the hemopoietic stem cell transplant center, solid organ transplant wards, hematology and oncology wards, and intensive care units during the outbreak period, 24 age- and sex-matched controls were chosen for the case-control analysis.
b The most immunosuppressed condition was chosen for analysis.
c Including 2 patients with post-hemopoietic stem cell transplantation, and 1 patient with myelodysplastic syndrome.
d Including 3 patients with acute leukemia and 2 patients with lymphoma.
e The hematological parameters were chosen on the date with lowest absolute neutrophil count before the diagnosis of zygomycosis and throughout hospitalization for cases and controls respectively.
f Leucopenia is defined as white cell count of <4 × 109/L.
g One hematology patient in case group also had end-stage renal failure on hemodialysis, which may explain a difference in hemoglobulin.
h Patient may receive more than one agent.
i Including central venous catheter, thoracotomy tube, and percutaneous transhepatic biliary drainage tube.
Characteristics of Age- and Sex-Matched Hospitalized Immunosuppressed Patients at Queen Mary Hospital With or Without Culture Documented Zygomycosisa
| . | Case (n = 6) . | Control (n = 24) . | P Value . |
|---|---|---|---|
| Age (mean ± SD) | 56 ± 13 | 55 ± 11 | .875 |
| Female sex | 4 (66.6%) | 14 (58.3%) | .709 |
| Predominant underlying diseaseb | |||
| Post-solid organ transplantation | 2 (33.3%) | 1 (4.2%) | .171 |
| Hematological malignancy | 3 (50%)c | 5 (20.8%)d | .353 |
| Solid organ malignancy with metastasis | 0 | 7 (29.2%) | .331 |
| Solid organ malignancy without metastasis | 0 | 9 (37.5%) | .195 |
| Autoimmune disease | 1 (16.7%) | 2 (8.3%) | .543 |
| Hematological parameterse | |||
| Total white cell count (mean ± SD) x109/L | 1.86 ± 1.04 | 4.37 ± 3.15 | .067 |
| Duration of leucopenia (mean day ± SD)f | 36.6 ± 15.0 | 7.7 ± 1.6 | .005 |
| Absolute neutrophil count (mean ± SD) ×109/L | 0.97 ± 0.86 | 2.88 ± 2.32 | .061 |
| Absolute lymphocyte count (mean ± SD) ×109/L | 0.20 ± 0.18 | 0.94 ± 0.74 | .024 |
| Hemoglobin (mean ± SD) g/dL | 6.7 ± 0.5 | 11.2 ± 2.6 | <.001g |
| Platelet count (mean ± SD) ×109/L | 79 ± 155 | 160 ± 114 | .163 |
| Use of immunosuppressive therapyh | |||
| Corticosteroids | 3 (50%) | 7 (29.2%) | .628 |
| Cyclosporin A/Mycophenolate/Sirolimus/Tacrolimus | 3 (50%) | 4 (16.7%) | .235 |
| Presence of percutaneous medical devicesi | 5 (83.3%) | 7 (29.2%) | .050 |
| Presence of skin lesions (ulcer or wound) | 3 (50%) | 0 | .004 |
| Duration of hospitalization (mean day ± SD) | 68.8 ± 59.4 | 8.8 ± 9.9 | <.001 |
| . | Case (n = 6) . | Control (n = 24) . | P Value . |
|---|---|---|---|
| Age (mean ± SD) | 56 ± 13 | 55 ± 11 | .875 |
| Female sex | 4 (66.6%) | 14 (58.3%) | .709 |
| Predominant underlying diseaseb | |||
| Post-solid organ transplantation | 2 (33.3%) | 1 (4.2%) | .171 |
| Hematological malignancy | 3 (50%)c | 5 (20.8%)d | .353 |
| Solid organ malignancy with metastasis | 0 | 7 (29.2%) | .331 |
| Solid organ malignancy without metastasis | 0 | 9 (37.5%) | .195 |
| Autoimmune disease | 1 (16.7%) | 2 (8.3%) | .543 |
| Hematological parameterse | |||
| Total white cell count (mean ± SD) x109/L | 1.86 ± 1.04 | 4.37 ± 3.15 | .067 |
| Duration of leucopenia (mean day ± SD)f | 36.6 ± 15.0 | 7.7 ± 1.6 | .005 |
| Absolute neutrophil count (mean ± SD) ×109/L | 0.97 ± 0.86 | 2.88 ± 2.32 | .061 |
| Absolute lymphocyte count (mean ± SD) ×109/L | 0.20 ± 0.18 | 0.94 ± 0.74 | .024 |
| Hemoglobin (mean ± SD) g/dL | 6.7 ± 0.5 | 11.2 ± 2.6 | <.001g |
| Platelet count (mean ± SD) ×109/L | 79 ± 155 | 160 ± 114 | .163 |
| Use of immunosuppressive therapyh | |||
| Corticosteroids | 3 (50%) | 7 (29.2%) | .628 |
| Cyclosporin A/Mycophenolate/Sirolimus/Tacrolimus | 3 (50%) | 4 (16.7%) | .235 |
| Presence of percutaneous medical devicesi | 5 (83.3%) | 7 (29.2%) | .050 |
| Presence of skin lesions (ulcer or wound) | 3 (50%) | 0 | .004 |
| Duration of hospitalization (mean day ± SD) | 68.8 ± 59.4 | 8.8 ± 9.9 | <.001 |
Abbreviation: SD, standard deviation.
a Of 413 hospitalized immunosuppressed patients who were admitted to the hemopoietic stem cell transplant center, solid organ transplant wards, hematology and oncology wards, and intensive care units during the outbreak period, 24 age- and sex-matched controls were chosen for the case-control analysis.
b The most immunosuppressed condition was chosen for analysis.
c Including 2 patients with post-hemopoietic stem cell transplantation, and 1 patient with myelodysplastic syndrome.
d Including 3 patients with acute leukemia and 2 patients with lymphoma.
e The hematological parameters were chosen on the date with lowest absolute neutrophil count before the diagnosis of zygomycosis and throughout hospitalization for cases and controls respectively.
f Leucopenia is defined as white cell count of <4 × 109/L.
g One hematology patient in case group also had end-stage renal failure on hemodialysis, which may explain a difference in hemoglobulin.
h Patient may receive more than one agent.
i Including central venous catheter, thoracotomy tube, and percutaneous transhepatic biliary drainage tube.
We sampled a total of 695 laundered linen items supplied by SWL, and 451 linen items from the storage of public hospitals supplied by 9 other laundries in Hong Kong were also sampled as controls. None (0%) of 451 linen items collected from the control hospitals were positive for zygomycetes (Supplementary Table 2), whereas 70 (27.8%) of the 252 clothing and 15 (3.4%) of the 443 nonclothing linen items collected from the SWL were positive for the implicated zygomycetes, with a significantly higher contamination rate compared with linens processed by other laundries (0%, n = 451; P < .001). In addition, the incidence of zygomycetes per 100 000 patient admission in case hospital (QMH) was significantly higher than that of the control hospitals (14.8 vs 0; P < .001) (Table 3). The clustering of R. microsporus isolates from patients, linens, and environment on the phylogenetic tree of their ITS1-5.8S-ITS2 rRNA gene cluster (ITS) region was illustrated in Supplementary Figure 2. No new cases of zygomycosis were identified between 1 August and 31 October 2015 since the change of laundry service provider on 20 July 2015, with no positive isolates in 3488 specimens collected from 1324 patients, which were processed for fungal culture during this time period.
Incidence of Zygomycosis and Linen Contamination by Implicated Zygomycetes in Case Hospital Supplied by Designated Laundry (SWL) and Control Hospitals Supplied by Other Laundries
| . | Case Hospitals With Linen Items Supplied by Designated Laundry (SWL) . | Control Hospitals With Linen Items Supplied by Other Laundries . | P Value . |
|---|---|---|---|
| Number of patient admission between 1 June 2015 and 31 July 2015 | 40 442 | 242 980 | |
| Number of patient with implicated zygomycosis between 1 June 2015 and 31 July 2015 | 6 | 0 | |
| Number of patient with implicated zygomycetes per 100 000 patient admission between 1 June 2015 and 31 July 2015 | 14.8 | 0 | <.001 |
| Laundered linen items tested for the presence of implicated zygomycetesa | |||
| Clothing | 70/252 (27.8%) | 0/180 (0%) | <.001 |
| Patients' clothes | 42/138 (30.4%) | 0/101 (0%) | |
| Patients' trousers | 28/114 (24.6%) | 0/79 (0%) | |
| Non-clothing linen items | 15/443 (3.4%) | 0/271 (0%) | <.001 |
| Pillow case | 1/132 (0.8%) | 0/90 (0%) | |
| Bed sheetb | 6/143 (4.2%) | 0/101 (0%) | |
| Draw sheetc | 1/124 (0.8%) | 0/80 (0%) | |
| Blanket | 2/7 (28.6%) | Not tested | |
| Towel | 5/7 (71.4%) | Not tested | |
| Collars and belts | 0/30 (0%) | Not tested | |
| Total laundered linen items | 85/695 (12.2%) | 0/451 (0%) | <.001 |
| . | Case Hospitals With Linen Items Supplied by Designated Laundry (SWL) . | Control Hospitals With Linen Items Supplied by Other Laundries . | P Value . |
|---|---|---|---|
| Number of patient admission between 1 June 2015 and 31 July 2015 | 40 442 | 242 980 | |
| Number of patient with implicated zygomycosis between 1 June 2015 and 31 July 2015 | 6 | 0 | |
| Number of patient with implicated zygomycetes per 100 000 patient admission between 1 June 2015 and 31 July 2015 | 14.8 | 0 | <.001 |
| Laundered linen items tested for the presence of implicated zygomycetesa | |||
| Clothing | 70/252 (27.8%) | 0/180 (0%) | <.001 |
| Patients' clothes | 42/138 (30.4%) | 0/101 (0%) | |
| Patients' trousers | 28/114 (24.6%) | 0/79 (0%) | |
| Non-clothing linen items | 15/443 (3.4%) | 0/271 (0%) | <.001 |
| Pillow case | 1/132 (0.8%) | 0/90 (0%) | |
| Bed sheetb | 6/143 (4.2%) | 0/101 (0%) | |
| Draw sheetc | 1/124 (0.8%) | 0/80 (0%) | |
| Blanket | 2/7 (28.6%) | Not tested | |
| Towel | 5/7 (71.4%) | Not tested | |
| Collars and belts | 0/30 (0%) | Not tested | |
| Total laundered linen items | 85/695 (12.2%) | 0/451 (0%) | <.001 |
a In sum, 85 samples were positive for zygomycetes, including Rhizopus microsporus (59, 69.4%), Lichtheimia species (24, 28.2%), Rhizopus oryzae (1, 1.2%), Syncephalastrum racemosum (1, 1.2%).
b Bed sheet is a rectangular cloth used to cover a mattress.
c Draw sheet is a small bed sheet placed across the rectangular cloth over the bed sheet underneath the patient's back to facilitate transfer of patients.
Incidence of Zygomycosis and Linen Contamination by Implicated Zygomycetes in Case Hospital Supplied by Designated Laundry (SWL) and Control Hospitals Supplied by Other Laundries
| . | Case Hospitals With Linen Items Supplied by Designated Laundry (SWL) . | Control Hospitals With Linen Items Supplied by Other Laundries . | P Value . |
|---|---|---|---|
| Number of patient admission between 1 June 2015 and 31 July 2015 | 40 442 | 242 980 | |
| Number of patient with implicated zygomycosis between 1 June 2015 and 31 July 2015 | 6 | 0 | |
| Number of patient with implicated zygomycetes per 100 000 patient admission between 1 June 2015 and 31 July 2015 | 14.8 | 0 | <.001 |
| Laundered linen items tested for the presence of implicated zygomycetesa | |||
| Clothing | 70/252 (27.8%) | 0/180 (0%) | <.001 |
| Patients' clothes | 42/138 (30.4%) | 0/101 (0%) | |
| Patients' trousers | 28/114 (24.6%) | 0/79 (0%) | |
| Non-clothing linen items | 15/443 (3.4%) | 0/271 (0%) | <.001 |
| Pillow case | 1/132 (0.8%) | 0/90 (0%) | |
| Bed sheetb | 6/143 (4.2%) | 0/101 (0%) | |
| Draw sheetc | 1/124 (0.8%) | 0/80 (0%) | |
| Blanket | 2/7 (28.6%) | Not tested | |
| Towel | 5/7 (71.4%) | Not tested | |
| Collars and belts | 0/30 (0%) | Not tested | |
| Total laundered linen items | 85/695 (12.2%) | 0/451 (0%) | <.001 |
| . | Case Hospitals With Linen Items Supplied by Designated Laundry (SWL) . | Control Hospitals With Linen Items Supplied by Other Laundries . | P Value . |
|---|---|---|---|
| Number of patient admission between 1 June 2015 and 31 July 2015 | 40 442 | 242 980 | |
| Number of patient with implicated zygomycosis between 1 June 2015 and 31 July 2015 | 6 | 0 | |
| Number of patient with implicated zygomycetes per 100 000 patient admission between 1 June 2015 and 31 July 2015 | 14.8 | 0 | <.001 |
| Laundered linen items tested for the presence of implicated zygomycetesa | |||
| Clothing | 70/252 (27.8%) | 0/180 (0%) | <.001 |
| Patients' clothes | 42/138 (30.4%) | 0/101 (0%) | |
| Patients' trousers | 28/114 (24.6%) | 0/79 (0%) | |
| Non-clothing linen items | 15/443 (3.4%) | 0/271 (0%) | <.001 |
| Pillow case | 1/132 (0.8%) | 0/90 (0%) | |
| Bed sheetb | 6/143 (4.2%) | 0/101 (0%) | |
| Draw sheetc | 1/124 (0.8%) | 0/80 (0%) | |
| Blanket | 2/7 (28.6%) | Not tested | |
| Towel | 5/7 (71.4%) | Not tested | |
| Collars and belts | 0/30 (0%) | Not tested | |
| Total laundered linen items | 85/695 (12.2%) | 0/451 (0%) | <.001 |
a In sum, 85 samples were positive for zygomycetes, including Rhizopus microsporus (59, 69.4%), Lichtheimia species (24, 28.2%), Rhizopus oryzae (1, 1.2%), Syncephalastrum racemosum (1, 1.2%).
b Bed sheet is a rectangular cloth used to cover a mattress.
c Draw sheet is a small bed sheet placed across the rectangular cloth over the bed sheet underneath the patient's back to facilitate transfer of patients.
Environmental and Microbiological Investigation at SWL
Before this incident, SWL regularly provided laundry service to 13 public hospitals and 2 outpatient clinics, with a marked increase in annual capacity output from 11.58 million pieces in 2011/2012 to 14.43 million pieces in 2014/15. The workflow of SWL is illustrated in Supplementary Figure 3. On-site inspection revealed an indoor temperature of 33°C and a relative humidity of 79%. The laundry site was not air-conditioned except in the office area. Most windows were closed. The general hygiene of the laundry was poor. All the fans on the walls, the outlets of air ducts on the ceiling, and the surface of calendering machines were covered with a thick layer of dust. Washing temperature was noted to be about 60°C by hand-held infrared thermometer. Linens felt moist and warm upon packing on the day of inspection, which could have encouraged fungal growth in the post-laundered linen.
Of 195 environmental samples taken at SWL, 119 (61.0%) were positive for zygomycetes (Table 4) indicating widespread environmental contamination in the laundry. Such widespread environmental contamination suggested that post-laundered linen items could be easily recontaminated by zygomycetes in the environment. SWL was closed for environmental disinfection. However, post-disinfection environmental culture performed 1 week later continue to yield zygomycetes in 64 (40.5%) of 158 samples (Table 4). Consequently, an alternative laundry has been sought to provide linen items for our hospital.
| . | 1st Visit on 20 July 2015 (Before Environmental Disinfection) . | 2nd Visit on 27 July 2015 (After Environmental Disinfection) . | ||
|---|---|---|---|---|
| Category of Sampling Sites . | Number of Samples Collected . | Number (Percentage) of Sample Positive for Zygomycetesa . | Number of Samples Collected . | Number (Percentage) of Sample Positive for Zygomycetesc . |
| Filters | ||||
| Filters of tumble dryer | 6 | 6 (100%) | 8 | 5 (62.5%) |
| Filters of batch dryer | 9 | 9 (100%) | 9 | 0 |
| Filters of portable airflow machine | 2 | 1 (50%) | 2 | 1 (50%) |
| Calendering machines | ||||
| Pre-iron linen items putting inside inlet conveyor belts | 20 | 1 (5%) | NAd | NAd |
| Inlet conveyor belts | 59 | 58 (98.3%) | 59 | 24 (40.7%) |
| Machine wheel adjacent to conveyor belts | 2 | 2 (100%) | 2 | 0 |
| Interior surfaces of calendering machines | 29 | 19 (65.5%) | 29 | 14 (48.3%) |
| Exhaust vent above the calendering machines | 7 | 7 (100%) | 9 | 9 (100%) |
| Outlet conveyor belts | 22 | 5 (22.7%) | 22 | 8 (36.4%) |
| Post-iron linen items coming out from outlet conveyor belt | 20 | 2 (10%) | NAd | NAd |
| Receiving table for post-iron linen items | 2 | 2 (100%) | 2 | 1 (50%) |
| Air sampling | ||||
| Near the portable airflow machine | 1 | 1 (100%)b | 1 | 0 |
| Near the calendering machine number 1 | 1 | 1 (100%)b | 1 | 0 |
| Near the calendering machine number 2 | 1 | 1 (100%)b | 1 | 0 |
| Others | ||||
| Interior surfaces of trolley containing laundered linen items | 5 | 4 (80%) | 5 | 0 |
| Patient clothing (without passing through calendering machine) | 5 | 0 | ||
| Laundry wax | 1 | 0 | 1 | 0 |
| Starch for fixation of clothing | 3 | 0 | ||
| Wall | NA | NA | 7 | 2 (28.6%) |
| Total | 195 | 119 (61%) | 158 | 64 (40.5%) |
| . | 1st Visit on 20 July 2015 (Before Environmental Disinfection) . | 2nd Visit on 27 July 2015 (After Environmental Disinfection) . | ||
|---|---|---|---|---|
| Category of Sampling Sites . | Number of Samples Collected . | Number (Percentage) of Sample Positive for Zygomycetesa . | Number of Samples Collected . | Number (Percentage) of Sample Positive for Zygomycetesc . |
| Filters | ||||
| Filters of tumble dryer | 6 | 6 (100%) | 8 | 5 (62.5%) |
| Filters of batch dryer | 9 | 9 (100%) | 9 | 0 |
| Filters of portable airflow machine | 2 | 1 (50%) | 2 | 1 (50%) |
| Calendering machines | ||||
| Pre-iron linen items putting inside inlet conveyor belts | 20 | 1 (5%) | NAd | NAd |
| Inlet conveyor belts | 59 | 58 (98.3%) | 59 | 24 (40.7%) |
| Machine wheel adjacent to conveyor belts | 2 | 2 (100%) | 2 | 0 |
| Interior surfaces of calendering machines | 29 | 19 (65.5%) | 29 | 14 (48.3%) |
| Exhaust vent above the calendering machines | 7 | 7 (100%) | 9 | 9 (100%) |
| Outlet conveyor belts | 22 | 5 (22.7%) | 22 | 8 (36.4%) |
| Post-iron linen items coming out from outlet conveyor belt | 20 | 2 (10%) | NAd | NAd |
| Receiving table for post-iron linen items | 2 | 2 (100%) | 2 | 1 (50%) |
| Air sampling | ||||
| Near the portable airflow machine | 1 | 1 (100%)b | 1 | 0 |
| Near the calendering machine number 1 | 1 | 1 (100%)b | 1 | 0 |
| Near the calendering machine number 2 | 1 | 1 (100%)b | 1 | 0 |
| Others | ||||
| Interior surfaces of trolley containing laundered linen items | 5 | 4 (80%) | 5 | 0 |
| Patient clothing (without passing through calendering machine) | 5 | 0 | ||
| Laundry wax | 1 | 0 | 1 | 0 |
| Starch for fixation of clothing | 3 | 0 | ||
| Wall | NA | NA | 7 | 2 (28.6%) |
| Total | 195 | 119 (61%) | 158 | 64 (40.5%) |
Abbreviation: NA, not available.
a In sum, 119samples were positive for zygomycetes, including Rhizopus microsporus (101, 84.9%), Lichtheimia species (15, 12.6%), Rhizopus oryzae (1, 0.8%), Syncephalastrum racemosum (1, 0.8%), Cunninghamella echinulata (1, 0.8%).
b 4 colony forming units of Rhizopus microsporus per m3 of air.
c 64 samples were positive zygomycetes, including Rhizopus microsporus (35, 54.7%), Lichtheimia species (29, 45.3%).
d Repeated culture at this site was not possible because the service was terminated.
| . | 1st Visit on 20 July 2015 (Before Environmental Disinfection) . | 2nd Visit on 27 July 2015 (After Environmental Disinfection) . | ||
|---|---|---|---|---|
| Category of Sampling Sites . | Number of Samples Collected . | Number (Percentage) of Sample Positive for Zygomycetesa . | Number of Samples Collected . | Number (Percentage) of Sample Positive for Zygomycetesc . |
| Filters | ||||
| Filters of tumble dryer | 6 | 6 (100%) | 8 | 5 (62.5%) |
| Filters of batch dryer | 9 | 9 (100%) | 9 | 0 |
| Filters of portable airflow machine | 2 | 1 (50%) | 2 | 1 (50%) |
| Calendering machines | ||||
| Pre-iron linen items putting inside inlet conveyor belts | 20 | 1 (5%) | NAd | NAd |
| Inlet conveyor belts | 59 | 58 (98.3%) | 59 | 24 (40.7%) |
| Machine wheel adjacent to conveyor belts | 2 | 2 (100%) | 2 | 0 |
| Interior surfaces of calendering machines | 29 | 19 (65.5%) | 29 | 14 (48.3%) |
| Exhaust vent above the calendering machines | 7 | 7 (100%) | 9 | 9 (100%) |
| Outlet conveyor belts | 22 | 5 (22.7%) | 22 | 8 (36.4%) |
| Post-iron linen items coming out from outlet conveyor belt | 20 | 2 (10%) | NAd | NAd |
| Receiving table for post-iron linen items | 2 | 2 (100%) | 2 | 1 (50%) |
| Air sampling | ||||
| Near the portable airflow machine | 1 | 1 (100%)b | 1 | 0 |
| Near the calendering machine number 1 | 1 | 1 (100%)b | 1 | 0 |
| Near the calendering machine number 2 | 1 | 1 (100%)b | 1 | 0 |
| Others | ||||
| Interior surfaces of trolley containing laundered linen items | 5 | 4 (80%) | 5 | 0 |
| Patient clothing (without passing through calendering machine) | 5 | 0 | ||
| Laundry wax | 1 | 0 | 1 | 0 |
| Starch for fixation of clothing | 3 | 0 | ||
| Wall | NA | NA | 7 | 2 (28.6%) |
| Total | 195 | 119 (61%) | 158 | 64 (40.5%) |
| . | 1st Visit on 20 July 2015 (Before Environmental Disinfection) . | 2nd Visit on 27 July 2015 (After Environmental Disinfection) . | ||
|---|---|---|---|---|
| Category of Sampling Sites . | Number of Samples Collected . | Number (Percentage) of Sample Positive for Zygomycetesa . | Number of Samples Collected . | Number (Percentage) of Sample Positive for Zygomycetesc . |
| Filters | ||||
| Filters of tumble dryer | 6 | 6 (100%) | 8 | 5 (62.5%) |
| Filters of batch dryer | 9 | 9 (100%) | 9 | 0 |
| Filters of portable airflow machine | 2 | 1 (50%) | 2 | 1 (50%) |
| Calendering machines | ||||
| Pre-iron linen items putting inside inlet conveyor belts | 20 | 1 (5%) | NAd | NAd |
| Inlet conveyor belts | 59 | 58 (98.3%) | 59 | 24 (40.7%) |
| Machine wheel adjacent to conveyor belts | 2 | 2 (100%) | 2 | 0 |
| Interior surfaces of calendering machines | 29 | 19 (65.5%) | 29 | 14 (48.3%) |
| Exhaust vent above the calendering machines | 7 | 7 (100%) | 9 | 9 (100%) |
| Outlet conveyor belts | 22 | 5 (22.7%) | 22 | 8 (36.4%) |
| Post-iron linen items coming out from outlet conveyor belt | 20 | 2 (10%) | NAd | NAd |
| Receiving table for post-iron linen items | 2 | 2 (100%) | 2 | 1 (50%) |
| Air sampling | ||||
| Near the portable airflow machine | 1 | 1 (100%)b | 1 | 0 |
| Near the calendering machine number 1 | 1 | 1 (100%)b | 1 | 0 |
| Near the calendering machine number 2 | 1 | 1 (100%)b | 1 | 0 |
| Others | ||||
| Interior surfaces of trolley containing laundered linen items | 5 | 4 (80%) | 5 | 0 |
| Patient clothing (without passing through calendering machine) | 5 | 0 | ||
| Laundry wax | 1 | 0 | 1 | 0 |
| Starch for fixation of clothing | 3 | 0 | ||
| Wall | NA | NA | 7 | 2 (28.6%) |
| Total | 195 | 119 (61%) | 158 | 64 (40.5%) |
Abbreviation: NA, not available.
a In sum, 119samples were positive for zygomycetes, including Rhizopus microsporus (101, 84.9%), Lichtheimia species (15, 12.6%), Rhizopus oryzae (1, 0.8%), Syncephalastrum racemosum (1, 0.8%), Cunninghamella echinulata (1, 0.8%).
b 4 colony forming units of Rhizopus microsporus per m3 of air.
c 64 samples were positive zygomycetes, including Rhizopus microsporus (35, 54.7%), Lichtheimia species (29, 45.3%).
d Repeated culture at this site was not possible because the service was terminated.
DISCUSSION
To our best knowledge, this is the first major outbreak of pulmonary and cutaneous zygomycosis in immunosuppressed patients due to linen items contaminated by spore-forming zygomycetes, predominantly R. microsporus. This occurred during the humid summer months in Hong Kong when the ambient temperature can go up to 34°C, similar to many linen-items related outbreaks caused by spore-forming Bacillus cereus [7]. The cutaneous nature in 3 cases and the diverse locations of the outbreak strongly suggested that medical items directly applied onto skin or linen items were the most likely source. Three (50%) of our adult patients died, compared with a 100% mortality in an outbreak due to R. delemar reported in pediatric immunocompromised patients in Louisiana, United States [6]. Although previously described zygomycosis outbreak due to contaminated linens lead to cutaneous zygomycosis [6], our outbreak suggested that inhalation of fungal spores from contaminated linens could result in pulmonary manifestations in immunosuppressed patients. The close proximity between contaminated pillow case, bed linens, and clothing with patients provides a convincing explanation for the pulmonary and cutaneous involvement in our patients. The duration of hospitalization was significantly associated with zygomycosis among the immunosuppressed patients. The finding strongly suggested that the duration of exposure to R. microsporus-contaminated linen items may be an important risk factor. Furthermore, the culture isolates from all patients, pulmonary and/or cutaneous zygomycosis, were phylogenetically associated with the environmental isolates cultured from freshly laundered linens supplied by SWL.
Epidemiological findings and microbiological sampling of the machineries and environment supported that SWL was the source of this outbreak. In contrast to the previously reported contaminated linen-related outbreaks that were associated with dust intrusion [6, 15, 16], contaminated washing machines [17–19], contaminated dryers [20], and failure to add bleach during laundering process [21], this outbreak was related to multiple factors. Major deficiencies in the physical environment (ambient temperature, lighting, ventilation, dustiness), transportation of linen (use of same transport for dirty and clean utility), and discrepancy between the temperature measured by the infrared thermometer and the preset temperature in the washing machines might have led to propagation of the implicated zygomycetes leading to heavy environmental contamination, which then recontaminate any freshly laundered linen items. Multiple deficiencies in the laundry process might have allowed the accumulation of relatively heat-resistant bacterial or fungal spores in the laundered linen items. It was also interesting that patients' clothing that was not calendared at 160°C had a higher positive R. microsporus-contamination rate than the calendared items such as pillow case, bed sheet, and draw sheet (Table 3).
The measured washing temperature of only 60°C during the initial investigation was not able to kill the spores of R. microsporus, which can survive at 65°C for 5 minutes and longer at lower temperature [2]. Even during calendaring where temperature should reach at least 160°C, 23% of the outlet conveyer belts and 10% of the post-calendered linen items remained positive for the implicated zygomycetes. During summer months, mold and bacterial spores readily germinate and multiply in the linen that contains organic materials supporting the growth of these microbes. Hence, poorly ventilated, hot and humid storage conditions would potentially amplify microbial proliferation in the laundry or hospital central storage and ward storages [22]. Therefore, a topping-up effect of spore-forming microbes was suspected to have occurred in SWL over time, with combination of heavy environmental recontamination of finished products and faulty disinfection during the laundry process.
Outbreaks of zygomycosis due to contaminated linens could not have been prevented under our current system. This is because there were no regular audits on the quality of linens, and the microbiological testing of clean healthcare textiles for “hygienically clean certification” purposes has not yet been implemented in our healthcare system [23]. “Hygienically clean” is defined as “free of pathogens in sufficient numbers to cause human illness,” whereby the total aerobic microbial count is ≤20 CFU/100 cm2 by RODAC plate count [23]. During our outbreak investigation, the laundered linens freshly supplied by SWL was found to have high total aerobic microbial counts, which signified potential contamination and revealed the need for revision of the entire laundering process. Notably, none of the RODAC plates recovered zygomycetes from the contaminated linen items whereas the sponge swabs did. Zygomycetes may be outgrown by environmental organism such as Bacillus species. Therefore, without the use of sponge swab and selective culture medium, the causative agents in this outbreak would have been overlooked. Sponge swab with selective culture medium is essential to facilitate isolation of any specific pathogenic microorganism of interest if there is epidemiological evidence suggestive of contaminated linen-related outbreak.
In summary, to prevent similar outbreaks, the concept of “hygienically clean” linen items should be introduced to the healthcare facilities with regular microbiological testing [7]. Thorough cleaning, disinfection, and de-dusting of facility's environment and delivering vehicles, including all surfaces and equipment, should be enforced and audited. The temperature sensors of washing machines should be checked and calibrated regularly by engineers to ensure appropriate washing temperature. The laundry process should be reviewed and monitored regularly, especially moisture control during drying and packing process. Additionally, there should be clear segregation, including linen, equipment and staff, between clean and dirty areas to avoid cross-contamination of processed items. At hospital level, linen storage conditions, for example, temperature and humidity, must not facilitate proliferation of spore-forming organisms. Linen consumption should follow the “first-in-first-out” principle, and topping up of linen items must not be allowed. Finally, clinicians should maintain a high index of suspicion for early diagnosis and treatment of zygomycosis in immunosuppressed patients [24].
Notes
Acknowledgments. We thank P. Y. Leung, Chief Executive of Hospital Authority for facilitating the outbreak investigation, and staff of Public Health Laboratory Services Branch, Centre for Health Protection, for verifying the microbiological findings in the study. We also thank Lisa Wong, Modissa Ng, Radley Ching, Doris Lee, Sara Ho, Wing Shan Li, Hon Kit Chui, Derek Hung, Wan Man Ting, Cyril Yip, and Rosana Poon for their assistance in the collection of linen samples, and Antonio Ngan, Toni Cheng, Betsy Chan, and Garnet Choi for performing laboratory identification of the zygomycetes.
Financial support. This work was partially supported by the Health and Medical Research Fund (HMRF), Food and Health Bureau, Hong Kong SAR Government (ref. no. HKM-15-M12B) and the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Disease for the HKSAR Department of Health.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
REFERENCES




