-
PDF
- Split View
-
Views
-
Cite
Cite
Fabienne Caby, Amélie Guihot, Sidonie Lambert-Niclot, Marguerite Guiguet, David Boutolleau, Rachid Agher, Marc-Antoine Valantin, Roland Tubiana, Vincent Calvez, Anne-Geneviève Marcelin, Guislaine Carcelain, Brigitte Autran, Dominique Costagliola, Christine Katlama, Determinants of a Low CD4/CD8 Ratio in HIV-1–Infected Individuals Despite Long-term Viral Suppression, Clinical Infectious Diseases, Volume 62, Issue 10, 15 May 2016, Pages 1297–1303, https://doi.org/10.1093/cid/ciw076
Close - Share Icon Share
Abstract
Background. A low CD4/CD8 ratio in human immunodeficiency virus (HIV)–infected individuals despite effective antiretroviral therapy (ART) reflects ongoing immune activation and has been linked to a higher risk of non-AIDS morbidity and mortality. Our aim was to describe the proportion of individuals with a persistent CD4/CD8 ratio <1 despite long-term viral suppression and to determine associated risk factors.
Methods. This cross-sectional study was conducted in 2012 in a single clinical center. HIV type 1 (HIV-1)–infected individuals were eligible if they had a plasma HIV-1 RNA level <50 copies/mL for at least 2 years on a stable ART regimen. Logistic regression was used to identify risk factors for a persistent CD4/CD8 ratio <1.
Results. We enrolled 719 individuals with a median CD4/CD8 ratio of 0.8 (interquartile range [IQR], 0.6–1.1), CD4 and CD8 T-cell counts of 565 (IQR, 435–742) cells/µL and 727 (IQR, 530–991) cells/µL respectively, and viral suppression for 5.4 (IQR, 3.3–9.1) years. Cytomegalovirus (CMV) serology was positive in 564 of 645 individuals (87%). Persistent CD4/CD8 ratio <1 was observed in 471 patients (66%). The following factors were independently associated with a CD4/CD8 ratio <1: CMV seropositivity (odds ratio [OR], 1.9 [95% confidence interval {CI}, 1.1–3.1]), ART initiation before 1997 (OR, 1.9 [95% CI, 1.2–3.0] compared with 2002 or later), a lower CD4 T-cell nadir (OR, 0.7 [95% CI, .7–.8] per log2 increment), and shorter duration of viral suppression (OR, 0.6 [95% CI, .5–.8] per 5 years).
Conclusions. Most HIV-infected individuals with long-term viral suppression still had a CD4/CD8 ratio <1. Early initiation and long-term effective ART appear to improve this ratio. CMV coinfection, which represents a potential target for therapeutic intervention, was strongly associated with a persistently suboptimal CD4/CD8 ratio.
Despite long-term viral suppression and CD4 T-cell recovery on effective antiretroviral therapy (ART), human immunodeficiency virus (HIV) infection is still associated with chronic immune activation and inflammation [1–3]. The latter appear to be largely responsible for premature aging of the immune system and for the onset of non-AIDS-defining conditions such as cardiovascular, renal, neurological, osseous, and malignant disorders [4–6].
An inverted CD4/CD8 ratio is characteristic of HIV infection and is linked both to CD4 T-cell depletion and to expansion of activated HIV-specific cytotoxic T lymphocytes [4]. The CD4/CD8 ratio usually rises during the first year of ART but rarely normalizes further, especially in case of late treatment [7–9]. A low CD4/CD8 ratio appears to be a reliable marker of systemic immune activation during successful ART; several studies have shown that a low ratio is associated with the expression of CD4 and CD8 T-cell markers of activation/exhaustion/senescence, such as CD38, HLA-DR, PD-1, and CD28–CD57, even in individuals with undetectable viremia and CD4 counts >500 cells/µL [7, 10–12]. High levels of plasma soluble markers of inflammation such as interleukin 6, soluble CD14, and high-sensitivity C-reactive protein have also been linked to a low CD4/CD8 ratio [7, 13].
An inverted CD4/CD8 ratio is associated with higher 4-year all-cause mortality in elderly HIV-seronegative individuals [14, 15], and also with disease progression and death among patients with untreated HIV infection, independent of the viral load and the CD4 T-cell count [16, 17]. Among individuals on effective ART, a low CD4/CD8 ratio has been linked to subclinical atherosclerosis and other markers of age-associated diseases [18–20]. Two case-control studies and 2 cohort studies of HIV-infected individuals have shown that a very low CD4/CD8 ratio (<0.5 or <0.3) is a risk factor for non-AIDS morbidity and mortality, especially when the CD4 count is high (≥350 cells/µL) [7, 8, 21, 22]. Furthermore, the CD4/CD8 ratio tends to be more stable over time than the CD4 or CD8 cell count, and its predictive value for non-AIDS events has been reported to be stronger than the absolute CD8 count in individuals with restored CD4 counts (>500 cells/µL) [7].
Here we identified determinants of an inverted CD4/CD8 ratio despite long-term HIV suppression, including in individuals with CD4 counts >500 cells/µL, to find potential therapeutic targets for further normalization of this ratio.
METHODS
Study Design and Population
This cross-sectional study took place in May 2012 in the Infectious Diseases Department of Pitié-Salpêtrière Hospital, Paris, France. Individuals were included if they had plasma HIV type 1 (HIV-1) RNA loads <50 copies/mL for at least 2 years, with a minimum of 2 measurements per year, on an antiretroviral regimen that had remained stable during the year before the study. The last plasma HIV-1 RNA value and the last CD4 and CD8 T-cell counts had to be available <6 months before the study.
Data Collection and Laboratory Measurements
All HIV-infected individuals receiving care in the Infectious Diseases Department of Pitié-Salpêtrière Hospital have their clinical, biological, and therapeutic data recorded prospectively in standardized electronic medical records (Nadis®, New Aids Data Information System). They are asked to provide their written informed consent to have their biological and clinical findings recorded in the database and to participate in subsequent clinical research.
Biological data obtained in the hospital, such as plasma HIV-1 RNA load and immunological parameters, are directly imported from the laboratory information system, thus minimizing collection bias. The quality of the database is ensured by automated checks during data capture and by regular controls and annual assessments. Routine blood tests are performed at each hospital visit, and residual plasma is stored frozen, identified by a serial number.
The percentages and absolute counts of lymphocyte subpopulations were determined in whole blood using Cyto-Stat tetraCHROME reagents (Beckman Coulter, Hialeah, Florida). CD4 T cells were defined as CD3+CD4+ lymphocytes, and CD8 T cells as CD3+CD8+ lymphocytes. Sample acquisition with Flow-Count Fluorospheres was performed on a FC500 flow cytometer (Beckman Coulter). The laboratory values in 101 healthy HIV-seronegative individuals were as follows (reported as median [10th–90th percentile]): CD4/CD8 ratio, 1.9 (1.2–3.1); CD4 T-cell count, 716 (510–1037) cells/µL; and CD8 T-cell count, 401 (258–615) cells/µL.
Plasma HIV-1 RNA was considered undetectable when it was below the detection limit of the method used (<200 copies/mL from 1996 to 2006, and <50 copies/mL thereafter).
In HIV-infected individuals with unknown cytomegalovirus (CMV) serostatus, the anti-CMV immunoglobulin G (IgG) antibody level was determined on a plasma sample stored within the year before the study, using the ARCHITECT CMV IgG chemoluminescence microparticle immunoassay (Abbott). CMV seropositivity was noted when the CMV IgG level was >15 antibody units per milliliter.
Statistical Methods
Continuous variables were expressed as the median and interquartile range (IQR), and categorical variables as counts and percentages. The normal range of the CD4/CD8 ratio is difficult to define. However, as a value <1 is associated with immunosenescence and increased mortality in the general elderly population, and with higher levels of immune activation in individuals treated for HIV infection, we used this cutoff to define a low ratio [7, 10, 11, 23]. Univariable and multivariable logistic regression analysis was used to identify factors associated with a CD4/CD8 ratio <1. Only individuals with known CMV serostatus were included in the univariable and multivariable models. The following variables were included in the analyses: age, sex, HIV risk group, body mass index, Centers for Disease Control and Prevention stage, CD4 T-cell nadir, duration of viral suppression, calendar period of ART introduction, and serostatus for hepatitis C virus, hepatitis B virus, and CMV. Univariable analysis was used to determine whether continuous variables were better modeled as continuous or categorical variables, based on Akaike information criterion. Variables with a P value <.20 in univariable analysis were included in the multivariable model.
To identify determinants of a low ratio in patients with optimal immunovirological status, we restricted the analysis first to patients with a CD4 count >500 cells/µL and then to those with viral suppression lasting at least 5 years. Finally, to better understand the role of CD8 T cells in the persistence of a low ratio, we examined factors associated with abnormally high CD8 counts (>1000 cells/µL) in individuals with CD4/CD8 ratios <1 and a normalized CD4 count (>500 cells/µL). All analyses were performed using R statistical software version 3.1.2.
RESULTS
Characteristics of the Patients
Among the 3129 HIV-infected individuals followed in the Pitié-Salpêtrière Infectious Diseases Department in May 2012, 719 individuals met the inclusion criteria. The current median CD4 and CD8 T-cell counts were 565 (IQR, 435–742) cells/µL and 727 (IQR, 530–991) cells/µL, respectively; the CD4/CD8 ratio was 0.8 (IQR, 0.6–1.1); and viral suppression had lasted for 5.4 (IQR, 3.3–9.1) years. CMV serostatus was available for 645 individuals: In 460 cases the information was extracted from the electronic medical records, and in the remaining 185 cases the CMV IgG level was determined on a stored plasma sample. Only the HIV risk group distribution differed between the 645 individuals with known CMV serostatus and the remaining 74 individuals; the latter individuals included more intravenous drug users and fewer men who have sex with men (MSM) (Table 1).
| Characteristics . | All (n = 719) . | Individuals With Available CMV Serology (n = 645) . | Individuals With No Available CMV Serology (n = 74) . | P Value . |
|---|---|---|---|---|
| Age, y | 49 (44–56) | 49 (44–56) | 49 (43–53) | .6905 |
| Male sex | 529 (74) | 478 (74) | 51 (69) | .3386 |
| HIV risk group | .9718 | |||
| MSM | 303 (48) | 303(53) | 0 (0) | |
| Heterosexual | 250 (40) | 222 (39) | 28 (38) | |
| IDU | 62 (10) | 43 (7) | 19 (26) | |
| Blood transfusion | 11 (2) | 8 (1) | 3 (4) | |
| Other/unknown | 93 (13) | 69 (11) | 24 (32) | |
| Body mass index, kg/m2 | 24 (21–26) | 24 (22–27) | 23 (20–25) | .0067 |
| CDC stage C | 168 (23) | 155 (24) | 13 (18) | .2158 |
| CD4 count nadir, cells/µL | 183 (80–276) | 183 (75–279) | 183 (125–245) | .7248 |
| CD8 count zenith, cells/µL | 1365 (1032–1843) | 1361 (1032–1861) | 1446 (920–1788) | .3862 |
| CD4 count, cells/µL | 565 (435–742) | 576 (432–756) | 520 (458–625) | .0843 |
| CD8 count, cells/µL | 727 (530–991) | 727 (529–986) | 717 (570–1097) | .2352 |
| CD4/CD8 ratio | 0.8 (0.6–1.1) | 0.8 (0.6–1.1) | 0.7 (0.5–1.0) | .0864 |
| Duration of viral suppressiona, y | 5.4 (3.3–9.1) | 5.5 (3.3–9.1) | 4.8 (3.0–7.9) | .1248 |
| ART introduction | .0669 | |||
| 2002 or later | 218 (30) | 203 (31) | 15 (20) | |
| 1997–2001 | 277 (39) | 245 (38) | 32 (43) | |
| Before 1997 | 224 (31) | 197 (31) | 27 (36) | |
| Current PI-containing regimen | 428 (60) | 380 (59) | 48 (65) | .3243 |
| Current NNRTI-containing regimen | 260 (36) | 240 (37) | 20 (27) | .0864 |
| Raltegravir-containing regimen | 95 (13) | 85 (13) | 10 (14) | .9357 |
| Maraviroc-containing regimen | 15 (2) | 13 (2) | 2 (3) | .6963 |
| Current NRTI-containing regimen | 665 (92) | 592 (92) | 73 (99) | .743 |
| Tenofovir | 395 (55) | 354 (55) | 41 (55) | |
| Abacavir | 186 (26) | 167 (26) | 19 (26) | |
| Zidovudine | 59 (8) | 51 (8) | 8 (11) | |
| Stavudine | 22 (3) | 18 (3) | 4 (5) | |
| Didanosine | 3 (0.4) | 2 (0.3) | 1 (1) | |
| Positive anti-HCV IgG | 105 (15) | 94 (15) | 11 (15) | .8685 |
| Positive HBsAg | 53 (8) | 49 (8) | 4 (5) | .4898 |
| Positive anti-CMV IgG (n = 645) | NA | 564 (87) | NA |
| Characteristics . | All (n = 719) . | Individuals With Available CMV Serology (n = 645) . | Individuals With No Available CMV Serology (n = 74) . | P Value . |
|---|---|---|---|---|
| Age, y | 49 (44–56) | 49 (44–56) | 49 (43–53) | .6905 |
| Male sex | 529 (74) | 478 (74) | 51 (69) | .3386 |
| HIV risk group | .9718 | |||
| MSM | 303 (48) | 303(53) | 0 (0) | |
| Heterosexual | 250 (40) | 222 (39) | 28 (38) | |
| IDU | 62 (10) | 43 (7) | 19 (26) | |
| Blood transfusion | 11 (2) | 8 (1) | 3 (4) | |
| Other/unknown | 93 (13) | 69 (11) | 24 (32) | |
| Body mass index, kg/m2 | 24 (21–26) | 24 (22–27) | 23 (20–25) | .0067 |
| CDC stage C | 168 (23) | 155 (24) | 13 (18) | .2158 |
| CD4 count nadir, cells/µL | 183 (80–276) | 183 (75–279) | 183 (125–245) | .7248 |
| CD8 count zenith, cells/µL | 1365 (1032–1843) | 1361 (1032–1861) | 1446 (920–1788) | .3862 |
| CD4 count, cells/µL | 565 (435–742) | 576 (432–756) | 520 (458–625) | .0843 |
| CD8 count, cells/µL | 727 (530–991) | 727 (529–986) | 717 (570–1097) | .2352 |
| CD4/CD8 ratio | 0.8 (0.6–1.1) | 0.8 (0.6–1.1) | 0.7 (0.5–1.0) | .0864 |
| Duration of viral suppressiona, y | 5.4 (3.3–9.1) | 5.5 (3.3–9.1) | 4.8 (3.0–7.9) | .1248 |
| ART introduction | .0669 | |||
| 2002 or later | 218 (30) | 203 (31) | 15 (20) | |
| 1997–2001 | 277 (39) | 245 (38) | 32 (43) | |
| Before 1997 | 224 (31) | 197 (31) | 27 (36) | |
| Current PI-containing regimen | 428 (60) | 380 (59) | 48 (65) | .3243 |
| Current NNRTI-containing regimen | 260 (36) | 240 (37) | 20 (27) | .0864 |
| Raltegravir-containing regimen | 95 (13) | 85 (13) | 10 (14) | .9357 |
| Maraviroc-containing regimen | 15 (2) | 13 (2) | 2 (3) | .6963 |
| Current NRTI-containing regimen | 665 (92) | 592 (92) | 73 (99) | .743 |
| Tenofovir | 395 (55) | 354 (55) | 41 (55) | |
| Abacavir | 186 (26) | 167 (26) | 19 (26) | |
| Zidovudine | 59 (8) | 51 (8) | 8 (11) | |
| Stavudine | 22 (3) | 18 (3) | 4 (5) | |
| Didanosine | 3 (0.4) | 2 (0.3) | 1 (1) | |
| Positive anti-HCV IgG | 105 (15) | 94 (15) | 11 (15) | .8685 |
| Positive HBsAg | 53 (8) | 49 (8) | 4 (5) | .4898 |
| Positive anti-CMV IgG (n = 645) | NA | 564 (87) | NA |
Results are expressed as median (IQR) or No. (%).
Abbreviations: ART, antiretroviral therapy; CDC, Centers for Disease Control and Prevention; CMV, cytomegalovirus; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, intravenous drug user; IgG, immunoglobulin G; IQR, interquartile range; MSM, men who have sex with men; NA, not available; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleos(t)ide reverse transcriptase inhibitor; PI, protease inhibitor.
a Viral suppression defined as a plasma HIV-1 RNA level <200 copies/mL or <50 copies/mL according to the method used (<200 copies/mL from 1996 to 2006, and <50 copies/mL thereafter).
| Characteristics . | All (n = 719) . | Individuals With Available CMV Serology (n = 645) . | Individuals With No Available CMV Serology (n = 74) . | P Value . |
|---|---|---|---|---|
| Age, y | 49 (44–56) | 49 (44–56) | 49 (43–53) | .6905 |
| Male sex | 529 (74) | 478 (74) | 51 (69) | .3386 |
| HIV risk group | .9718 | |||
| MSM | 303 (48) | 303(53) | 0 (0) | |
| Heterosexual | 250 (40) | 222 (39) | 28 (38) | |
| IDU | 62 (10) | 43 (7) | 19 (26) | |
| Blood transfusion | 11 (2) | 8 (1) | 3 (4) | |
| Other/unknown | 93 (13) | 69 (11) | 24 (32) | |
| Body mass index, kg/m2 | 24 (21–26) | 24 (22–27) | 23 (20–25) | .0067 |
| CDC stage C | 168 (23) | 155 (24) | 13 (18) | .2158 |
| CD4 count nadir, cells/µL | 183 (80–276) | 183 (75–279) | 183 (125–245) | .7248 |
| CD8 count zenith, cells/µL | 1365 (1032–1843) | 1361 (1032–1861) | 1446 (920–1788) | .3862 |
| CD4 count, cells/µL | 565 (435–742) | 576 (432–756) | 520 (458–625) | .0843 |
| CD8 count, cells/µL | 727 (530–991) | 727 (529–986) | 717 (570–1097) | .2352 |
| CD4/CD8 ratio | 0.8 (0.6–1.1) | 0.8 (0.6–1.1) | 0.7 (0.5–1.0) | .0864 |
| Duration of viral suppressiona, y | 5.4 (3.3–9.1) | 5.5 (3.3–9.1) | 4.8 (3.0–7.9) | .1248 |
| ART introduction | .0669 | |||
| 2002 or later | 218 (30) | 203 (31) | 15 (20) | |
| 1997–2001 | 277 (39) | 245 (38) | 32 (43) | |
| Before 1997 | 224 (31) | 197 (31) | 27 (36) | |
| Current PI-containing regimen | 428 (60) | 380 (59) | 48 (65) | .3243 |
| Current NNRTI-containing regimen | 260 (36) | 240 (37) | 20 (27) | .0864 |
| Raltegravir-containing regimen | 95 (13) | 85 (13) | 10 (14) | .9357 |
| Maraviroc-containing regimen | 15 (2) | 13 (2) | 2 (3) | .6963 |
| Current NRTI-containing regimen | 665 (92) | 592 (92) | 73 (99) | .743 |
| Tenofovir | 395 (55) | 354 (55) | 41 (55) | |
| Abacavir | 186 (26) | 167 (26) | 19 (26) | |
| Zidovudine | 59 (8) | 51 (8) | 8 (11) | |
| Stavudine | 22 (3) | 18 (3) | 4 (5) | |
| Didanosine | 3 (0.4) | 2 (0.3) | 1 (1) | |
| Positive anti-HCV IgG | 105 (15) | 94 (15) | 11 (15) | .8685 |
| Positive HBsAg | 53 (8) | 49 (8) | 4 (5) | .4898 |
| Positive anti-CMV IgG (n = 645) | NA | 564 (87) | NA |
| Characteristics . | All (n = 719) . | Individuals With Available CMV Serology (n = 645) . | Individuals With No Available CMV Serology (n = 74) . | P Value . |
|---|---|---|---|---|
| Age, y | 49 (44–56) | 49 (44–56) | 49 (43–53) | .6905 |
| Male sex | 529 (74) | 478 (74) | 51 (69) | .3386 |
| HIV risk group | .9718 | |||
| MSM | 303 (48) | 303(53) | 0 (0) | |
| Heterosexual | 250 (40) | 222 (39) | 28 (38) | |
| IDU | 62 (10) | 43 (7) | 19 (26) | |
| Blood transfusion | 11 (2) | 8 (1) | 3 (4) | |
| Other/unknown | 93 (13) | 69 (11) | 24 (32) | |
| Body mass index, kg/m2 | 24 (21–26) | 24 (22–27) | 23 (20–25) | .0067 |
| CDC stage C | 168 (23) | 155 (24) | 13 (18) | .2158 |
| CD4 count nadir, cells/µL | 183 (80–276) | 183 (75–279) | 183 (125–245) | .7248 |
| CD8 count zenith, cells/µL | 1365 (1032–1843) | 1361 (1032–1861) | 1446 (920–1788) | .3862 |
| CD4 count, cells/µL | 565 (435–742) | 576 (432–756) | 520 (458–625) | .0843 |
| CD8 count, cells/µL | 727 (530–991) | 727 (529–986) | 717 (570–1097) | .2352 |
| CD4/CD8 ratio | 0.8 (0.6–1.1) | 0.8 (0.6–1.1) | 0.7 (0.5–1.0) | .0864 |
| Duration of viral suppressiona, y | 5.4 (3.3–9.1) | 5.5 (3.3–9.1) | 4.8 (3.0–7.9) | .1248 |
| ART introduction | .0669 | |||
| 2002 or later | 218 (30) | 203 (31) | 15 (20) | |
| 1997–2001 | 277 (39) | 245 (38) | 32 (43) | |
| Before 1997 | 224 (31) | 197 (31) | 27 (36) | |
| Current PI-containing regimen | 428 (60) | 380 (59) | 48 (65) | .3243 |
| Current NNRTI-containing regimen | 260 (36) | 240 (37) | 20 (27) | .0864 |
| Raltegravir-containing regimen | 95 (13) | 85 (13) | 10 (14) | .9357 |
| Maraviroc-containing regimen | 15 (2) | 13 (2) | 2 (3) | .6963 |
| Current NRTI-containing regimen | 665 (92) | 592 (92) | 73 (99) | .743 |
| Tenofovir | 395 (55) | 354 (55) | 41 (55) | |
| Abacavir | 186 (26) | 167 (26) | 19 (26) | |
| Zidovudine | 59 (8) | 51 (8) | 8 (11) | |
| Stavudine | 22 (3) | 18 (3) | 4 (5) | |
| Didanosine | 3 (0.4) | 2 (0.3) | 1 (1) | |
| Positive anti-HCV IgG | 105 (15) | 94 (15) | 11 (15) | .8685 |
| Positive HBsAg | 53 (8) | 49 (8) | 4 (5) | .4898 |
| Positive anti-CMV IgG (n = 645) | NA | 564 (87) | NA |
Results are expressed as median (IQR) or No. (%).
Abbreviations: ART, antiretroviral therapy; CDC, Centers for Disease Control and Prevention; CMV, cytomegalovirus; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, intravenous drug user; IgG, immunoglobulin G; IQR, interquartile range; MSM, men who have sex with men; NA, not available; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleos(t)ide reverse transcriptase inhibitor; PI, protease inhibitor.
a Viral suppression defined as a plasma HIV-1 RNA level <200 copies/mL or <50 copies/mL according to the method used (<200 copies/mL from 1996 to 2006, and <50 copies/mL thereafter).
CD4/CD8 Ratio
The CD4/CD8 ratio was >1.2 in 143 individuals (20%), 1–1.2 in 105 individuals (14%), and <1 in 471 individuals (66%). Among the latter individuals, the ratio was 0.8–0.99 in 114 cases (24%), 0.5–0.79 in 205 cases (44%), 0.3–0.49 in 118 cases (25%), and <0.3 in 35 cases (7%). Figure 1 shows the distribution of CD4 and CD8 T-cell counts according to the CD4/CD8 ratio. Overall, a CD4/CD8 ratio >1 was associated with normalization of both the CD4 count (>500 cells/µL) and the CD8 count (<600 cells/µL). A CD4 count >500 cells/µL was not sufficient to ensure a CD4/CD8 ratio >1. Indeed, 61% of the individuals with a ratio between 0.5 and 0.99 had normal CD4 T-cell counts, showing that a high CD8 T-cell count accounts for most CD4/CD8 ratios persistently <1 (Figure 1). Only individuals with a ratio ≥1.5 achieved an apparently normal median CD8 count: 429 (IQR, 345–512) cells/µL vs 401 (IQR, 306–519) cells/µL in healthy HIV-seronegative individuals (see “Data Collection and Laboratory Measurements” in the “Methods” section).
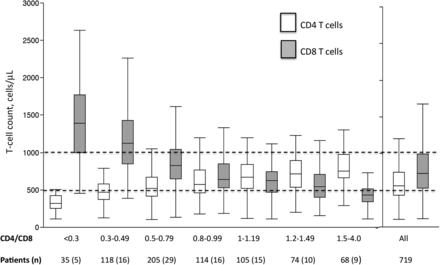
Absolute CD4 and CD8 T-cell counts according to CD4/CD8 ratio quantiles. Data are presented as box plots, with the central line indicating the median, the box the interquartile range, and the whiskers the 10th and 90th percentiles. The horizontal dotted lines indicate counts of 500 cells/µL and 1000 cells/µL. CD4/CD8 denotes CD4 to CD8 ratio.
Factors Associated With a CD4/CD8 Ratio <1
Factors independently associated with a CD4/CD8 ratio persistently <1 despite suppressive ART were CMV seropositivity (OR, 1.9 [95% confidence interval {CI}, 1.1–3.1]), ART initiation before 1997 (OR, 1.9 [95% CI, 1.2–3.0] compared with starting ART in 2002 or later), a lower CD4 T-cell nadir (OR, 0.7 [95% CI, .7–.8] per log2 increment), and shorter duration of viral suppression (OR, 0.6 [95% CI, .5–.8] per 5 years) (Table 2).
Risk Factors for a CD4/CD8 Ratio <1 in Individuals With Available Cytomegalovirus Serology: Univariable and Multivariable Analyses (n = 645)
| Variable . | CD4/CD8 Ratio <1 (n = 416) . | CD4/CD8 Ratio ≥1 (n = 229) . | Univariable Analysis . | Multivariable Analysisa . | ||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) . | P Value . | Odds Ratio (95% CI) . | P Value . | |||
| Ageb, y | 48 (42–56) | 51 (45–57) | 0.8 (.7–.9) | .005 | 0.8 (.7–1.0) | .064 |
| Male sex | 300 (72) | 178 (78) | 0.7 (.5–1.0) | .061 | 0.9 (.6–1.5) | |
| Body mass index, kg/m2 | 24 (22–27) | 23 (21–26) | 1.0 (1.0–1.1) | .418 | ||
| HIV risk group | ||||||
| MSM | 186 (45) | 117 (51) | 1 | .226 | ||
| Heterosexual | 153 (37) | 69 (30) | 1.4 (1.0–2.1) | |||
| IDU | 26 (6) | 17 (8) | 1.0 (.6–6.5) | |||
| Other/unknown | 51 (12) | 26 (11) | 1.4 (.9–2.4) | |||
| CD4 count nadirc, cells/µL | 153 (56–240) | 233 (141–315) | 0.7 (.6–.8) | <.001 | 0.7 (.7–.8) | <.001 |
| Duration of viral suppressiond, y | 4.9 (3.1–8.5) | 6.3 (3.7–10.1) | 0.7 (.5–.8) | <.001 | 0.6 (.5–.8) | <.001 |
| ART introduction | ||||||
| 2002 or later | 123 (30) | 80 (35) | 1 | .004 | 1 | .009 |
| 1997–2001 | 150 (36) | 95 (41) | 1.1 (.8–1.6) | 1.5 (1.0–2.3) | ||
| Before 1997 | 143 (34) | 54 (24) | 1.9 (1.2–2.8) | 1.9 (1.2–3.0) | ||
| Positive anti-HCV IgG | 68 (16) | 26 (11) | 1.4 (.9–2.2) | .159 | 1.4 (.8–2.3) | .224 |
| Positive HBsAg | 36 (9) | 13 (6) | 1.5 (.8–2.8) | .21 | ||
| Positive anti-CMV IgG | 375 (90) | 189 (83) | 1.9 (1.2–3.1) | .005 | 1.9 (1.1–3.1) | .003 |
| Variable . | CD4/CD8 Ratio <1 (n = 416) . | CD4/CD8 Ratio ≥1 (n = 229) . | Univariable Analysis . | Multivariable Analysisa . | ||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) . | P Value . | Odds Ratio (95% CI) . | P Value . | |||
| Ageb, y | 48 (42–56) | 51 (45–57) | 0.8 (.7–.9) | .005 | 0.8 (.7–1.0) | .064 |
| Male sex | 300 (72) | 178 (78) | 0.7 (.5–1.0) | .061 | 0.9 (.6–1.5) | |
| Body mass index, kg/m2 | 24 (22–27) | 23 (21–26) | 1.0 (1.0–1.1) | .418 | ||
| HIV risk group | ||||||
| MSM | 186 (45) | 117 (51) | 1 | .226 | ||
| Heterosexual | 153 (37) | 69 (30) | 1.4 (1.0–2.1) | |||
| IDU | 26 (6) | 17 (8) | 1.0 (.6–6.5) | |||
| Other/unknown | 51 (12) | 26 (11) | 1.4 (.9–2.4) | |||
| CD4 count nadirc, cells/µL | 153 (56–240) | 233 (141–315) | 0.7 (.6–.8) | <.001 | 0.7 (.7–.8) | <.001 |
| Duration of viral suppressiond, y | 4.9 (3.1–8.5) | 6.3 (3.7–10.1) | 0.7 (.5–.8) | <.001 | 0.6 (.5–.8) | <.001 |
| ART introduction | ||||||
| 2002 or later | 123 (30) | 80 (35) | 1 | .004 | 1 | .009 |
| 1997–2001 | 150 (36) | 95 (41) | 1.1 (.8–1.6) | 1.5 (1.0–2.3) | ||
| Before 1997 | 143 (34) | 54 (24) | 1.9 (1.2–2.8) | 1.9 (1.2–3.0) | ||
| Positive anti-HCV IgG | 68 (16) | 26 (11) | 1.4 (.9–2.2) | .159 | 1.4 (.8–2.3) | .224 |
| Positive HBsAg | 36 (9) | 13 (6) | 1.5 (.8–2.8) | .21 | ||
| Positive anti-CMV IgG | 375 (90) | 189 (83) | 1.9 (1.2–3.1) | .005 | 1.9 (1.1–3.1) | .003 |
Results are expressed as median (IQR) or No. (%).
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; CMV, cytomegalovirus; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, intravenous drug user; IgG, immunoglobulin G; IQR, interquartile range; MSM, men who have sex with men.
a Statistically significant variables in univariable analysis (P < .20) were included in the multivariable analysis model.
b Odds ratios are expressed per 10 years of age.
c Odds ratios are expressed per log2 CD4 cell nadir.
d Viral suppression is defined as a plasma HIV-1 RNA level <200 copies/mL or <50 copies/mL according to the method used (<200 copies/mL from 1996 to 2006, and <50 copies/mL thereafter). Odds ratios are expressed per 5-year increments.
Risk Factors for a CD4/CD8 Ratio <1 in Individuals With Available Cytomegalovirus Serology: Univariable and Multivariable Analyses (n = 645)
| Variable . | CD4/CD8 Ratio <1 (n = 416) . | CD4/CD8 Ratio ≥1 (n = 229) . | Univariable Analysis . | Multivariable Analysisa . | ||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) . | P Value . | Odds Ratio (95% CI) . | P Value . | |||
| Ageb, y | 48 (42–56) | 51 (45–57) | 0.8 (.7–.9) | .005 | 0.8 (.7–1.0) | .064 |
| Male sex | 300 (72) | 178 (78) | 0.7 (.5–1.0) | .061 | 0.9 (.6–1.5) | |
| Body mass index, kg/m2 | 24 (22–27) | 23 (21–26) | 1.0 (1.0–1.1) | .418 | ||
| HIV risk group | ||||||
| MSM | 186 (45) | 117 (51) | 1 | .226 | ||
| Heterosexual | 153 (37) | 69 (30) | 1.4 (1.0–2.1) | |||
| IDU | 26 (6) | 17 (8) | 1.0 (.6–6.5) | |||
| Other/unknown | 51 (12) | 26 (11) | 1.4 (.9–2.4) | |||
| CD4 count nadirc, cells/µL | 153 (56–240) | 233 (141–315) | 0.7 (.6–.8) | <.001 | 0.7 (.7–.8) | <.001 |
| Duration of viral suppressiond, y | 4.9 (3.1–8.5) | 6.3 (3.7–10.1) | 0.7 (.5–.8) | <.001 | 0.6 (.5–.8) | <.001 |
| ART introduction | ||||||
| 2002 or later | 123 (30) | 80 (35) | 1 | .004 | 1 | .009 |
| 1997–2001 | 150 (36) | 95 (41) | 1.1 (.8–1.6) | 1.5 (1.0–2.3) | ||
| Before 1997 | 143 (34) | 54 (24) | 1.9 (1.2–2.8) | 1.9 (1.2–3.0) | ||
| Positive anti-HCV IgG | 68 (16) | 26 (11) | 1.4 (.9–2.2) | .159 | 1.4 (.8–2.3) | .224 |
| Positive HBsAg | 36 (9) | 13 (6) | 1.5 (.8–2.8) | .21 | ||
| Positive anti-CMV IgG | 375 (90) | 189 (83) | 1.9 (1.2–3.1) | .005 | 1.9 (1.1–3.1) | .003 |
| Variable . | CD4/CD8 Ratio <1 (n = 416) . | CD4/CD8 Ratio ≥1 (n = 229) . | Univariable Analysis . | Multivariable Analysisa . | ||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) . | P Value . | Odds Ratio (95% CI) . | P Value . | |||
| Ageb, y | 48 (42–56) | 51 (45–57) | 0.8 (.7–.9) | .005 | 0.8 (.7–1.0) | .064 |
| Male sex | 300 (72) | 178 (78) | 0.7 (.5–1.0) | .061 | 0.9 (.6–1.5) | |
| Body mass index, kg/m2 | 24 (22–27) | 23 (21–26) | 1.0 (1.0–1.1) | .418 | ||
| HIV risk group | ||||||
| MSM | 186 (45) | 117 (51) | 1 | .226 | ||
| Heterosexual | 153 (37) | 69 (30) | 1.4 (1.0–2.1) | |||
| IDU | 26 (6) | 17 (8) | 1.0 (.6–6.5) | |||
| Other/unknown | 51 (12) | 26 (11) | 1.4 (.9–2.4) | |||
| CD4 count nadirc, cells/µL | 153 (56–240) | 233 (141–315) | 0.7 (.6–.8) | <.001 | 0.7 (.7–.8) | <.001 |
| Duration of viral suppressiond, y | 4.9 (3.1–8.5) | 6.3 (3.7–10.1) | 0.7 (.5–.8) | <.001 | 0.6 (.5–.8) | <.001 |
| ART introduction | ||||||
| 2002 or later | 123 (30) | 80 (35) | 1 | .004 | 1 | .009 |
| 1997–2001 | 150 (36) | 95 (41) | 1.1 (.8–1.6) | 1.5 (1.0–2.3) | ||
| Before 1997 | 143 (34) | 54 (24) | 1.9 (1.2–2.8) | 1.9 (1.2–3.0) | ||
| Positive anti-HCV IgG | 68 (16) | 26 (11) | 1.4 (.9–2.2) | .159 | 1.4 (.8–2.3) | .224 |
| Positive HBsAg | 36 (9) | 13 (6) | 1.5 (.8–2.8) | .21 | ||
| Positive anti-CMV IgG | 375 (90) | 189 (83) | 1.9 (1.2–3.1) | .005 | 1.9 (1.1–3.1) | .003 |
Results are expressed as median (IQR) or No. (%).
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; CMV, cytomegalovirus; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, intravenous drug user; IgG, immunoglobulin G; IQR, interquartile range; MSM, men who have sex with men.
a Statistically significant variables in univariable analysis (P < .20) were included in the multivariable analysis model.
b Odds ratios are expressed per 10 years of age.
c Odds ratios are expressed per log2 CD4 cell nadir.
d Viral suppression is defined as a plasma HIV-1 RNA level <200 copies/mL or <50 copies/mL according to the method used (<200 copies/mL from 1996 to 2006, and <50 copies/mL thereafter). Odds ratios are expressed per 5-year increments.
Sensitivity Analyses
Among the 401 individuals with CD4 counts >500 cells/µL, 210 (52%) had a CD4/CD8 ratio <1. The factors associated with a CD4/CD8 ratio <1 in this subgroup were the same as in the whole group—namely, CMV seropositivity (OR, 2.2 [95% CI, 1.2–4.1]), ART initiation before 1997 (OR, 2.1 [95% CI, 1.1–3.8]), a lower CD4 T-cell nadir (OR, 0.8 [95% CI, .7–.9]), and shorter duration of viral suppression (OR, 0.6 [95% CI, .4–.8]) (Supplementary Table).
Among the 383 individuals with viral suppression lasting at least 5 years, 231 (60%) had a CD4/CD8 ratio <1. Again, the factors associated with a CD4/CD8 ratio <1 were CMV seropositivity (OR, 3.4 [95% CI, 1.8–6.7]), ART initiation before 1997 (OR, 1.6 [95% CI, .8–3.1]), a lower CD4 T-cell nadir (OR, 0.7 [95% CI, .6–.8] per log2 increment), and shorter duration of viral suppression (OR, 0.6 [95% CI, .4–.9] per 5 years) (data not shown).
Among the 210 individuals with a CD4/CD8 ratio <1 despite normalization of the CD4 count (>500 cells/µL), 102 patients (50%) had a CD8 count that remained abnormally high (>1000 cells/µL). In multivariable analysis, factors associated with this profile were CMV seropositivity (OR, 1.6 [95% CI, .6–4.4]) and male sex (OR, 0.5 [95% CI, .3–1.0]), although neither factor reached statistical significance (data not shown).
CD4/CD8 Ratio and Distribution of CD4 and CD8 T-Cell Counts According to CMV Serostatus
The median CD4 cell count did not differ significantly between CMV-seropositive and -seronegative individuals (573 [IQR, 425–753] cells/µL vs 605 [IQR, 461–806] cells/µL; P = .16), whereas the median CD8 cell count was higher in the seropositive individuals (742 [IQR, 539–997] cells/µL vs 630 [IQR, 440–833] cells/µL; P = .02) (Figure 2). Overall, a lower median ratio was observed in CMV-seropositive than in CMV-seronegative individuals: 0.8 (IQR, 0.5–1.1) vs 1.0 (IQR, 0.6–1.4), respectively (P < .001). The frequency of ratios <0.5, 0.5–0.99, 1–1.49, and ≥1.5 were 21%, 45%, 25%, and 8% among the 567 CMV-seropositive individuals and 12%, 38%, 27%, and 23% in the 81 CMV-seronegative individuals, respectively (P < .001). However, within each ratio quantile, no difference was observed in the distribution of the CD4 or CD8 T-cell count between CMV-seropositive and -seronegative individuals (Figure 2).

Absolute CD4 (A) and CD8 (B) T-cell counts according to CD4/CD8 ratio quantiles and cytomegalovirus (CMV) serostatus. The horizontal dotted line indicates T-cell counts of 500 cells/µL (A) and 1000 cells/µL (B). Data are presented as dotted lines for CMV-seronegative individuals and as full lines for CMV-seropositive individuals. Data are presented as box plots, with the central line indicating the median, the box the interquartile range, and the whiskers the 10th and 90th percentiles. Student t test was used to compare the CMV-seronegative and CMV-seropositive subgroups. CD4/CD8 denotes CD4 to CD8 ratio.
DISCUSSION
Despite long-term viral suppression (median, 5.4 [IQR, 3.3–9.1] years), two-thirds of HIV-infected individuals with a median age of 49 (IQR, 44–56) years still had a CD4/CD8 ratio <1, including more than half (53%) of those with CD4 counts >500 cells/µL. In the general population, only 8% of 20- to 60-year-olds and 16%–20% of 60- to 95-year-olds are reported to have a CD4/CD8 ratio <1 [23, 24]. It is particularly noteworthy that 153 (21%) individuals in our study had extremely low ratios (<0.5), which have been linked to a higher risk of non-AIDS morbidity and mortality [8, 21, 22].
We identified 4 independent risk factors for a low CD4/CD8 ratio—namely, CMV seropositivity, ART initiation before 1997, a lower CD4 T-cell nadir, and shorter duration of viral suppression. The same factors were identified in individuals with a CD4 count >500 cells/µL and in those with viral suppression lasting at least 5 years.
As expected, a low CD4 T-cell nadir, reflecting previously advanced HIV disease, was associated with a CD4/CD8 ratio <1 despite long-term viral suppression. Indeed, a low CD4 cell count at ART initiation is associated with slower immune recovery, even when viremia is fully suppressed for several years [25, 26].
The CD4/CD8 ratio tended to improve with the duration of viral suppression. We and others have previously demonstrated that a positive slope of CD4 recovery is maintained after 7 or even 10 years of suppressive ART, even in patients with the lowest nadir values [27, 28]. The few available data on long-term changes in the CD4/CD8 ratio during effective ART suggest that it may take longer to recover a normal CD4/CD8 ratio than a normal CD4 cell count [8].
ART initiation before 1997, and even before 2002, was associated with a lower likelihood of achieving a CD4/CD8 ratio >1, independent of the CD4 T-cell nadir and the duration of viral suppression. Previous antiretroviral drugs were less tolerable and less potent. Before 1997, available single- or dual-drug regimens were more prone to virological failure, resulting in lengthier periods of immune activation than at present [8, 29]. Our results are reminiscent of a study showing a link between cumulative HIV viremia and the CD4/CD8 ratio [30].
CMV seropositivity was the fourth factor independently influencing the CD4/CD8 ratio, mostly by increasing the CD8 count, suggesting that CMV coinfection may also result in persistent immune activation. A CD4/CD8 ratio <0.5 was twice as likely in the case of CMV seropositivity, while a ratio >1.5 was 3 times more likely in the case of CMV seronegativity. These results are in line with previously published reports [31, 32]. Of note, when we compared the CD4 and the CD8 counts between CMV-seropositive and CMV-seronegative individuals within each ratio quantile (<0.5, 0.5–0.99, 1–1.49, and ≥1.5), no difference was observed in the distribution of the CD4 or CD8 cell counts (Figure 2). Although this may be explained by a lack of power, it could also reflect that a low ratio is driven by the same mechanism in CMV-seropositive and in CMV-seronegative individuals. HIV-infected individuals have a far higher CMV seroprevalence than the general population, along with remarkably high levels of CMV-specific effector cells [33–35]. Recent studies show that CMV seropositivity and higher anti-CMV antibody titers are associated with increased non-AIDS morbidity and mortality, especially with respect to cardiovascular and cerebrovascular events [34]. Other observations showed how CMV coinfection was associated with increased systemic inflammation, chronic immune activation, and immunosenescence in HIV-infected individuals despite effective ART [31, 32, 36, 37]. An 8-week course of valganciclovir has been shown to lead to a 20% reduction in activated CD8 T cells (CD38+HLA-DR+), suggesting that CMV is a targetable cause of persistent immune activation in this setting, especially with newer, better-tolerated drugs [38, 39]. In accordance with these data, our findings suggest that the CD4/CD8 ratio might be strongly influenced by the CMV-specific inflammatory response, which provokes CD8 T-cell activation.
Our study has several limitations. First, as in any cross-sectional study, causality between the observed risk factors and a low CD4/CD8 ratio cannot be properly established. Second, the CMV serology was not available in 74 study participants. In the latter, we observed a higher frequency of intravenous drug users and a lower frequency of MSM, which might have slightly overestimated the CMV seroprevalence in the study population. However it is unlikely that this influenced the strength of the association between CMV seropositivity and the CD4/CD8 ratio, as individuals with no available CMV serology did not differ from others, especially with regard to risk factors that we found to be associated with a low CD4/CD8 ratio. Third, we could not study markers of inflammation and asymptomatic CMV replication, precluding mechanistic insights into the complex interaction between CMV coinfection and the CD4/CD8 ratio. Finally, several parameters that may impact inflammation and systemic immune activation could not be included in our analyses, in particular, smoking status, which was recently shown to increase immune activation and to impair T-cell function in treated HIV-infected patients [40].
In conclusion, a large proportion of HIV-infected individuals continue to have an abnormal CD4/CD8 ratio despite long-term viral suppression and CD4 T-cell recovery. Early ART based on recent drugs should help to improve this ratio, while new anti-CMV drugs might represent an interesting therapeutic option for CMV-seropositive individuals with the lowest CD4/CD8 ratios.
Note
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




