-
PDF
- Split View
-
Views
-
Cite
Cite
Martina Oneko, Simon Kariuki, Vincent Muturi-Kioi, Kephas Otieno, Vincent O. Otieno, John M. Williamson, Jason Folster, Michele B. Parsons, Laurence Slutsker, Barbara E. Mahon, Mary J. Hamel, Emergence of Community-Acquired, Multidrug-Resistant Invasive Nontyphoidal Salmonella Disease in Rural Western Kenya, 2009–2013, Clinical Infectious Diseases, Volume 61, Issue suppl_4, November 2015, Pages S310–S316, https://doi.org/10.1093/cid/civ674
Close - Share Icon Share
Abstract
Background. Nontyphoidal Salmonella (NTS), mainly serotypes Typhimurium and Enteritidis, cause invasive infections with high mortality in children in sub-Saharan Africa. Multidrug resistance is common, and resistance to third-generation cephalosporins has emerged.
Methods. We reviewed clinical features, outcomes, and antimicrobial resistance patterns in invasive NTS infections among children aged 6 weeks to 5 years participating in malaria vaccine studies in an area of high malaria and human immunodeficiency virus (HIV) transmission in Siaya, western Kenya. Blood culture was performed in hospitalized children and pediatric outpatients with prolonged fever.
Results. From July 2009 to December 2013, 1696 children aged 6 weeks to 17 months were enrolled into vaccine trials and followed for up to 53 months. We obtained 1692 blood cultures from 847 children. Of 134 bacterial pathogens isolated, 102 (76.1%) were Salmonella serogroup B or D. Invasive NTS disease occurred in 94 (5.5%) children, with an incidence of 1870, 4134, and 6510 episodes per 100 000 person-years overall, in infants, and in HIV-infected children, respectively. Malaria infection within the past 2 weeks occurred in 18.8% (3/16) of invasive NTS episodes in HIV-infected and 66.2% (53/80) in HIV-uninfected children. Case fatality rate was 3.1%. Salmonella group B resistant to ceftriaxone emerged in 2009 and 2010 (6.2% [2/32 isolates]), rising to 56.5% (13/23 isolates) in 2012 and 2013.
Conclusions. Incidence of invasive NTS disease was high in this area of high malaria and HIV transmission, especially in HIV-infected children. Rapidly emerging resistance against ceftriaxone requires urgent reevaluation of antibiotic recommendations and primary prevention of exposure to Salmonella.
Invasive nontyphoidal Salmonella (NTS) disease—an infection caused by Salmonella serovars other than Typhi— is a major cause of childhood morbidity and mortality in sub-Saharan Africa. Serovar Salmonella Typhimurium is most commonly identified, followed by Salmonella Enteritidis [1]. Because microbiology services are limited, suspected cases of bacteremia are often treated empirically. The emergence of antimicrobial resistance frequently remains undetected for many months [2, 3].
Invasive NTS (iNTS) disease in African children is strongly associated with malaria infection [4, 5], and dramatic reductions in iNTS disease incidence have been documented following scale-up of effective malaria control measures [6]. The case fatality rate (CFR) is high, ranging from 21% to 36% in hospitalized children in East Africa, highest in infants [3, 7, 8]. Strains of NTS that are multidrug resistant to recommended first-line antibiotics, including chloramphenicol and ampicillin, have emerged in several African countries over the past 20 years [9, 10]; however, resistance to ceftriaxone and ciprofloxacin had not been documented until recently [11, 12]. A distinct phylogenetic lineage of Salmonella Typhimurium, sequence type 313 (ST313), is a significant cause of iNTS disease in Africa. ST313 often harbors plasmids encoding genes for antibiotic resistance, virulence, and invasion [13]. Treatment failure and complications are associated with failure to identify and appropriately treat resistant strains [14, 15].
We recently conducted 2 malaria vaccine trials in western Kenya that included systematic microbiologic evaluation of fever in children hospitalized with severe disease. These trials provided an opportunity to identify and characterize iNTS disease in a large cohort of children and to document the emergence of resistance to ceftriaxone. This work was conducted under the Kenya Medical Research Institute (KEMRI) and Centers for Disease Control and Prevention (CDC) public health and research collaboration.
METHODS
Malaria Vaccine Trial Design
The previously described phase 3 multisite RTS,S/AS01 malaria vaccine trial was conducted in 11 sites in 7 African countries [16]. Children were enrolled in 2 age categories: 6–12 weeks and 5–17 months. Systematic human immunodeficiency virus (HIV) testing was not required per protocol; however, Kenyan national guidelines recommend provider-initiated HIV counseling and testing for all patients; therefore, most parents of study participants were offered HIV testing for their children. HIV-infected children received cotrimoxazole prophylaxis through most of the study period, and HIV-exposed but uninfected children received it for varying time periods. A second trial of the RTS,S/AS01 vaccine was conducted in HIV-infected children between 6 weeks and 17 months of age meeting World Health Organization criteria for stage 1 or 2 HIV disease at 2 study sites in the same area of western Kenya. In both trials, participants requiring hospitalization were managed according to standardized diagnostic algorithms including a blood culture at admission [16]. Here we describe findings limited to cases of iNTS disease among children enrolled at the KEMRI/CDC site in western Kenya.
Patients and Setting
The study cohort was recruited and followed from July 2009 to December 2013. In this region, HIV prevalence was 16.1% in pregnant women in 2012 [17] and Plasmodium falciparum parasitemia among children under 5 is 42% (KEMRI/CDC 2011, unpublished data). All children enrolled in the 2 malaria vaccine trials in Siaya County who received at least 1 study vaccination were included in this analysis. Caretakers were encouraged to seek care for their child's illness at study clinics; transport reimbursement was provided. Children requiring admission were referred to Siaya District Hospital. A blood culture was performed for all hospitalized participants and for children at outpatient clinics with prolonged fever. Study participants with a positive blood culture for NTS were defined as having iNTS disease. Sepsis was defined as bacteremia in a child with at least 2 of the following: axillary temperature >38°C or <35.5°C, abnormal leukocyte count, tachycardia (>180 beats per minute <1 year; >140 beats per minute 1–5 years), or tachypnea (≥50 breaths per minute 2 months to 1 year; ≥40 breaths per minute >1 year); at least 1 of the signs must be an elevated temperature or abnormal leukocyte count [18]. Relapse of iNTS disease was defined as fever recurrence and isolation of the same pathogen in blood culture within 4 weeks of initial clinical cure.
Microbiology
A total of 1–3 mL of blood was obtained aseptically by venous puncture and inoculated into a pediatric culture bottle. Standard blood culture methods were followed using Bactec incubators (Becton Dickinson). Positive cultures were subcultured following standard methods [19, 20]. A blood culture was considered positive if a pathogen was grown or if a bacterium that could be pathogen or contaminant (eg, Enterococcus faecalis) was isolated within 48 hours of incubation [21]. Salmonella serogroups were determined by slide agglutination tests using specific O and H antisera (Becton Dickinson). The Kirby–Bauer disk diffusion method was used for antimicrobial susceptibility testing of the isolate with Escherichia coli ATCC 25922 as control strain tested concurrently as per Clinical and Laboratory Standards Institute guidelines [22]. A convenience sample of 12 isolates determined at the KEMRI laboratory to be serogroup B and ceftriaxone resistant underwent confirmatory testing at the CDC in Atlanta. Serotyping was conducted using standard methods [22, 23], and broth microdilution was used for antimicrobial susceptibility testing [24] (Ulzii-Orshikh Luvsansharav, CDC, unpublished data).
Other Laboratory Data
All children had a blood sample collected for malaria diagnosis by microscopy or rapid diagnostic test, and a complete blood count when they presented with measured or reported fever. Recent or concurrent malaria infection was defined as a positive blood smear for any plasmodium species or a positive rapid diagnostic test during the 2 weeks prior to fever onset or at the time of diagnosis of bacteremia.
HIV-exposed children >18 months of age were tested using rapid HIV antibody tests; children <18 months of age were tested by HIV DNA polymerase chain reaction (PCR). A child was considered to be HIV uninfected if a negative antibody test of the mother and/or a negative PCR or antibody test in the child were documented. If no HIV test for mother or child was available, or an HIV-exposed child had no confirmatory test after cessation of breastfeeding, the status was considered unknown. Children with unknown HIV status were included with children who were HIV uninfected in this analysis.
Antibiotic Treatment
Children admitted to hospital with suspected bacterial infection were treated with antibiotics, usually penicillins with gentamicin or chloramphenicol [25, 26]. Antibiotic regimens were adjusted according to blood culture and antibiotic sensitivity results. After recognizing that most Salmonella isolates were resistant to first-line antibiotics, we began initiating treatment with ceftriaxone as soon as gram-negative bacilli were identified in the blood culture.
Statistical Analysis
Incidence was calculated by dividing the number of incident cases by person-years of follow-up, from consent until study end, death, or loss to follow-up. Incidence ratios were calculated by comparing incidence between HIV groups using Poisson regression. Poisson regression was used to model iNTS disease incidence and generalized estimating equations to control for correlated observations on the same child. A Cox proportional hazards model was fit for time to iNTS disease using sex, clinical malaria in the preceding 2 weeks, and HIV status as predictors. CFRs were compared using Pearson χ2, exact version.
Ethics Review
The vaccine trials were approved by the ethical review board of KEMRI and CDC as well as the Western Institutional Review Board.
RESULTS
A total of 1696 children aged between 6 weeks and 17 months were enrolled and followed for a total of 5119 person-years. Relevant baseline characteristics and follow-up time are shown in Table 1. HIV status was confirmed to be positive in 7.7% and negative in 85.1%.
Baseline Characteristics of Study Population in Malaria Vaccine Studies, 2009–2014
| Characteristic . | Value . |
|---|---|
| Follow-up time, y, mean (median; range) | 3.02 (3.34; 0.01–4.50) |
| Age at enrollment | |
| 6 wk–4 mo | 838 (49.4) |
| 5–17 mo | 858 (50.6) |
| Sex, female | 50.4% |
| HIV status | |
| Uninfected | 1444 (85.1) |
| Infected | 131 (7.7) |
| Unknown | 121 (7.1) |
| Nutritional status at enrollment | |
| Mean weight-for-age z score (range) | −0.69 (−5.4 to 3.4) |
| Mean height-for-age z score (range) | −1.0 (−7 to 5) |
| Hemoglobin, g/dL | |
| Mean (range) | 10.8 (5.1–17.9) |
| <8 g/dL | 116 (6.8) |
| Characteristic . | Value . |
|---|---|
| Follow-up time, y, mean (median; range) | 3.02 (3.34; 0.01–4.50) |
| Age at enrollment | |
| 6 wk–4 mo | 838 (49.4) |
| 5–17 mo | 858 (50.6) |
| Sex, female | 50.4% |
| HIV status | |
| Uninfected | 1444 (85.1) |
| Infected | 131 (7.7) |
| Unknown | 121 (7.1) |
| Nutritional status at enrollment | |
| Mean weight-for-age z score (range) | −0.69 (−5.4 to 3.4) |
| Mean height-for-age z score (range) | −1.0 (−7 to 5) |
| Hemoglobin, g/dL | |
| Mean (range) | 10.8 (5.1–17.9) |
| <8 g/dL | 116 (6.8) |
Data are presented as No. (%) unless otherwise specified.
Abbreviation: HIV, human immunodeficiency virus.
Baseline Characteristics of Study Population in Malaria Vaccine Studies, 2009–2014
| Characteristic . | Value . |
|---|---|
| Follow-up time, y, mean (median; range) | 3.02 (3.34; 0.01–4.50) |
| Age at enrollment | |
| 6 wk–4 mo | 838 (49.4) |
| 5–17 mo | 858 (50.6) |
| Sex, female | 50.4% |
| HIV status | |
| Uninfected | 1444 (85.1) |
| Infected | 131 (7.7) |
| Unknown | 121 (7.1) |
| Nutritional status at enrollment | |
| Mean weight-for-age z score (range) | −0.69 (−5.4 to 3.4) |
| Mean height-for-age z score (range) | −1.0 (−7 to 5) |
| Hemoglobin, g/dL | |
| Mean (range) | 10.8 (5.1–17.9) |
| <8 g/dL | 116 (6.8) |
| Characteristic . | Value . |
|---|---|
| Follow-up time, y, mean (median; range) | 3.02 (3.34; 0.01–4.50) |
| Age at enrollment | |
| 6 wk–4 mo | 838 (49.4) |
| 5–17 mo | 858 (50.6) |
| Sex, female | 50.4% |
| HIV status | |
| Uninfected | 1444 (85.1) |
| Infected | 131 (7.7) |
| Unknown | 121 (7.1) |
| Nutritional status at enrollment | |
| Mean weight-for-age z score (range) | −0.69 (−5.4 to 3.4) |
| Mean height-for-age z score (range) | −1.0 (−7 to 5) |
| Hemoglobin, g/dL | |
| Mean (range) | 10.8 (5.1–17.9) |
| <8 g/dL | 116 (6.8) |
Data are presented as No. (%) unless otherwise specified.
Abbreviation: HIV, human immunodeficiency virus.
Among the 121 (7.1%) children with unknown HIV status, 29 (24%) were followed up for <1 year due to migration (n = 20) or consent withdrawal (n = 9); 13 were HIV exposed without confirmatory testing after cessation of breastfeeding. A sensitivity analysis categorizing them as HIV infected did not alter the results.
Microbiology Results
We obtained 1692 blood samples for culture from 847 participants; 97% were inpatients. In total, 7.9% (n = 134) of the cultures yielded pathogens, with 76% of positive cultures identified as NTS group B (n = 72) or D (n = 30), resulting in an overall NTS isolation rate 6% (102/1692). Other pathogens were Streptococcus pneumoniae (n = 7), Enterococcus (n = 3), Escherichia coli (n = 6), Streptococcus viridans (n = 3), and others (n = 13). Salmonella Typhi was not isolated from any patient.
From 2009, all iNTS of both serogroups showed resistance to ≥3 antibiotics including chloramphenicol and amoxicillin-clavulanic acid (Table 2). We isolated Salmonella group B resistant to ceftriaxone once in 2009 (1/11) and once in 2010 (1/21), both in repeat blood cultures of children under treatment with ceftriaxone. In 2012 and 2013, resistance of community-acquired Salmonella group B isolates against ceftriaxone increased to 50% (9/18) and 86% (6/7), respectively (Figure 1). CDC testing of ceftriaxone-resistant serogroup B isolates identified all as serotype Typhimurium; antimicrobial susceptibility testing confirmed resistance to antibiotics from ≥6 antibiotic classes. All Typhimurium isolates were resistant to ceftriaxone (third-generation cephalosporin), and 1 isolate also showed resistance to ciprofloxacin (fluoroquinolone). All isolates were susceptible to carbapenems.
Antibiotic Resistance Pattern in Blood Cultures From Participants of Malaria Vaccine Trials in Western Kenya
| Antibiotic . | Resistant/Tested, No. (%) . | |
|---|---|---|
| Salmonella B (n = 72)a . | Salmonella D (n = 30) . | |
| Ampicillin | 64/68 (94.1) | 27/30 (90) |
| Chloramphenicol | 54/72 (75) | 28/30 (93.3) |
| Amoxicillin + clavulanate | 44/67 (65.7) | 13/27 (48.1) |
| Ceftriaxone | 17/72 (23.6) | 0/30 (0) |
| Ciprofloxacin | 1/71 (1.4%) | 0/30 (0) |
| Imipenem | 0/42 (0) | 0/15 (0) |
| A, Au, C | 36/64 (56.3) | 13/30 (43.3) |
| A, Au, C, Gen, Cx | 17/64 (26.6) | 0/30 (0) |
| Antibiotic . | Resistant/Tested, No. (%) . | |
|---|---|---|
| Salmonella B (n = 72)a . | Salmonella D (n = 30) . | |
| Ampicillin | 64/68 (94.1) | 27/30 (90) |
| Chloramphenicol | 54/72 (75) | 28/30 (93.3) |
| Amoxicillin + clavulanate | 44/67 (65.7) | 13/27 (48.1) |
| Ceftriaxone | 17/72 (23.6) | 0/30 (0) |
| Ciprofloxacin | 1/71 (1.4%) | 0/30 (0) |
| Imipenem | 0/42 (0) | 0/15 (0) |
| A, Au, C | 36/64 (56.3) | 13/30 (43.3) |
| A, Au, C, Gen, Cx | 17/64 (26.6) | 0/30 (0) |
Abbreviations: A, ampicillin; Au, amoxicillin-clavulanic acid; C, chloramphenicol; Cx, ceftriaxone; Gen, gentamycin (only those isolates tested against all included).
a Includes 2 cultures repeated with hospital-acquired ceftriaxone resistance, and 4 relapses.
Antibiotic Resistance Pattern in Blood Cultures From Participants of Malaria Vaccine Trials in Western Kenya
| Antibiotic . | Resistant/Tested, No. (%) . | |
|---|---|---|
| Salmonella B (n = 72)a . | Salmonella D (n = 30) . | |
| Ampicillin | 64/68 (94.1) | 27/30 (90) |
| Chloramphenicol | 54/72 (75) | 28/30 (93.3) |
| Amoxicillin + clavulanate | 44/67 (65.7) | 13/27 (48.1) |
| Ceftriaxone | 17/72 (23.6) | 0/30 (0) |
| Ciprofloxacin | 1/71 (1.4%) | 0/30 (0) |
| Imipenem | 0/42 (0) | 0/15 (0) |
| A, Au, C | 36/64 (56.3) | 13/30 (43.3) |
| A, Au, C, Gen, Cx | 17/64 (26.6) | 0/30 (0) |
| Antibiotic . | Resistant/Tested, No. (%) . | |
|---|---|---|
| Salmonella B (n = 72)a . | Salmonella D (n = 30) . | |
| Ampicillin | 64/68 (94.1) | 27/30 (90) |
| Chloramphenicol | 54/72 (75) | 28/30 (93.3) |
| Amoxicillin + clavulanate | 44/67 (65.7) | 13/27 (48.1) |
| Ceftriaxone | 17/72 (23.6) | 0/30 (0) |
| Ciprofloxacin | 1/71 (1.4%) | 0/30 (0) |
| Imipenem | 0/42 (0) | 0/15 (0) |
| A, Au, C | 36/64 (56.3) | 13/30 (43.3) |
| A, Au, C, Gen, Cx | 17/64 (26.6) | 0/30 (0) |
Abbreviations: A, ampicillin; Au, amoxicillin-clavulanic acid; C, chloramphenicol; Cx, ceftriaxone; Gen, gentamycin (only those isolates tested against all included).
a Includes 2 cultures repeated with hospital-acquired ceftriaxone resistance, and 4 relapses.
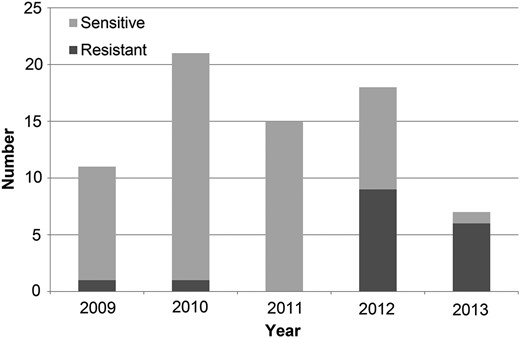
Resistance of Salmonella group B to ceftriaxone among participants of malaria vaccine trials in western Kenya, 2009–2013.
Incidence
Ninety-four children (5.5%) experienced a total of 100 episodes of iNTS disease. Four children (2 HIV infected) with group B relapsed, 1 child had 2 independent group B infections, and 1 child had 1 episode each of group B and D infection. Overall, the incidence was 1870 (95% confidence interval [CI], 1540–2280) per 100 000 person-years. Infants had the highest disease burden of any age group, with an incidence of 4134 (95% CI, 2982–5731) per 100 000 person-years (Figure 2). The incidence of iNTS disease in HIV-infected participants was higher than in participants with HIV-uninfected/unknown status: 6510 (95% CI, 4060–10 440) vs 1640 (95% CI, 1320–2040) episodes per 100 000 person-years (P < .001); however, when controlling for malaria infection and sex, this association was no longer statistically significant (hazard ratio = 2.08 [95% CI, .91–4.75; P = .084]).
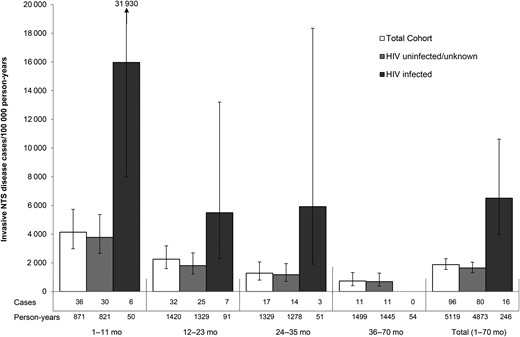
Incidence of invasive nontyphoidal Salmonella (NTS) disease by age group and human immunodeficiency virus (HIV) status in participants of malaria vaccine trials in western Kenya.
Clinical Findings
Among children who developed iNTS disease, 65.6% (63/96) had fever of >3 days’ duration, frequently persisting after completion of antimalarial treatment, and 81.3% (78/96) fulfilled sepsis criteria (Table 3).
Characteristics and Clinical Findings in Children With Invasive Nontyphoidal Salmonella Disease in Western Kenya (n = 96 Episodes)
| Characteristic . | Value . |
|---|---|
| Age at time of iNTS disease, mo | |
| Mean | 18.7 |
| Median; range | 17.5; 2.2–53 |
| Female sex | 52/94 (55.3) |
| Nutritional status at disease onset | |
| Weight-for-age z score, mean (range) | −2.07 (−5.4 to 2.8) |
| Height-for-age z core, mean (range) | −1.3 (−4.7 to 2) |
| Weight-for-height/length z score <−2 | 21 (21.9) |
| Weight-for-height/length z score <−3 | 8 (8.3) |
| Clinical findings | |
| Malaria 2 wk prior to iNTS | 56/96 (58) |
| In HIV uninfected (n = 80 episodes) | 53/80 (66.3) |
| In HIV infected (n = 16 episodes) | 3/16 (18.8) |
| History of fever during this illness | 96 (100) |
| Measured fever ≥37.5°C during this illness | 95 (99) |
| Fever >3 d | 63 (65.6) |
| Mean duration of fever, d (range) | 4.0 (0–14) |
| Mean axillary temperature, °C (range) | 39.6 (36.8–41.4) |
| Diarrhea | 44 (45.8) |
| Tachypneaa | 66 (68.8) |
| Tachycardiab | 49 (51) |
| Abnormal WBCc (n = 95) | 30 (31.6) |
| Mean hemoglobin, g/dL, (range) | 8.2 (3.9–14.1) |
| Hemoglobin <8 g/dL | 31 (32.3) |
| Neurological symptoms | 23 (24) |
| Seizures | 13 (13.5) |
| Reduced level of consciousness | 7 (7.3) |
| Two sepsis criteriad | 78 (81.3) |
| Four sepsis criteriad | 14 (14.6) |
| Complications | |
| Meningitis | 2 (2.1) |
| Septic arthritis/pyomyositis | 7 (7.3) |
| Death | 3 (3.1) |
| Characteristic . | Value . |
|---|---|
| Age at time of iNTS disease, mo | |
| Mean | 18.7 |
| Median; range | 17.5; 2.2–53 |
| Female sex | 52/94 (55.3) |
| Nutritional status at disease onset | |
| Weight-for-age z score, mean (range) | −2.07 (−5.4 to 2.8) |
| Height-for-age z core, mean (range) | −1.3 (−4.7 to 2) |
| Weight-for-height/length z score <−2 | 21 (21.9) |
| Weight-for-height/length z score <−3 | 8 (8.3) |
| Clinical findings | |
| Malaria 2 wk prior to iNTS | 56/96 (58) |
| In HIV uninfected (n = 80 episodes) | 53/80 (66.3) |
| In HIV infected (n = 16 episodes) | 3/16 (18.8) |
| History of fever during this illness | 96 (100) |
| Measured fever ≥37.5°C during this illness | 95 (99) |
| Fever >3 d | 63 (65.6) |
| Mean duration of fever, d (range) | 4.0 (0–14) |
| Mean axillary temperature, °C (range) | 39.6 (36.8–41.4) |
| Diarrhea | 44 (45.8) |
| Tachypneaa | 66 (68.8) |
| Tachycardiab | 49 (51) |
| Abnormal WBCc (n = 95) | 30 (31.6) |
| Mean hemoglobin, g/dL, (range) | 8.2 (3.9–14.1) |
| Hemoglobin <8 g/dL | 31 (32.3) |
| Neurological symptoms | 23 (24) |
| Seizures | 13 (13.5) |
| Reduced level of consciousness | 7 (7.3) |
| Two sepsis criteriad | 78 (81.3) |
| Four sepsis criteriad | 14 (14.6) |
| Complications | |
| Meningitis | 2 (2.1) |
| Septic arthritis/pyomyositis | 7 (7.3) |
| Death | 3 (3.1) |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: HIV, human immunodeficiency virus; iNTS, invasive nontyphoidal Salmonella; WBC, white blood cell.
a Tachypnea: respiratory rate ≥50 breaths per minute (2 months to 1 year old) or ≥40 breaths per minute (>1 year of age).
b Tachycardia: heart rate >180 beats per minute (<1 year of age) or >140 beats per minute (1–5 years of age).
c WBC>17.5 or <5 × 103 cells/µL (<1 year of age) or >15.5 or <6 × 103 cells/µL (1–5 years of age).
d See sepsis definition in “Methods” section.
Characteristics and Clinical Findings in Children With Invasive Nontyphoidal Salmonella Disease in Western Kenya (n = 96 Episodes)
| Characteristic . | Value . |
|---|---|
| Age at time of iNTS disease, mo | |
| Mean | 18.7 |
| Median; range | 17.5; 2.2–53 |
| Female sex | 52/94 (55.3) |
| Nutritional status at disease onset | |
| Weight-for-age z score, mean (range) | −2.07 (−5.4 to 2.8) |
| Height-for-age z core, mean (range) | −1.3 (−4.7 to 2) |
| Weight-for-height/length z score <−2 | 21 (21.9) |
| Weight-for-height/length z score <−3 | 8 (8.3) |
| Clinical findings | |
| Malaria 2 wk prior to iNTS | 56/96 (58) |
| In HIV uninfected (n = 80 episodes) | 53/80 (66.3) |
| In HIV infected (n = 16 episodes) | 3/16 (18.8) |
| History of fever during this illness | 96 (100) |
| Measured fever ≥37.5°C during this illness | 95 (99) |
| Fever >3 d | 63 (65.6) |
| Mean duration of fever, d (range) | 4.0 (0–14) |
| Mean axillary temperature, °C (range) | 39.6 (36.8–41.4) |
| Diarrhea | 44 (45.8) |
| Tachypneaa | 66 (68.8) |
| Tachycardiab | 49 (51) |
| Abnormal WBCc (n = 95) | 30 (31.6) |
| Mean hemoglobin, g/dL, (range) | 8.2 (3.9–14.1) |
| Hemoglobin <8 g/dL | 31 (32.3) |
| Neurological symptoms | 23 (24) |
| Seizures | 13 (13.5) |
| Reduced level of consciousness | 7 (7.3) |
| Two sepsis criteriad | 78 (81.3) |
| Four sepsis criteriad | 14 (14.6) |
| Complications | |
| Meningitis | 2 (2.1) |
| Septic arthritis/pyomyositis | 7 (7.3) |
| Death | 3 (3.1) |
| Characteristic . | Value . |
|---|---|
| Age at time of iNTS disease, mo | |
| Mean | 18.7 |
| Median; range | 17.5; 2.2–53 |
| Female sex | 52/94 (55.3) |
| Nutritional status at disease onset | |
| Weight-for-age z score, mean (range) | −2.07 (−5.4 to 2.8) |
| Height-for-age z core, mean (range) | −1.3 (−4.7 to 2) |
| Weight-for-height/length z score <−2 | 21 (21.9) |
| Weight-for-height/length z score <−3 | 8 (8.3) |
| Clinical findings | |
| Malaria 2 wk prior to iNTS | 56/96 (58) |
| In HIV uninfected (n = 80 episodes) | 53/80 (66.3) |
| In HIV infected (n = 16 episodes) | 3/16 (18.8) |
| History of fever during this illness | 96 (100) |
| Measured fever ≥37.5°C during this illness | 95 (99) |
| Fever >3 d | 63 (65.6) |
| Mean duration of fever, d (range) | 4.0 (0–14) |
| Mean axillary temperature, °C (range) | 39.6 (36.8–41.4) |
| Diarrhea | 44 (45.8) |
| Tachypneaa | 66 (68.8) |
| Tachycardiab | 49 (51) |
| Abnormal WBCc (n = 95) | 30 (31.6) |
| Mean hemoglobin, g/dL, (range) | 8.2 (3.9–14.1) |
| Hemoglobin <8 g/dL | 31 (32.3) |
| Neurological symptoms | 23 (24) |
| Seizures | 13 (13.5) |
| Reduced level of consciousness | 7 (7.3) |
| Two sepsis criteriad | 78 (81.3) |
| Four sepsis criteriad | 14 (14.6) |
| Complications | |
| Meningitis | 2 (2.1) |
| Septic arthritis/pyomyositis | 7 (7.3) |
| Death | 3 (3.1) |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: HIV, human immunodeficiency virus; iNTS, invasive nontyphoidal Salmonella; WBC, white blood cell.
a Tachypnea: respiratory rate ≥50 breaths per minute (2 months to 1 year old) or ≥40 breaths per minute (>1 year of age).
b Tachycardia: heart rate >180 beats per minute (<1 year of age) or >140 beats per minute (1–5 years of age).
c WBC>17.5 or <5 × 103 cells/µL (<1 year of age) or >15.5 or <6 × 103 cells/µL (1–5 years of age).
d See sepsis definition in “Methods” section.
Three of 96 children died, giving an overall case fatality of 3.1% (95% CI, .7%–8.9%). Among the children who died, 1 had mixed growth of Salmonella group B and E. coli. Two of the 3 children who died were HIV infected; thus, CFR in HIV-infected children compared with HIV-uninfected children was 12.5% (2/16) and 1.3% (1/80), respectively (P = .076).
Temporal Relationship of iNTS Disease to Malaria
Of the children diagnosed with iNTS disease, 56 of 96 (58%) had malaria infection within 2 weeks before onset of iNTS disease. This association was stronger in HIV-uninfected children, of whom 66.3% (53/80) had malaria within 2 weeks before iNTS disease, compared with 18.8% (3/16) of HIV-infected children (odds ratio, 7.22 [95% CI, 1.90–27.43]). The adjusted hazard ratio comparing incidence of iNTS disease during the 2 weeks following a malaria infection (0.064) vs incidence of iNTS disease overall (0.019) was 3.67 (95% CI, 2.95–4.56; P < .0001).
DISCUSSION
This study documents extremely high incidence of iNTS disease in young children in a rural region in Kenya: close to 2000 per 100 000 person-years overall, >4000 per 100 000 person-years in infants, and >6000 per 100 000 person-years in HIV-infected children. Incidence in these trials is much higher than in previous reports from other sub-Saharan African settings of 88–300 per 100 000 person-years [5, 27]. The study methodology used in the malaria vaccine trials makes these data particularly robust: Children were followed longitudinally, diagnostic algorithms were consistently applied to all children, and quality assurance methods ensured high-quality microbiological diagnostics, resulting in a reliable estimate of the incidence of iNTS disease in this population. We likely detected many infections that would otherwise have gone undiagnosed and possibly treated empirically in other contexts; the high proportion of children meeting criteria for sepsis shows that most, if not all, of these infections were clinically meaningful. Facilitated access to high-quality healthcare may have resulted in lower mortality than reported in the literature, comparable to that reported for patients admitted at a private hospital in Kenya where CFR among children aged 1–3 years was <1% compared with 12% in a public hospital [10]. The lower mortality rate demonstrates the value of early detection, knowledge of current resistance patterns to guide empiric therapy, and timely microbiological results to further modify antimicrobial therapy. Our findings underscore the urgent need for primary prevention of iNTS infection, yet little is known about the sources of NTS causing invasive infections in Africa. The relative contributions of exposure to NTS through food, water, direct animal contact, and person-to-person spread are unclear. Interventions to improve sanitation and hygiene that reduce spread from any of these sources could reduce transmission of other enteric infections, too. However, achieving lasting improvements in hygiene and sanitation is challenging. An effective vaccine may be needed to supplement efforts to prevent iNTS infection.
Malaria infection is a known risk factor for iNTS disease, and the high incidence of malaria in Siaya County (4.27 episodes of malaria/year in the infant control group of the vaccine trial [28]) likely contributed to the high incidence of iNTS disease. In this study population, the risk of being diagnosed with iNTS disease increased >3-fold during the 2 weeks following an episode of malaria infection, consistent with findings from previous studies that showed recent malaria infection to be an important risk factor [4, 5]. The overall incidence of iNTS disease in HIV-infected children was higher than in noninfected children, as has also been reported in adults [25]. However, in multivariate analysis the relationship between HIV and iNTS disease did not achieve statistical significance, possibly due to small sample size and the potential suppressive effect of cotrimoxazole prophylaxis on malaria infection [29, 30].
More than 80% of children with iNTS disease had serious illness fulfilling sepsis criteria, but because the study protocol included blood culture in hospitalized children regardless of whether sepsis-related symptoms were present, a relatively mild form of iNTS disease was also identified. Whether these children would have progressed to more serious illness had the bacteremia not been detected early is unknown. In most sub-Saharan African rural clinics, blood cultures are not available for persistently febrile children, but our data indicate that iNTS disease is commonly preceded by malaria and should be suspected in children with fever that persists.
A limitation of this study is that whole-genome sequencing was not performed on NTS isolates from these patients. However, it was conducted on ceftriaxone-resistant Typhimurium isolates from patients with invasive infections in western Kenya around the time of this study. Whole-genome sequencing confirmed ST313 and identified multiple antimicrobial resistance genes on 2 plasmids, IncHI2 and IncFIIS, with ceftriaxone resistance conferred by blaCTX-M-15 on IncHI2. (Ulzii-Orshikh Luvsansharav, CDC, unpublished data). Similar findings were reported for NTS isolated in Nairobi, about 400 km east of the study area [10] and from Malawi, almost 2000 km south, suggesting that this strain may be a common and widespread cause of invasive infections in sub-Saharan Africa [12, 31]. Additional subtyping of NTS isolates will be done later to help elucidate the role of specific subtypes in the observed trends.
Emerging multidrug resistance of NTS makes it challenging to recommend empiric treatment of children with suspected sepsis in whom a definitive microbiological diagnosis is unavailable. In our study, treatment alternatives for these resistant bacteria included ciprofloxacin and antibiotics from the carbapenem group, which are either not routinely recommended in children or unaffordable for most patients and health systems in sub-Saharan Africa. Adding these to hospital formularies for use as first-line antibiotic treatment of suspected bacteremia would be a costly undertaking.
Our analysis was conducted on a self-selected cohort of children enrolled into 2 vaccine trials, and therefore our incidence may not be representative of the population as a whole. This cohort of children had good access to high-quality healthcare, provided according to Kenyan national guidelines; although this allowed us to reliably capture invasive NTS incidence, the CFR in our study is likely lower than would occur under usual conditions, when barriers to high-quality care exist. We did not compare differences in iNTS disease incidence among children receiving RTS,S with those receiving the comparator vaccines; this analysis will be done across all sites at a later date. A known risk factor for iNTS disease, sickle cell disease, was not evaluated in this study.
CONCLUSIONS
We describe a high incidence of community-acquired invasive infections with multidrug-resistant NTS in a cohort of children living in rural western Kenya. The emerging multidrug resistance of this pathogen might have remained undiagnosed had a trial not been under way, which included the systematic evaluation of febrile children with high-quality microbiological laboratory methods. To measure the incidence of iNTS disease, and to capture the emergence of resistant pathogens early, microbiological services are needed in resource-limited settings. Furthermore, to optimally reduce morbidity and mortality from common bacterial infections, the resistance profile must be known at all hospitals, for the rational selection of empirical antibiotic treatment, based on local resistance data. Efforts to develop rapid diagnostic bedside tests for NTS and other pathogens should be accelerated to improve diagnosis and treatment of iNTS where microbiology services cannot be offered. Perhaps most important, efforts to prevent iNTS disease are needed. Improved understanding of the sources of NTS causing invasive infections could help tailor such strategies, but waiting for new information is not necessary for prudent action. Salmonellosis is an enteric infection, so measures to improve sanitation and food and water quality are likely to be effective, as they are for other enteric infections. Efforts to develop an effective vaccine should be intensified.
Notes
Acknowledgments. We thank the sponsors of the RTS,S malaria vaccine trials, GlaxoSmithKline and PATH Malaria Vaccine Initiative; the study participants and study staff at KEMRI/CDC; and the Director of the Center For Global Health Research, KEMRI.
Disclaimer. The findings and conclusions in this publication are those of the authors and do not necessarily reflect the official position of the US CDC. This supplement is published with approval of the KEMRI Director.
Financial support. This publication was made possible by a grant from the Bill & Melinda Gates Foundation (OPP1125993).
Supplement sponsorship. This article appears as part of the supplement “Invasive Salmonella Disease in Africa,” sponsored by the University of Otago.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- antibiotics
- primary prevention
- hiv
- ceftriaxone
- cephalosporins
- fever
- africa south of the sahara
- child
- child, hospitalized
- drug resistance, microbial
- drug resistance, multiple
- infant
- kenya
- malaria
- malaria vaccines
- outpatients
- pediatrics
- signs and symptoms
- infections
- mortality
- salmonella
- pathogenic organism
- hiv infections
- vaccine clinical trial
- blood culture
- case fatality rate
- hiv transmission
- community
- serotype
- serogroup




