-
PDF
- Split View
-
Views
-
Cite
Cite
Andrew Lau, Catriona S. Bradshaw, Dyani Lewis, Christopher K. Fairley, Marcus Y. Chen, Fabian Y. S. Kong, Jane S. Hocking, The Efficacy of Azithromycin for the Treatment of Genital Mycoplasma genitalium: A Systematic Review and Meta-analysis, Clinical Infectious Diseases, Volume 61, Issue 9, 1 November 2015, Pages 1389–1399, https://doi.org/10.1093/cid/civ644
Close - Share Icon Share
Abstract
Background. Mycoplasma genitalium (MG) is associated with nongonococcal urethritis in men and cervicitis in women. Current guidelines recommend treatment with 1 gram of azithromycin; however, treatment failure has increasingly been reported. This meta-analysis estimates treatment efficacy following treatment with 1 gram of azithromycin.
Methods. Electronic databases were searched for articles published to the end of February 2015 using the following search terms: (Mycoplasma genitalium) AND (azithromycin OR zithromax OR [treatment efficacy]). Studies were included if they were English language, had participants aged ≥12 years diagnosed with urogenital MG, and had microbial cure measured within 12 months of treatment. Treatment efficacy was measured as microbial cure at last follow-up after treatment.
Results. A total of 21 studies, including 1490 participants, fulfilled the inclusion criteria. Most studies were observational, with only 5 controlled trials identified. The random-effects pooled microbial cure was 77.2% (95% confidence interval [CI], 71.1%–83.4%; I2 = 80.8%, P < .01). For the 12 studies conducted prior to 2009, pooled microbial cure was 85.3% (CI, 82.3%–88.3%; I2 = 19.7%, P = .25); for the 9 studies conducted since the beginning of 2009, pooled microbial cure was 67.0% (CI, 57.0%–76.9%; I2 = 80.9%, P < .01).
Conclusions. The efficacy of a single dose of 1 gram of azithromycin for the treatment of urogenital MG has decreased to approach 60%. Even though most of the available evidence is based on observational studies that have considerable variability in sample size and timing of microbial cure, this low efficacy is of considerable concern. It is vital that new treatment options for MG are investigated.
(See the Editorial Commentary by Horner on pages 1400–2.)
Mycoplasma genitalium (MG) causes nongonococcal urethritis (NGU) in men [1] and is detected in 10%–30% of men presenting with nonchlamydial NGU and in up to 40% of those with chronic NGU [1]. Morbidity in women is less well established, but studies suggest an association with urethritis and cervicitis [2], endometritis [3], pelvic inflammatory disease (PID) [4], and possibly tubal factor infertility [5, 6].
Prevalence estimates for MG vary greatly worldwide, with fewer community-based data for men than for women. Community-based studies of women estimate an MG prevalence of 2.4% in Australia [7], between 2.3% and 3.3% in the United Kingdom [8, 9], 0.5% in Norway [10], between 1% and 2.3% in Denmark [11, 12], and 0.8% in the United States [13]. Among men, community-based estimates of MG prevalence of about 1.1% have been reported in Norway [10], the United States [13], and Denmark [12].
With the advantages of superior cell penetration and ease of single-dose administration, 1 gram (g) of azithromycin has been recommended as standard treatment for MG by the Centers for Disease Control and Prevention [14] and the Australasian Sexual Health Alliance [15]. However, there is growing evidence of azithromycin treatment failure, including macrolide resistance mutations. Some of the earliest azithromycin failure was reported by Bradshaw et al in 2006 where 28% of an Australian cohort of symptomatic men reported treatment failure attributable to resistance in vivo [16, 17]. Since then, macrolide resistance has been reported worldwide in France [18], New Zealand [19], Greenland [20], Japan [21], and elsewhere in Australia [17]. Mutations of region V of the 23S ribosomal RNA (rRNA) gene have been identified in association with elevated minimum inhibitory concentrations (MICs) for azithromycin and treatment failure [17].
In 2011, Manhart and colleagues published a review of the clinical treatment of MG [22], but to date no meta-analysis of the efficacy of azithromycin has been published. We present the results of a systematic review and meta-analysis of the efficacy of 1 g of azithromycin for the treatment of urogenital MG.
METHODS
Search Strategy
This systematic review and meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses [23]. We searched for published, peer-reviewed studies reporting on microbial cure following a 1 g single dose of azithromycin for the treatment of genital MG in men and women for the period up to the end of February 2015.
The search was performed on the electronic online databases Embase, Medline, PubMed, and Cochrane Central Register of Controlled Trials. Search terms included Mycoplasma genitalium AND (azithromycin OR zithromax OR [treatment efficacy]). Medical subject headings were used where possible.
Eligible studies were English language and included participants aged ≥12 years who were treated for urogenital MG with 1 g of azithromycin. MG was diagnosed using a nucleic acid amplification test (NAAT), and microbiological cure was measured within 12 months. Studies were excluded if participants were undergoing treatment for prostatitis or PID, or were opinion or review articles. Microbial cure was defined as negative MG result determined by NAAT during follow-up.
Data Extraction
Variables extracted included study design, number of participants, sex, loss to follow-up, duration of follow-up, microbial cure, and specimen type (swab, first-void urine, or both). Wherever possible, sex-specific microbial cure rates and 95% confidence intervals (CIs) were also extracted. Where 95% CIs for microbial cure were not reported, they were calculated using binomial methods. One review author (A. L.) extracted the data and a second author (J. S. H.) checked the extracted data. Disagreements were resolved by discussion between the 2 authors and consultation with a third author (C. S. B.) until consensus was reached.
Outcome
The primary outcome was azithromycin efficacy measured as MG microbial cure following treatment with a single 1 g dose of azithromycin. Microbial cure was calculated as follows: numerator = number of participants treated by 1 g of azithromycin who were microbiologically cured of MG (defined as a negative NAAT at follow-up); denominator = all those treated with 1 g of azithromycin for MG and tested at follow-up. For both the denominator and numerator, only those who were followed up were included. The outcome at last point of follow-up was reported.
Analysis
Meta-analysis was used to calculate a pooled estimate of microbial cure of 1 g of azithromycin for the treatment of MG. The I2 test was used to calculate the proportion of total variability in microbial cure estimates that could be attributed to underlying study heterogeneity rather than chance alone [24]. For the primary outcome, we pooled the data depending on the level of heterogeneity: for I2 < 25%, fixed-effects meta-analysis was used to estimate the pooled microbial cure (95% CI); for I2 > 25%, random-effects meta-analysis was used to estimate the pooled microbial cure (95% CI). Data were analyzed using Stata software version 13 (StataCorp, College Station, Texas).
Subgroup Analyses
Possible reasons for heterogeneity were explored by stratifying by sex, study design, study period, symptomatic status of participants, and time after treatment when microbial cure was measured. For the variable “study period,” studies were categorized as study conducted prior to 2009, or study conducted in 2009 or later. The year 2009 was chosen arbitrarily as efficacy appeared to attenuate after 2009 upon visual inspection of the data.
Assessment of Bias and Quality
We assessed publication bias using funnel plots. Asymmetry was statistically evaluated using the Egger correlation test by regressing treatment efficacy by its standard error. Assessment of within-study bias for observational studies was undertaken using the evaluation criteria adopted by Sanderson et al in their systematic review of tools used to assess bias in observational studies [25].
RESULTS
Study Selection
The review process is shown in Figure 1 and the selected studies summarized in Table 1. Of the 364 references identified, 151 were duplicates, yielding 213 unique references. An additional 3 references were identified through other sources [26–28]. One identified abstract was excluded, as the full article was subsequently published during the observation period [29].
| Study, First Author . | Design . | Study Period . | Population . | Azithromycin Treatment . | Symptomatic . | Duration of Follow-up . | Specimen Type . | Loss to Follow-up, No. (%) . | Microbial Cure, % (No. Cured/Tested) . |
|---|---|---|---|---|---|---|---|---|---|
| Gambini [35] | Observational | 1998–1999 | MG-positive patients attending STI clinic in Italy | M: 17 | Mixed | 1 wk | Urethral swab | 0/17 (0) | M: 82 (14/17) |
| Takahashi [37] | Observational | 2004 | Men from urology clinics | M: 3 | Mixed | <2 wk | FVU | 0/3 (0) | M: 100 (3/3) |
| Wikström [38] | Observational | 2002–2004 | Men attending an STI clinic in Sweden | M: 3 | Yes | 3 wk | FVU | 1/3 (33) | M: 100 (3/3) |
| Bradshaw [16] | Observational | 2004–2005 | MG-positive patients attending STI clinic in Australia | M: 34 | Yes | 17–59 d; median 31 d | FVU | 2/34 (6) | M: 72 (23/32) |
| Anagrius [36] | Observational | 1998–2005 | MG-positive patients attending STI clinic in Sweden | M: 72 W: 62 | Yes | 4–52 wk | FVU; urethral swab; cervical swab | 17/134 (13) | M: 88 (57/65) W: 96 (50/52) T: 91 (107/117) |
| Jernberg [39] | Observational | 2005–2006 | MG-positive patients attending STI clinic in Norway | M/W: 232 | Mixed | 7 d | FVU | 49/232 (21) | T: 79 (144/183) |
| Bradshaw [40] | Observational | 2005–2007 | MG-positive patients attending STI clinic in Australia | M: 161 W: 30 | Yes | 13–373 d; median 36 d | FVU; urethral swab; rectal swab | 71/191 (37) | T: 84 (101/120) |
| Hagiwara [41] | Observational | 2004–2007 | MG-positive patients attending 5 urology clinics in Japan | M: 30 | Yes | 2–4 wk | FVU | 0/30 (0) | T: 84 (101/120) |
| Walker [7] | Observational | 2007–2008 | Women attending primary care and sexual health/family planning clinics in Australia | W: 41 | Mixed | 4 wk | Self-collected vaginal swab | 9/41 (22) | W: 91 (29/32) |
| Terada [42] | Observational | 2008–2010 | MG-positive women in Japan | W: 42 | Yes | 14 d | Cervical swab | 0/42 (0) | W: 86 (36/42) |
| Couldwell [28] | Observational | 2008–2011 | MG-positive patients attending STI clinic in Australia | M/W: 34 episodes | Mixed | 12–273 d; median 46 d | FVU; cervical swab | 8/34 (24) | T: 46 (12/26) |
| Bissessor [29] | Observational | 2012–2013 | MG-positive patients attending STI clinic in Australia | M/W: 160 | Mixed | 4 wk | FVU; cervical swab | 5/160 (3) | T: 61 (95/155) |
| Henning [27] | Observational | 2012 | Primary healthcare patients attending a health clinic in Australia | M: 2 W: 3 | No | 4 wk | FVU | 3/5 (60) | T: 50 (1/2) |
| Kissinger [26] | Observational | Not specified | MG-positive patients from STI clinics in the United States | M: 205 | Yes | 4–10 wk | Not specified | 70/205 (34) | M: 65 (89/135) |
| Daley [43] | Observational | 2012–2013 | MG-positive incarcerated men in Australia | M: 8 | Mixed | 4 wk | FVU | 0/8 (0) | M: 100 (8/8) |
| Gundevia [44] | Observational | 2009–2013 | MG-positive patients attending a sexual health clinic in Australia | M: 111 W: 49 | Mixed | 4 wk | FVU; cervical swab | 73/160 (46) | M: 79 (44/56) W: 65 (20/31) |
| Björnelius [34] | Controlled trial | 2002–2004 | MG-positive patients attending 6 STI clinics in Norway and Sweden | M: 39 W: 17 | Yes | 20–56 d | FVU; cervical swab | 0/56 (0) | M: 85 (33/39) W: 88 (15/17) T: 86 (48/56) |
| Stamm [30] | RCT | 2003–2004 | MG-positive patients attending 11 STI or public health clinics in the United States | M: 11 | Yes | 2–5 wk | FVU | 4/11 (36) | M: 87 (20/23) |
| Mena [31] | RCT | 2002–2004 | MG-positive patients attending STI clinic in the United States | M: 36 | Yes | 9–21 d | FVU; urethral swab | 13/36 (36) | M: 86 (6/7) |
| Schwebke [32] | RCT | 2006–2009 | MG-positive patients attending 5 STI clinics in the United States | M: 50 | Yes | 2–6 wk | FVU | 5/50 (10) | M: 67 (30/45) |
| Manhart [33] | RCT | 2007–2011 | MG-positive patients attending STI clinic in the United States | M: 38 | Yes | 3–5 wk | FVU | 0/38 (0) | M: 39 (15/38) |
| Study, First Author . | Design . | Study Period . | Population . | Azithromycin Treatment . | Symptomatic . | Duration of Follow-up . | Specimen Type . | Loss to Follow-up, No. (%) . | Microbial Cure, % (No. Cured/Tested) . |
|---|---|---|---|---|---|---|---|---|---|
| Gambini [35] | Observational | 1998–1999 | MG-positive patients attending STI clinic in Italy | M: 17 | Mixed | 1 wk | Urethral swab | 0/17 (0) | M: 82 (14/17) |
| Takahashi [37] | Observational | 2004 | Men from urology clinics | M: 3 | Mixed | <2 wk | FVU | 0/3 (0) | M: 100 (3/3) |
| Wikström [38] | Observational | 2002–2004 | Men attending an STI clinic in Sweden | M: 3 | Yes | 3 wk | FVU | 1/3 (33) | M: 100 (3/3) |
| Bradshaw [16] | Observational | 2004–2005 | MG-positive patients attending STI clinic in Australia | M: 34 | Yes | 17–59 d; median 31 d | FVU | 2/34 (6) | M: 72 (23/32) |
| Anagrius [36] | Observational | 1998–2005 | MG-positive patients attending STI clinic in Sweden | M: 72 W: 62 | Yes | 4–52 wk | FVU; urethral swab; cervical swab | 17/134 (13) | M: 88 (57/65) W: 96 (50/52) T: 91 (107/117) |
| Jernberg [39] | Observational | 2005–2006 | MG-positive patients attending STI clinic in Norway | M/W: 232 | Mixed | 7 d | FVU | 49/232 (21) | T: 79 (144/183) |
| Bradshaw [40] | Observational | 2005–2007 | MG-positive patients attending STI clinic in Australia | M: 161 W: 30 | Yes | 13–373 d; median 36 d | FVU; urethral swab; rectal swab | 71/191 (37) | T: 84 (101/120) |
| Hagiwara [41] | Observational | 2004–2007 | MG-positive patients attending 5 urology clinics in Japan | M: 30 | Yes | 2–4 wk | FVU | 0/30 (0) | T: 84 (101/120) |
| Walker [7] | Observational | 2007–2008 | Women attending primary care and sexual health/family planning clinics in Australia | W: 41 | Mixed | 4 wk | Self-collected vaginal swab | 9/41 (22) | W: 91 (29/32) |
| Terada [42] | Observational | 2008–2010 | MG-positive women in Japan | W: 42 | Yes | 14 d | Cervical swab | 0/42 (0) | W: 86 (36/42) |
| Couldwell [28] | Observational | 2008–2011 | MG-positive patients attending STI clinic in Australia | M/W: 34 episodes | Mixed | 12–273 d; median 46 d | FVU; cervical swab | 8/34 (24) | T: 46 (12/26) |
| Bissessor [29] | Observational | 2012–2013 | MG-positive patients attending STI clinic in Australia | M/W: 160 | Mixed | 4 wk | FVU; cervical swab | 5/160 (3) | T: 61 (95/155) |
| Henning [27] | Observational | 2012 | Primary healthcare patients attending a health clinic in Australia | M: 2 W: 3 | No | 4 wk | FVU | 3/5 (60) | T: 50 (1/2) |
| Kissinger [26] | Observational | Not specified | MG-positive patients from STI clinics in the United States | M: 205 | Yes | 4–10 wk | Not specified | 70/205 (34) | M: 65 (89/135) |
| Daley [43] | Observational | 2012–2013 | MG-positive incarcerated men in Australia | M: 8 | Mixed | 4 wk | FVU | 0/8 (0) | M: 100 (8/8) |
| Gundevia [44] | Observational | 2009–2013 | MG-positive patients attending a sexual health clinic in Australia | M: 111 W: 49 | Mixed | 4 wk | FVU; cervical swab | 73/160 (46) | M: 79 (44/56) W: 65 (20/31) |
| Björnelius [34] | Controlled trial | 2002–2004 | MG-positive patients attending 6 STI clinics in Norway and Sweden | M: 39 W: 17 | Yes | 20–56 d | FVU; cervical swab | 0/56 (0) | M: 85 (33/39) W: 88 (15/17) T: 86 (48/56) |
| Stamm [30] | RCT | 2003–2004 | MG-positive patients attending 11 STI or public health clinics in the United States | M: 11 | Yes | 2–5 wk | FVU | 4/11 (36) | M: 87 (20/23) |
| Mena [31] | RCT | 2002–2004 | MG-positive patients attending STI clinic in the United States | M: 36 | Yes | 9–21 d | FVU; urethral swab | 13/36 (36) | M: 86 (6/7) |
| Schwebke [32] | RCT | 2006–2009 | MG-positive patients attending 5 STI clinics in the United States | M: 50 | Yes | 2–6 wk | FVU | 5/50 (10) | M: 67 (30/45) |
| Manhart [33] | RCT | 2007–2011 | MG-positive patients attending STI clinic in the United States | M: 38 | Yes | 3–5 wk | FVU | 0/38 (0) | M: 39 (15/38) |
Abbreviations: FVU, first-void urine; M, men; MG, Mycoplasma genitalium; mixed, both symptomatic and asymptomatic participants; RCT, randomized controlled trial; STI, sexually transmitted infection; T, total (men and women); W, women.
| Study, First Author . | Design . | Study Period . | Population . | Azithromycin Treatment . | Symptomatic . | Duration of Follow-up . | Specimen Type . | Loss to Follow-up, No. (%) . | Microbial Cure, % (No. Cured/Tested) . |
|---|---|---|---|---|---|---|---|---|---|
| Gambini [35] | Observational | 1998–1999 | MG-positive patients attending STI clinic in Italy | M: 17 | Mixed | 1 wk | Urethral swab | 0/17 (0) | M: 82 (14/17) |
| Takahashi [37] | Observational | 2004 | Men from urology clinics | M: 3 | Mixed | <2 wk | FVU | 0/3 (0) | M: 100 (3/3) |
| Wikström [38] | Observational | 2002–2004 | Men attending an STI clinic in Sweden | M: 3 | Yes | 3 wk | FVU | 1/3 (33) | M: 100 (3/3) |
| Bradshaw [16] | Observational | 2004–2005 | MG-positive patients attending STI clinic in Australia | M: 34 | Yes | 17–59 d; median 31 d | FVU | 2/34 (6) | M: 72 (23/32) |
| Anagrius [36] | Observational | 1998–2005 | MG-positive patients attending STI clinic in Sweden | M: 72 W: 62 | Yes | 4–52 wk | FVU; urethral swab; cervical swab | 17/134 (13) | M: 88 (57/65) W: 96 (50/52) T: 91 (107/117) |
| Jernberg [39] | Observational | 2005–2006 | MG-positive patients attending STI clinic in Norway | M/W: 232 | Mixed | 7 d | FVU | 49/232 (21) | T: 79 (144/183) |
| Bradshaw [40] | Observational | 2005–2007 | MG-positive patients attending STI clinic in Australia | M: 161 W: 30 | Yes | 13–373 d; median 36 d | FVU; urethral swab; rectal swab | 71/191 (37) | T: 84 (101/120) |
| Hagiwara [41] | Observational | 2004–2007 | MG-positive patients attending 5 urology clinics in Japan | M: 30 | Yes | 2–4 wk | FVU | 0/30 (0) | T: 84 (101/120) |
| Walker [7] | Observational | 2007–2008 | Women attending primary care and sexual health/family planning clinics in Australia | W: 41 | Mixed | 4 wk | Self-collected vaginal swab | 9/41 (22) | W: 91 (29/32) |
| Terada [42] | Observational | 2008–2010 | MG-positive women in Japan | W: 42 | Yes | 14 d | Cervical swab | 0/42 (0) | W: 86 (36/42) |
| Couldwell [28] | Observational | 2008–2011 | MG-positive patients attending STI clinic in Australia | M/W: 34 episodes | Mixed | 12–273 d; median 46 d | FVU; cervical swab | 8/34 (24) | T: 46 (12/26) |
| Bissessor [29] | Observational | 2012–2013 | MG-positive patients attending STI clinic in Australia | M/W: 160 | Mixed | 4 wk | FVU; cervical swab | 5/160 (3) | T: 61 (95/155) |
| Henning [27] | Observational | 2012 | Primary healthcare patients attending a health clinic in Australia | M: 2 W: 3 | No | 4 wk | FVU | 3/5 (60) | T: 50 (1/2) |
| Kissinger [26] | Observational | Not specified | MG-positive patients from STI clinics in the United States | M: 205 | Yes | 4–10 wk | Not specified | 70/205 (34) | M: 65 (89/135) |
| Daley [43] | Observational | 2012–2013 | MG-positive incarcerated men in Australia | M: 8 | Mixed | 4 wk | FVU | 0/8 (0) | M: 100 (8/8) |
| Gundevia [44] | Observational | 2009–2013 | MG-positive patients attending a sexual health clinic in Australia | M: 111 W: 49 | Mixed | 4 wk | FVU; cervical swab | 73/160 (46) | M: 79 (44/56) W: 65 (20/31) |
| Björnelius [34] | Controlled trial | 2002–2004 | MG-positive patients attending 6 STI clinics in Norway and Sweden | M: 39 W: 17 | Yes | 20–56 d | FVU; cervical swab | 0/56 (0) | M: 85 (33/39) W: 88 (15/17) T: 86 (48/56) |
| Stamm [30] | RCT | 2003–2004 | MG-positive patients attending 11 STI or public health clinics in the United States | M: 11 | Yes | 2–5 wk | FVU | 4/11 (36) | M: 87 (20/23) |
| Mena [31] | RCT | 2002–2004 | MG-positive patients attending STI clinic in the United States | M: 36 | Yes | 9–21 d | FVU; urethral swab | 13/36 (36) | M: 86 (6/7) |
| Schwebke [32] | RCT | 2006–2009 | MG-positive patients attending 5 STI clinics in the United States | M: 50 | Yes | 2–6 wk | FVU | 5/50 (10) | M: 67 (30/45) |
| Manhart [33] | RCT | 2007–2011 | MG-positive patients attending STI clinic in the United States | M: 38 | Yes | 3–5 wk | FVU | 0/38 (0) | M: 39 (15/38) |
| Study, First Author . | Design . | Study Period . | Population . | Azithromycin Treatment . | Symptomatic . | Duration of Follow-up . | Specimen Type . | Loss to Follow-up, No. (%) . | Microbial Cure, % (No. Cured/Tested) . |
|---|---|---|---|---|---|---|---|---|---|
| Gambini [35] | Observational | 1998–1999 | MG-positive patients attending STI clinic in Italy | M: 17 | Mixed | 1 wk | Urethral swab | 0/17 (0) | M: 82 (14/17) |
| Takahashi [37] | Observational | 2004 | Men from urology clinics | M: 3 | Mixed | <2 wk | FVU | 0/3 (0) | M: 100 (3/3) |
| Wikström [38] | Observational | 2002–2004 | Men attending an STI clinic in Sweden | M: 3 | Yes | 3 wk | FVU | 1/3 (33) | M: 100 (3/3) |
| Bradshaw [16] | Observational | 2004–2005 | MG-positive patients attending STI clinic in Australia | M: 34 | Yes | 17–59 d; median 31 d | FVU | 2/34 (6) | M: 72 (23/32) |
| Anagrius [36] | Observational | 1998–2005 | MG-positive patients attending STI clinic in Sweden | M: 72 W: 62 | Yes | 4–52 wk | FVU; urethral swab; cervical swab | 17/134 (13) | M: 88 (57/65) W: 96 (50/52) T: 91 (107/117) |
| Jernberg [39] | Observational | 2005–2006 | MG-positive patients attending STI clinic in Norway | M/W: 232 | Mixed | 7 d | FVU | 49/232 (21) | T: 79 (144/183) |
| Bradshaw [40] | Observational | 2005–2007 | MG-positive patients attending STI clinic in Australia | M: 161 W: 30 | Yes | 13–373 d; median 36 d | FVU; urethral swab; rectal swab | 71/191 (37) | T: 84 (101/120) |
| Hagiwara [41] | Observational | 2004–2007 | MG-positive patients attending 5 urology clinics in Japan | M: 30 | Yes | 2–4 wk | FVU | 0/30 (0) | T: 84 (101/120) |
| Walker [7] | Observational | 2007–2008 | Women attending primary care and sexual health/family planning clinics in Australia | W: 41 | Mixed | 4 wk | Self-collected vaginal swab | 9/41 (22) | W: 91 (29/32) |
| Terada [42] | Observational | 2008–2010 | MG-positive women in Japan | W: 42 | Yes | 14 d | Cervical swab | 0/42 (0) | W: 86 (36/42) |
| Couldwell [28] | Observational | 2008–2011 | MG-positive patients attending STI clinic in Australia | M/W: 34 episodes | Mixed | 12–273 d; median 46 d | FVU; cervical swab | 8/34 (24) | T: 46 (12/26) |
| Bissessor [29] | Observational | 2012–2013 | MG-positive patients attending STI clinic in Australia | M/W: 160 | Mixed | 4 wk | FVU; cervical swab | 5/160 (3) | T: 61 (95/155) |
| Henning [27] | Observational | 2012 | Primary healthcare patients attending a health clinic in Australia | M: 2 W: 3 | No | 4 wk | FVU | 3/5 (60) | T: 50 (1/2) |
| Kissinger [26] | Observational | Not specified | MG-positive patients from STI clinics in the United States | M: 205 | Yes | 4–10 wk | Not specified | 70/205 (34) | M: 65 (89/135) |
| Daley [43] | Observational | 2012–2013 | MG-positive incarcerated men in Australia | M: 8 | Mixed | 4 wk | FVU | 0/8 (0) | M: 100 (8/8) |
| Gundevia [44] | Observational | 2009–2013 | MG-positive patients attending a sexual health clinic in Australia | M: 111 W: 49 | Mixed | 4 wk | FVU; cervical swab | 73/160 (46) | M: 79 (44/56) W: 65 (20/31) |
| Björnelius [34] | Controlled trial | 2002–2004 | MG-positive patients attending 6 STI clinics in Norway and Sweden | M: 39 W: 17 | Yes | 20–56 d | FVU; cervical swab | 0/56 (0) | M: 85 (33/39) W: 88 (15/17) T: 86 (48/56) |
| Stamm [30] | RCT | 2003–2004 | MG-positive patients attending 11 STI or public health clinics in the United States | M: 11 | Yes | 2–5 wk | FVU | 4/11 (36) | M: 87 (20/23) |
| Mena [31] | RCT | 2002–2004 | MG-positive patients attending STI clinic in the United States | M: 36 | Yes | 9–21 d | FVU; urethral swab | 13/36 (36) | M: 86 (6/7) |
| Schwebke [32] | RCT | 2006–2009 | MG-positive patients attending 5 STI clinics in the United States | M: 50 | Yes | 2–6 wk | FVU | 5/50 (10) | M: 67 (30/45) |
| Manhart [33] | RCT | 2007–2011 | MG-positive patients attending STI clinic in the United States | M: 38 | Yes | 3–5 wk | FVU | 0/38 (0) | M: 39 (15/38) |
Abbreviations: FVU, first-void urine; M, men; MG, Mycoplasma genitalium; mixed, both symptomatic and asymptomatic participants; RCT, randomized controlled trial; STI, sexually transmitted infection; T, total (men and women); W, women.
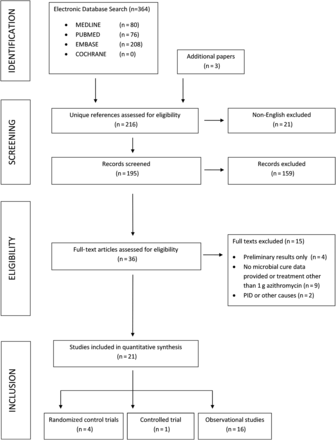
Flowchart of inclusions and exclusions from the systematic review. Abbreviation: PID, pelvic inflammatory disease.
Study Characteristics
A total of 21 studies were eligible and included in the meta-analysis: 4 randomized trials [30–33], 1 controlled trial [34], and 16 observational studies [7, 16, 26–29, 35–44] (Table 1). Duration of follow-up ranged from 1 to 52 weeks. Loss to follow-up ranged from 0% to 60%; no loss to follow-up data were available for 7 studies [33–35, 37, 41–43]. Microbial cure estimates for women were provided in 5 studies [7, 34, 36, 42, 44], microbial cure estimates for men were provided in 14 studies [16, 26, 30–38, 41, 43, 44], and 5 studies did not present sex-specific microbial cure estimates [27–29, 39, 40]. In total, 1490 participants were included—820 men, 244 women, and 426 for whom participant sex was not reported separately [28, 29, 39]. Sample sizes in each study ranged from 2 to 232.
| Analysis . | No. . | Microbial Cure, % (95% CI) . | Test for Heterogeneity, I2%, P Value . | Effects Model (Random/Fixed) . |
|---|---|---|---|---|
| Overall | 1152 | 77.2 (71.1–83.4) | 80.8, P < .01 | Random |
| Women | 174 | 86.8 (77.5–96.1) | 67.7, P = .02 | Random |
| Men | 500 | 78.9 (71.2–86.6) | 70.5, P < .01 | Random |
| Controlled trials | 169 | 72.2 (53.5–90.8) | 84.4, P < .01 | Random |
| Observational | 983 | 78.8 (72.2–85.4) | 80.3, P < .01 | Random |
| Conducted <2009 | 622 | 85.3 (82.3–88.3) | 19.7, P = .25 | Fixed |
| Conducted ≥2009 | 530 | 67.0 (57.0–76.9) | 80.9, P < .01 | Random |
| Symptomatic | 527 | 77.2 (67.4–86.7) | 82.9, P < .01 | Random |
| Asymptomatic | 2 | 50.0 (7.0–93.1) | NAa | NAa |
| Symptomatic + asymptomatic | 623 | 78.3 (69.6–86.9) | 81.1, P < .01 | Random |
| Microbial cure at ≤4 wk | 576 | 81.4 (74.0–88.8) | 68.7, P < .01 | Random |
| Microbial cure at >4–8 wk | 178 | 69.4 (52.2–86.6) | 83.4, P < .01 | Random |
| Microbial cure at >8 wk | 398 | 74.2 (59.4–89.0) | 92.1, P < .01 | Random |
| Analysis . | No. . | Microbial Cure, % (95% CI) . | Test for Heterogeneity, I2%, P Value . | Effects Model (Random/Fixed) . |
|---|---|---|---|---|
| Overall | 1152 | 77.2 (71.1–83.4) | 80.8, P < .01 | Random |
| Women | 174 | 86.8 (77.5–96.1) | 67.7, P = .02 | Random |
| Men | 500 | 78.9 (71.2–86.6) | 70.5, P < .01 | Random |
| Controlled trials | 169 | 72.2 (53.5–90.8) | 84.4, P < .01 | Random |
| Observational | 983 | 78.8 (72.2–85.4) | 80.3, P < .01 | Random |
| Conducted <2009 | 622 | 85.3 (82.3–88.3) | 19.7, P = .25 | Fixed |
| Conducted ≥2009 | 530 | 67.0 (57.0–76.9) | 80.9, P < .01 | Random |
| Symptomatic | 527 | 77.2 (67.4–86.7) | 82.9, P < .01 | Random |
| Asymptomatic | 2 | 50.0 (7.0–93.1) | NAa | NAa |
| Symptomatic + asymptomatic | 623 | 78.3 (69.6–86.9) | 81.1, P < .01 | Random |
| Microbial cure at ≤4 wk | 576 | 81.4 (74.0–88.8) | 68.7, P < .01 | Random |
| Microbial cure at >4–8 wk | 178 | 69.4 (52.2–86.6) | 83.4, P < .01 | Random |
| Microbial cure at >8 wk | 398 | 74.2 (59.4–89.0) | 92.1, P < .01 | Random |
Abbreviations: CI, confidence interval; NA, not applicable.
a Only 1 study.
| Analysis . | No. . | Microbial Cure, % (95% CI) . | Test for Heterogeneity, I2%, P Value . | Effects Model (Random/Fixed) . |
|---|---|---|---|---|
| Overall | 1152 | 77.2 (71.1–83.4) | 80.8, P < .01 | Random |
| Women | 174 | 86.8 (77.5–96.1) | 67.7, P = .02 | Random |
| Men | 500 | 78.9 (71.2–86.6) | 70.5, P < .01 | Random |
| Controlled trials | 169 | 72.2 (53.5–90.8) | 84.4, P < .01 | Random |
| Observational | 983 | 78.8 (72.2–85.4) | 80.3, P < .01 | Random |
| Conducted <2009 | 622 | 85.3 (82.3–88.3) | 19.7, P = .25 | Fixed |
| Conducted ≥2009 | 530 | 67.0 (57.0–76.9) | 80.9, P < .01 | Random |
| Symptomatic | 527 | 77.2 (67.4–86.7) | 82.9, P < .01 | Random |
| Asymptomatic | 2 | 50.0 (7.0–93.1) | NAa | NAa |
| Symptomatic + asymptomatic | 623 | 78.3 (69.6–86.9) | 81.1, P < .01 | Random |
| Microbial cure at ≤4 wk | 576 | 81.4 (74.0–88.8) | 68.7, P < .01 | Random |
| Microbial cure at >4–8 wk | 178 | 69.4 (52.2–86.6) | 83.4, P < .01 | Random |
| Microbial cure at >8 wk | 398 | 74.2 (59.4–89.0) | 92.1, P < .01 | Random |
| Analysis . | No. . | Microbial Cure, % (95% CI) . | Test for Heterogeneity, I2%, P Value . | Effects Model (Random/Fixed) . |
|---|---|---|---|---|
| Overall | 1152 | 77.2 (71.1–83.4) | 80.8, P < .01 | Random |
| Women | 174 | 86.8 (77.5–96.1) | 67.7, P = .02 | Random |
| Men | 500 | 78.9 (71.2–86.6) | 70.5, P < .01 | Random |
| Controlled trials | 169 | 72.2 (53.5–90.8) | 84.4, P < .01 | Random |
| Observational | 983 | 78.8 (72.2–85.4) | 80.3, P < .01 | Random |
| Conducted <2009 | 622 | 85.3 (82.3–88.3) | 19.7, P = .25 | Fixed |
| Conducted ≥2009 | 530 | 67.0 (57.0–76.9) | 80.9, P < .01 | Random |
| Symptomatic | 527 | 77.2 (67.4–86.7) | 82.9, P < .01 | Random |
| Asymptomatic | 2 | 50.0 (7.0–93.1) | NAa | NAa |
| Symptomatic + asymptomatic | 623 | 78.3 (69.6–86.9) | 81.1, P < .01 | Random |
| Microbial cure at ≤4 wk | 576 | 81.4 (74.0–88.8) | 68.7, P < .01 | Random |
| Microbial cure at >4–8 wk | 178 | 69.4 (52.2–86.6) | 83.4, P < .01 | Random |
| Microbial cure at >8 wk | 398 | 74.2 (59.4–89.0) | 92.1, P < .01 | Random |
Abbreviations: CI, confidence interval; NA, not applicable.
a Only 1 study.
| Study . | Type of Bias [25] . | |||
|---|---|---|---|---|
| Selection . | Measurement . | Loss to Follow-up . | Sample Size . | |
| Gambini [35] | ++ | +++ | + | +++ |
| Takahashi [37] | ++ | ++/+++ | + | +++ |
| Wikström [38] | ++ | + | +++ | +++ |
| Bradshaw [16] | ++ | + | + | ++ |
| Anagrius [36] | ++ | +/++ | + | + |
| Jernberg [39] | + | + | ++ | + |
| Bradshaw [40] | ++ | + | +++ | + |
| Hagiwara [41] | ++ | +/++ | + | ++ |
| Walker [7] | + | + | ++ | +/++ |
| Terada [42] | ++ | ++ | + | +/++ |
| Couldwell [28] | +++ | +/++ | ++/+++ | ++ |
| Bissessor [29] | +/++ | + | + | + |
| Henning [27] | +/++ | + | +++ | +++ |
| Kissinger [26] | ++ | + | +++ | + |
| Daley [43] | ++ | + | + | +++ |
| Gundevia [44] | ++ | + | ++/+++ | + |
| Björnelius [34] | ++ | + | + | + |
| Stamm [30] | +/++ | + | ++/+++ | +++ |
| Mena [31] | +/++ | +/++ | ++ | ++ |
| Schwebke [32] | +/++ | + | + | +/++ |
| Manhart [33] | +/++ | + | + | +/++ |
| Study . | Type of Bias [25] . | |||
|---|---|---|---|---|
| Selection . | Measurement . | Loss to Follow-up . | Sample Size . | |
| Gambini [35] | ++ | +++ | + | +++ |
| Takahashi [37] | ++ | ++/+++ | + | +++ |
| Wikström [38] | ++ | + | +++ | +++ |
| Bradshaw [16] | ++ | + | + | ++ |
| Anagrius [36] | ++ | +/++ | + | + |
| Jernberg [39] | + | + | ++ | + |
| Bradshaw [40] | ++ | + | +++ | + |
| Hagiwara [41] | ++ | +/++ | + | ++ |
| Walker [7] | + | + | ++ | +/++ |
| Terada [42] | ++ | ++ | + | +/++ |
| Couldwell [28] | +++ | +/++ | ++/+++ | ++ |
| Bissessor [29] | +/++ | + | + | + |
| Henning [27] | +/++ | + | +++ | +++ |
| Kissinger [26] | ++ | + | +++ | + |
| Daley [43] | ++ | + | + | +++ |
| Gundevia [44] | ++ | + | ++/+++ | + |
| Björnelius [34] | ++ | + | + | + |
| Stamm [30] | +/++ | + | ++/+++ | +++ |
| Mena [31] | +/++ | +/++ | ++ | ++ |
| Schwebke [32] | +/++ | + | + | +/++ |
| Manhart [33] | +/++ | + | + | +/++ |
Abbreviations: +, low risk of bias; ++, medium risk of bias; +++, high risk of bias.
| Study . | Type of Bias [25] . | |||
|---|---|---|---|---|
| Selection . | Measurement . | Loss to Follow-up . | Sample Size . | |
| Gambini [35] | ++ | +++ | + | +++ |
| Takahashi [37] | ++ | ++/+++ | + | +++ |
| Wikström [38] | ++ | + | +++ | +++ |
| Bradshaw [16] | ++ | + | + | ++ |
| Anagrius [36] | ++ | +/++ | + | + |
| Jernberg [39] | + | + | ++ | + |
| Bradshaw [40] | ++ | + | +++ | + |
| Hagiwara [41] | ++ | +/++ | + | ++ |
| Walker [7] | + | + | ++ | +/++ |
| Terada [42] | ++ | ++ | + | +/++ |
| Couldwell [28] | +++ | +/++ | ++/+++ | ++ |
| Bissessor [29] | +/++ | + | + | + |
| Henning [27] | +/++ | + | +++ | +++ |
| Kissinger [26] | ++ | + | +++ | + |
| Daley [43] | ++ | + | + | +++ |
| Gundevia [44] | ++ | + | ++/+++ | + |
| Björnelius [34] | ++ | + | + | + |
| Stamm [30] | +/++ | + | ++/+++ | +++ |
| Mena [31] | +/++ | +/++ | ++ | ++ |
| Schwebke [32] | +/++ | + | + | +/++ |
| Manhart [33] | +/++ | + | + | +/++ |
| Study . | Type of Bias [25] . | |||
|---|---|---|---|---|
| Selection . | Measurement . | Loss to Follow-up . | Sample Size . | |
| Gambini [35] | ++ | +++ | + | +++ |
| Takahashi [37] | ++ | ++/+++ | + | +++ |
| Wikström [38] | ++ | + | +++ | +++ |
| Bradshaw [16] | ++ | + | + | ++ |
| Anagrius [36] | ++ | +/++ | + | + |
| Jernberg [39] | + | + | ++ | + |
| Bradshaw [40] | ++ | + | +++ | + |
| Hagiwara [41] | ++ | +/++ | + | ++ |
| Walker [7] | + | + | ++ | +/++ |
| Terada [42] | ++ | ++ | + | +/++ |
| Couldwell [28] | +++ | +/++ | ++/+++ | ++ |
| Bissessor [29] | +/++ | + | + | + |
| Henning [27] | +/++ | + | +++ | +++ |
| Kissinger [26] | ++ | + | +++ | + |
| Daley [43] | ++ | + | + | +++ |
| Gundevia [44] | ++ | + | ++/+++ | + |
| Björnelius [34] | ++ | + | + | + |
| Stamm [30] | +/++ | + | ++/+++ | +++ |
| Mena [31] | +/++ | +/++ | ++ | ++ |
| Schwebke [32] | +/++ | + | + | +/++ |
| Manhart [33] | +/++ | + | + | +/++ |
Abbreviations: +, low risk of bias; ++, medium risk of bias; +++, high risk of bias.
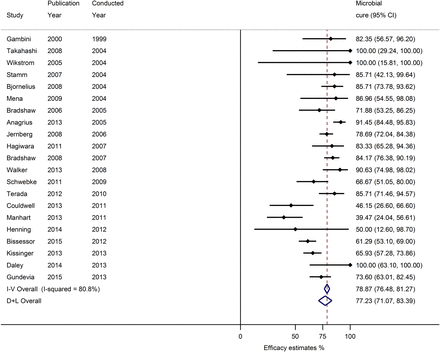
Microbial cure following treatment with 1 g of azithromycin for urogenital Mycoplasma genitalium infection. Abbreviations: CI, confidence interval; D+L, DerSimonian and Laird method; I-V, inverse-variance method.
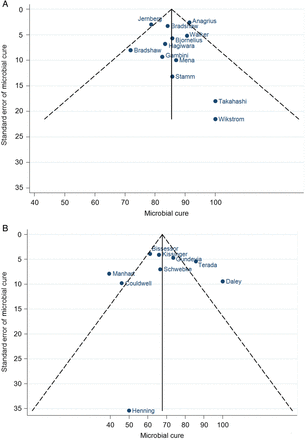
Funnel plots of microbial cure following treatment of urogenital Mycoplasma genitalium infection by 1 g of azithromycin. A, Studies conducted prior to 2009. B, Studies conducted since 2009.
Azithromycin Efficacy
Overall random-effects pooled microbial cure was 77.2% (95% CI, 71.1%–83.4%) with high heterogeneity (I2 = 80.8%, P < .01) (Figure 2).
Subgroup Analyses
When stratified by sex, the pooled microbial cure for men was 78.9% (95% CI, 71.2%–86.6%) with moderately high heterogeneity (I2 = 70.5%, P < .01). The pooled microbial cure for women was 86.8% (95% CI, 77.5%–96.1%), with moderately high heterogeneity (I2 = 67.6%, P = .02) (Table 2).
Pooled microbial cure was similar between observational studies (78.8% [95% CI, 72.2%–85.4%]; I2 = 80.3%, P < .01) and controlled trials (72.2% [95% CI, 53.5%–90.8%]; I2 = 84.4%, P < .01). However, pooled microbial cure was considerably higher for the 12 studies conducted before 2009 (85.3% [95% CI, 82.3%–88.3%]; I2 = 19.7%, P = .25) compared with the 9 studies conducted since the beginning of 2009 (67.0% [95% CI, 57.0%–76.9%]; I2 = 80.9%, P < .01). There was no difference in pooled efficacy between those studies that included only symptomatic participants and those that included both symptomatic and asymptomatic participants. Pooled efficacy was higher for studies that measured microbial cure within 4 weeks after treatment (81.4% [95% CI, 74.0%–88.8%]; I2 = 68.7%, P < .01) compared with studies that measured microbial cure between 4 and 8 weeks after treatment (69.4% [95% CI, 52.2%–86.6%]; I2 = 83.4%, P < .01) and studies that measured it up to 52 weeks after treatment (74.2% [95% CI, 59.4%–89.0%]; I2 = 92.1%, P < .01).
Between-Study Bias
Separate funnel plots were generated for studies conducted prior to and after 2009 because of the differences in microbial cure observed in the subgroup analyses above (Figure 3). Funnel plot analysis showed an absence of published small studies conducted prior to 2009 reporting a low microbial cure, but the Egger test found no evidence of publication bias with a coefficient of −0.02 (95% CI, −1.52 to 1.50; P = .98). Since 2009, funnel plot analysis showed there have been no published small studies finding a larger microbial cure, but no evidence of publication bias was found (−0.35 [95% CI, −5.22 to 4.52]; P = .87).
Within-Study Bias
Nineteen of the 21 studies included participants from high-risk clinical settings or at correctional facilities, whereas only 2 studies enrolled participants from a lower-risk general practice/primary care–based setting [7, 27], raising concern about a bias toward symptomatic cases and reducing the generalizability of the study results (Table 3). However, study population and clinical procedures were clearly defined within studies, and the risk of intrastudy selection bias was considered low. There was some potential for measurement bias, with a few studies assessing microbial cure either too early or too late after treatment, increasing the risk of either false-positive diagnoses or reinfection, respectively. The risk of loss to follow-up bias was considered to be high, with 10 studies reporting loss to follow-up between 21% and 60% [7, 26–28, 30, 31, 38–40, 44]. Only 3 studies reported sample size calculations for MG treatment efficacy [29, 32, 33] (Supplementary Data).
DISCUSSION
To our knowledge, this is the first meta-analysis of the efficacy of 1 g of azithromycin for the treatment of MG and estimates a pooled efficacy of 77.2%. However, this must be interpreted with caution because of considerable heterogeneity in the estimate (I2 > 80%). Our subgroup analysis identified that year of study was an important source of heterogeneity—with only mild heterogeneity between studies conducted prior to 2009 (I2, approximately 20%) and marked heterogeneity in studies conducted since the beginning of 2009 (I2, approximately 80%). This subgroup analysis also found that overall efficacy has decreased from 85.3% in studies conducted prior to 2009 to 67.0% in studies conducted since then, suggesting that azithromycin has become less effective for MG over time. This has implications for clinical management and treatment guidelines.
The reduced efficacy observed since 2009 is likely attributable to an increasing proportion of MG infections possessing single-nucleotide polymorphism (SNP) macrolide resistance–mediating mutations [17, 36]. These SNPs in region V of the 23S rRNA gene of MG were first reported in 2008 and found to be strongly associated with increased MICs to azithromycin in clinical isolates and treatment failure [17]. The proportion of cases of MG with these mutations has increased in recent years; data from Sweden showed the proportion of infections with a mutation increasing from 0% in 2006 to 6% in 2009, 14% in 2010, and 21% in 2011 [36], data from Japan showed an increase from 0% in 2011 and 2012 to 29% in 2013 [45], and data from Australia showed an increase from about 20% between 2007 and 2009 to 36% between 2012 and 2013 [46, 47].
There is some evidence that these SNPs may be being induced in vivo during treatment with 1 g of azithromycin [21, 36, 40, 17], although further research, including deep genomic sequencing, is ongoing to determine whether resistance is truly being induced during treatment, or whether minority resistance species are emerging under antibiotic pressure. The former hypothesis has led some investigators to use an extended dose of azithromycin, in the belief that it may be less likely to induce resistance than a single 1 g dose. A retrospective case series comparing a single 1 g dose of azithromycin with an extended 1.5-g dose of azithromycin over 5 days (500 mg on day 1 followed by 250 mg once daily for 4 days) found that macrolide resistance developed in 100% (7/7) of cases treated with 1 g of azithromycin compared with none (0/25) among those treated with an extended azithromycin dose [37]. This study also found however, that extended azithromycin was of little benefit when the SNP associated with macrolide resistance was present before treatment. In a controlled trial, Björnelius et al investigated efficacy of extended azithromycin in those who had failed treatment with 100 mg of doxycycline twice daily for 15 days and found an efficacy of 95%, higher than the efficacy of 85% for men treated initially with 1 g of azithromycin, although statistical significance was not reached [34]. Given that there are already high levels of macrolide resistance circulating in many clinical settings, widespread adoption of an extended dose of azithromycin for MG infections is therefore unlikely to achieve significant increases in cure.
An association between MG organism load and azithromycin treatment failure has been previously reported in a small number of women [7]. This was further explored in a larger cohort of men infected with MG that found that the odds of azithromycin failure increased by 22% for every log10 increase in load, although this was not significant [29]. However, this study did find that cases that acquired a mutation after treatment appeared to have a higher pretreatment organism load than fully susceptible strains, although this analysis was limited by the small sample size. These data suggest that organism load may influence the efficacy of azithromycin. High-load infections that appear fully susceptible could harbor low copy numbers of resistant mutants that may emerge under selective pressure [17, 48].
Efficacy of 1 g of azithromycin was higher for women than men (86.8% vs 78.9%), but there are fewer data assessing treatment efficacy in women. The difference in efficacy between the sexes could not be explained by the year of the study. Björnelius and colleagues observed a similar difference in efficacy between men and women treated with doxycycline for MG and speculated that men might harbor MG in compartments such as the prostate gland where antibiotic concentrations are insufficient to eliminate the infection [34]. Azithromycin has superior tissue absorption compared with doxycycline, even in prostate tissue, suggesting that this is less likely to impact on azithromycin efficacy for MG [49]. The men in the studies reviewed were more likely to be symptomatic than women and, although it is unclear whether symptoms are associated with a higher MG organism load as can be seen with chlamydia [50], it is possible that as men were more often symptomatic they had a higher organism load than women, making them potentially more susceptible to treatment failure.
What do the results of this meta-analysis mean for MG treatment? Although macrolide resistance appears to be rapidly emerging, there has not been support for discontinuation of azithromycin [36] and there remains a lack of affordable, accessible, and effective alternatives [51]. Moxifloxacin (400 mg for 7–10 days) is often used as second-line treatment [46, 52]. However, this agent is unsuitable as initial treatment for MG due to its considerable expense and risk of serious adverse events including hepatotoxicity [1]. Furthermore, there has recently been evidence of sporadic moxifloxacin failures with fluoroquinolone resistance mutations associated with treatment failure in Australia and Japan [45, 46, 52, 53], which suggests that its use as an effective treatment for infections with macrolide resistance may be short lived. Instead, recommendations have been for continued monitoring of relevant resistance mutations and treatment efficacy while new drug options are explored. High-throughput combined diagnostic resistance assays are emerging and will be of considerable benefit for management of MG. Twin et al developed a high-resolution melt analysis assay that has been shown to be effective in detecting resistance-facilitating mutations in pre- and posttreatment samples of MG [47]. Salado-Rasmussen et al have a polymerase chain reaction–based combined diagnostic resistance assay already in use in Denmark [54]. Such applications can facilitate rapid administration of more effective second-line agents when macrolide mutations are detected, thereby shortening the duration of untreated infection and potentially limiting symptoms, ongoing transmission, and MG-associated sequelae such as PID and infertility [47].
Our meta-analysis has a number of strengths including a subgroup analysis to investigate causes of heterogeneity, evaluation of potential publication bias, and assessment of data quality. There are also a number of limitations. First, there was considerable heterogeneity between studies (>80%), reducing the reliability of the pooled estimates, although all but 3 studies [37, 38, 43] had treatment efficacy of <95%, the usual trigger for a change in treatment recommendations [55]. Second, most studies included were observational with variation in sample size and timing of when microbial cure was measured. If microbial cure was measured too early, there is an increased chance that nonviable DNA will be detected and a false-positive result will occur. However, if microbial cure is measured too late, there is a greater likelihood of reinfection. We investigated this in our subgroup analyses and found that efficacy was lower in those studies which measured cure >4 weeks after treatment, suggesting that reinfection may have contributed to a declining efficacy over time. This makes interpretation of the results difficult. Our review was limited to published English-language studies, potentially reducing the generalizability of our findings. Most studies were set in STI clinics and included mainly symptomatic patients, who are less likely to be representative of everyone who becomes infected with genital MG. Last, we cannot rule out the impact of publication bias, and given that there is increasing discussion in the medical literature [56] about MG treatment failure, it is possible that studies that report lower efficacy for azithromycin are being preferentially submitted for publication.
CONCLUSIONS
Our meta-analysis found that the efficacy of 1 g of azithromycin for genital MG has decreased over the last 5 years, and is now approaching 60%. Even though most of the evidence is based on data from observational studies that have considerable variability in sample size and timing of microbial cure, this low efficacy is of concern and is well below the 95% threshold recommended by the World Health Organization for STI treatments [55]. However, given that the prevalence of resistance mutations is increasing, it is vital that new treatment regimens for MG are investigated. A major barrier to improving the management of MG is that the majority of MG infections are presumptively exposed to 1 g of azithromycin during management of associated syndromes. This raises the broader and more complex issue of whether it is now time to address the presumptive use of azithromycin in MG-associated syndromes.
Notes
Financial support. A. L. undertook this review as part of the requirements for a Master of Science (Epidemiology) at University of Melbourne. J. S. H. was supported by a National Health and Medical Research Council Senior Research Fellowship (APP1042907).
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




