-
PDF
- Split View
-
Views
-
Cite
Cite
Kimberly G. Blumenthal, Robert A. Parker, Erica S. Shenoy, Rochelle P. Walensky, Improving Clinical Outcomes in Patients With Methicillin-Sensitive Staphylococcus aureus Bacteremia and Reported Penicillin Allergy, Clinical Infectious Diseases, Volume 61, Issue 5, 1 September 2015, Pages 741–749, https://doi.org/10.1093/cid/civ394
Close - Share Icon Share
Abstract
Background. Methicillin-sensitive Staphylococcus aureus (MSSA) bacteremia is a morbid infection. First-line MSSA therapies (nafcillin, oxacillin, cefazolin) are generally avoided in the 10% of patients reporting penicillin (PCN) allergy, but most of these patients are not truly allergic. We used a decision tree with sensitivity analyses to determine the optimal evaluation and treatment for patients with MSSA bacteremia and reported PCN allergy.
Methods. Our model simulates 3 strategies: (1) no allergy evaluation, give vancomycin (Vanc); (2) allergy history–guided treatment: if history excludes anaphylactic features, give cefazolin (Hx-Cefaz); and (3) complete allergy evaluation with history-appropriate PCN skin testing: if skin test negative, give cefazolin (ST-Cefaz). Model outcomes included 12-week MSSA cure, recurrence, and death; allergic reactions including major, minor, and potentially iatrogenic; and adverse drug reactions.
Results. Vanc results in the fewest patients achieving MSSA cure and the highest rate of recurrence (67.3%/14.8% vs 83.4%/9.3% for Hx-Cefaz and 84.5%/8.9% for ST-Cefaz) as well as the greatest frequency of allergic reactions (3.0% vs 2.4% for Hx-Cefaz and 1.7% for ST-Cefaz) and highest rates of adverse drug reactions (5.2% vs 4.6% for Hx-Cefaz and 4.7% for ST-Cefaz). Even in a “best case for Vanc” scenario, Vanc yields the poorest outcomes. ST-Cefaz is preferred to Hx-Cefaz although sensitive to input variations.
Conclusions. Patients with MSSA bacteremia and a reported PCN allergy should have the allergy addressed for optimal treatment. Full allergy evaluation with skin testing seems to be preferred, although more data are needed.
Staphylococcus aureus is a leading cause of bacteremia, with two-thirds being methicillin-sensitive (MSSA) [1–4]. Patients with MSSA bacteremia can develop complications, such as endocarditis or osteomyelitis [5, 6]. Mortality rates in MSSA bacteremia range from 9% to 50% [1, 2, 5–10].
Prolonged courses of the β-lactam antibiotics—nafcillin, oxacillin, and cefazolin—provide the greatest chance of MSSA bacteremia cure [7–11]. Vancomycin is a second-line agent because of its slower microbicidal activity, higher failure rates, and higher associated morbidity and mortality [9, 12, 13]. Guidelines indicate that vancomycin is inferior to β-lactams for MSSA bacteremia [14–16].
The reported prevalence of penicillin (PCN) allergy is 10%–15% among inpatients [17–22]. Once reported, nafcillin, oxacillin, and cefazolin are generally avoided, even in infections such as MSSA where they are clearly superior [11, 17–19, 23]. However, 90%–99% of patients with a reported PCN allergy are not allergic [21, 24–27]. This large discrepancy between reported allergy and true allergy is attributable both to the waning natural history of PCN allergy and misclassification of the original reaction [17, 28].
Infectious disease experts recommend cefazolin for MSSA treatment in patients with PCN allergy unless the reaction to PCN is anaphylactic [5, 14]. However, patients without an anaphylactic allergy history can have future anaphylactic reactions to PCN [29–31]. Because there is 2%–4% cross-reactivity between PCN and first-generation cephalosporins [32–35], allergy practice advises PCN skin testing prior to cefazolin administration, or if skin testing is not available, administration of cefazolin only in patients without symptoms of an immunoglobulin E–mediated reaction by an observed graded challenge [17, 29, 30, 36, 37]. To determine the optimal treatment for patients with MSSA bacteremia and reported PCN allergy, we synthesized the most relevant data from the primary literature, using a decision analysis model.
METHODS
Analytic Overview
The decision analysis model compares 3 treatment strategies for patients with MSSA bacteremia and reported PCN allergy: (1) no allergy evaluation, give vancomycin (Vanc); (2) allergy history-guided treatment: if detailed history excludes anaphylactic features, give cefazolin (Hx-Cefaz); and (3) complete allergy evaluation with history-appropriate PCN skin testing: if skin test (ST) negative, give cefazolin (ST-Cefaz).
Model outcomes at 12 weeks include (1) MSSA cure, recurrence, and death; (2) allergic reactions classified as major or minor and, separately, as potentially iatrogenic; and (3) adverse drug reactions (ADRs; drug toxicity or intolerance). We defined “potentially iatrogenic” as all allergic reactions from cefazolin in patients with PCN allergy history [38].
We assessed which strategy is optimal for each individual outcome. We then created a composite outcome where we summed outcomes using the following weights: cure, +50; recurrence of infection, −20; death, −50; major allergic reactions, −10; minor allergic reactions, −2; and ADRs, −6. A composite score of 50 would indicate a strategy where patients are cured without allergic reactions or ADRs. We examined key input parameters in a series of 1-way, multiway, and probabilistic sensitivity analyses (PSA); we also conducted sensitivity analyses on weights for the composite outcome.
Model Description and Strategies
The decision tree (TreeAge Pro 2014, TreeAge Software, Inc, Williamstown, Massachusetts) includes 3 competing strategies for the patient with MSSA bacteremia and reported PCN allergy (Figure 1). Within each strategy, an alternative strategy may be considered, depending on allergic and adverse outcomes. We limited the model so that no patient receives >3 sequential antibiotics. At the end of a patient's course through the decision tree, he or she is treated with vancomycin, cefazolin, nafcillin, or an alternative non–β-lactam drug (eg, daptomycin). Model outcomes are defined after 12 weeks, at the rightmost end of the tree. If a tree course does not have the outcome of interest, the probabilities are multiplied by a payoff value of 0. If the tree course has the outcome of interest, the probabilities are multiplied by a payoff value of 1. This structure allows us to obtain the percentages of patients in each tree strategy who had each outcome.
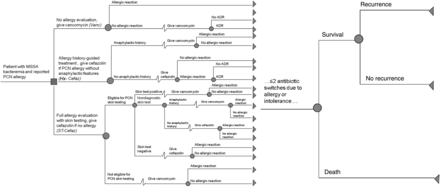
Simplified decision tree for patients with methicillin-sensitive Staphylococcus aureus (MSSA) bacteremia and reported penicillin (PCN) allergy. The decision tree, read from left to right, displays the 12-week course of a patient with MSSA bacteremia and reported PCN allergy. Squares represent decision nodes; circles represent the chance nodes where the probabilities are defined by the input parameters. The tree's 3 prominent branches are the strategies evaluated by the model: (1) no allergy evaluation, give vancomycin (Vanc); (2) allergy history–guided treatment: if history excludes anaphylactic features, give cefazolin (Hx-Cefaz); and (3) complete allergy evaluation with history-appropriate PCN skin testing; if skin test negative, give cefazolin (ST-Cefaz). In each branch, therapy can be altered based on allergic or adverse drug reactions (ADRs); no patient in the tree is treated with >3 drugs in his/her 12-week course. Once a definitive antibiotic course is identified, the patient experiences cure, recurrence, or death, represented by the rightmost part of the figure.
In the Vanc strategy, all patients with MSSA bacteremia and reported PCN allergy receive vancomycin treatment without allergy evaluation. Vancomycin-treated patients may develop an allergic reaction (major or minor) or an ADR. Upon an allergic reaction or ADR to vancomycin, clinicians reconsider therapy options, and the patients have an equal chance of getting (1) an alternative non–β-lactam drug; (2) a history-driven allergy evaluation where cefazolin is given if the PCN allergy history is not anaphylactic; or (3) a full PCN allergy evaluation with history-appropriate skin testing, with cefazolin given if there is no allergy. The latter strategies are similar to the Hx-Cefaz and ST-Cefaz branches of the tree, although, because these patients suffered an ADR or allergic reaction to vancomycin, they do not receive vancomycin again.
In the Hx-Cefaz strategy, patients with a history of PCN allergy with anaphylactic features receive vancomycin; patients with milder allergy histories receive cefazolin. Patients may have an allergic reaction to cefazolin necessitating a drug change to vancomycin. Patients who do not tolerate cefazolin but did not have an allergic reaction have an equal chance of receiving vancomycin therapy or a full allergy evaluation with PCN skin testing. The latter strategy is similar to the ST-Cefaz branch, although if these patients are ST negative, they receive another first-line MSSA antimicrobial (nafcillin). If they are ST positive, they receive vancomycin. Patients with an anaphylactic history receive vancomycin, and may have an allergic reaction or an ADR to vancomycin necessitating a drug change to an alternative non–β-lactam drug.
In the ST-Cefaz arm, patients eligible for skin testing receive PCN skin testing. Patients ineligible for skin testing are given vancomycin. Allergy specialists generally define patients ineligible for skin testing as those with anaphylaxis to PCN in the last 10 years, with a history of a Gell and Coombs type II–IV hypersensitivity reaction to PCN, or currently taking medications that interfere with ST results (eg, antihistamines) [17]. For patients skin tested to PCN, those who are ST negative receive cefazolin, those who are ST positive receive vancomycin, and those with a nondiagnostic ST receive history-guided therapy (as in Hx-Cefaz). In this strategy, patients ineligible for ST who develop an allergic reaction to vancomycin have an equal chance of being given cefazolin guided by allergy history alone or given an alternative non–β-lactam drug.
Input Parameters
The model uses data from literature sources as input parameters (Table 1). These data, reported as fractions and frequencies in the primary literature, are converted into probabilities for inclusion into the decision tree model at the chance nodes (Figure 1). We weighted the outcomes from sources based on cohort numbers when >1 source was available for our base case values (Table 1). Reported probabilities are conditional upon prior outcomes, as indicated by tree structure.
Input Parameters for Decision Analysis Model of Methicillin-Sensitive Staphylococcus aureus and Reported Penicillin Allergy
| Description . | Baseline Value, % . | Range for Sensitivity Analyses, % . | Source . |
|---|---|---|---|
| Treatment efficacya | |||
| Vancomycin | |||
| MSSA cure | 62.0 | 36.0–90.0 | [7, 8, 16] |
| MSSA recurrence | 18.8 | 0.0–23.2 | [7, 8] |
| MSSA mortality | 19.2 | 10.0–41.0 | [7, 8, 16] |
| Cefazolin | |||
| MSSA cure | 85.0 | 80.0–88.3 | [7, 8, 16] |
| MSSA recurrence | 9.1 | 0.0–10.0 | [7] |
| MSSA mortality | 5.9 | 2.0–6.0 | [7, 16] |
| Alternativeb | |||
| MSSA cure | 86.4 | 36.0–90.0 | [7, 8, 16, 23] |
| MSSA recurrence | 13.2 | 0.0–23.2 | [7, 8, 23] |
| MSSA mortality | 0.4 | 0.0–41.0 | [7, 8, 16, 23] |
| Allergic reactions | |||
| Vanc | |||
| Allergic reaction to vancomycinc | 3.0 | 2.0–6.4 | [39, 40] |
| Allergic reaction to vancomycin considered major | 8.3 | 0.0–10.0 | [40] |
| Hx-Cefaz | |||
| Patients with PCN allergy whose reaction has anaphylactic features | 5.6 | 0.0–11.2 | PEARd |
| Patients without anaphylactic PCN allergy history who react to cefazoline | 2.2f | 0.2–5.6 | [17, 30, 32, 41–44] |
| ST-Cefaz | |||
| Patients not eligible for PCN skin testing | 9.1 | 0.0–11.0 | [20, 21] |
| Patients with a positive PCN skin test | 1.2 | 0.0–10.0 | [20, 21, 24, 25, 45] |
| Patients with a nondiagnostic PCN skin test | 3.2 | 0.0–10.4 | [20, 21, 24, 25, 45] |
| Allergic reaction to cefazolin or nafcillin given a negative skin test to penicilline | 1.6 | 0.1–1.8 | [20, 21, 24, 25, 45] |
| General | |||
| Allergic reaction to cefazolin considered major | 4.7 | 0.0–5.6 | [26, 36] |
| Death from allergic reaction given a major allergic reaction occurs | 0.2 | 0.0–3.8 | [46–49] |
| Adverse drug reactions | |||
| Vancomycin | 5.3 | 0.0–43.0 | [40, 50, 51] |
| Cefazolin | 4.4 | 0.0–7.0 | [40,51,52] |
| Description . | Baseline Value, % . | Range for Sensitivity Analyses, % . | Source . |
|---|---|---|---|
| Treatment efficacya | |||
| Vancomycin | |||
| MSSA cure | 62.0 | 36.0–90.0 | [7, 8, 16] |
| MSSA recurrence | 18.8 | 0.0–23.2 | [7, 8] |
| MSSA mortality | 19.2 | 10.0–41.0 | [7, 8, 16] |
| Cefazolin | |||
| MSSA cure | 85.0 | 80.0–88.3 | [7, 8, 16] |
| MSSA recurrence | 9.1 | 0.0–10.0 | [7] |
| MSSA mortality | 5.9 | 2.0–6.0 | [7, 16] |
| Alternativeb | |||
| MSSA cure | 86.4 | 36.0–90.0 | [7, 8, 16, 23] |
| MSSA recurrence | 13.2 | 0.0–23.2 | [7, 8, 23] |
| MSSA mortality | 0.4 | 0.0–41.0 | [7, 8, 16, 23] |
| Allergic reactions | |||
| Vanc | |||
| Allergic reaction to vancomycinc | 3.0 | 2.0–6.4 | [39, 40] |
| Allergic reaction to vancomycin considered major | 8.3 | 0.0–10.0 | [40] |
| Hx-Cefaz | |||
| Patients with PCN allergy whose reaction has anaphylactic features | 5.6 | 0.0–11.2 | PEARd |
| Patients without anaphylactic PCN allergy history who react to cefazoline | 2.2f | 0.2–5.6 | [17, 30, 32, 41–44] |
| ST-Cefaz | |||
| Patients not eligible for PCN skin testing | 9.1 | 0.0–11.0 | [20, 21] |
| Patients with a positive PCN skin test | 1.2 | 0.0–10.0 | [20, 21, 24, 25, 45] |
| Patients with a nondiagnostic PCN skin test | 3.2 | 0.0–10.4 | [20, 21, 24, 25, 45] |
| Allergic reaction to cefazolin or nafcillin given a negative skin test to penicilline | 1.6 | 0.1–1.8 | [20, 21, 24, 25, 45] |
| General | |||
| Allergic reaction to cefazolin considered major | 4.7 | 0.0–5.6 | [26, 36] |
| Death from allergic reaction given a major allergic reaction occurs | 0.2 | 0.0–3.8 | [46–49] |
| Adverse drug reactions | |||
| Vancomycin | 5.3 | 0.0–43.0 | [40, 50, 51] |
| Cefazolin | 4.4 | 0.0–7.0 | [40,51,52] |
Abbreviations: Hx-Cefaz, allergy history-guided treatment: if history excludes anaphylactic features, give cefazolin; MSSA, methicillin-sensitive Staphylococcus aureus; PCN, penicillin; PEAR, Partners Enterprise Allergy Repository; ST-Cefaz, complete allergy evaluation with penicillin skin testing, give cefazolin if negative; Vanc, no allergy evaluation, give vancomycin.
a Outcomes defined 12 weeks after initial blood culture.
b Base case outcomes based on 1 study [23]; examined range for outcomes from vancomycin therapy in sensitivity analyses.
c Excludes infusion reactions (eg, “red man syndrome”).
d Partners Enterprise Allergy Repository, accessed November 2013.
e The sum of these reactions were considered potentially iatrogenic allergic reactions.
f Mathematical computation = ∑ (patients who react to cefazolin who are skin test positive + patients who react to cefazolin who are skin test negative) = ∑ [{ proportion of patients skin test positive (0.49)} × {positive predictive value of PCN skin testing (0.4)} × {the cross-reactivity of PCN and cefazolin (0.029)} + {patients who react to cefazolin who are skin test negative (0.016)}] = (0.49 × 0.4 × 0.029) + 0.016 = 0.022 = 2.2%.
Input Parameters for Decision Analysis Model of Methicillin-Sensitive Staphylococcus aureus and Reported Penicillin Allergy
| Description . | Baseline Value, % . | Range for Sensitivity Analyses, % . | Source . |
|---|---|---|---|
| Treatment efficacya | |||
| Vancomycin | |||
| MSSA cure | 62.0 | 36.0–90.0 | [7, 8, 16] |
| MSSA recurrence | 18.8 | 0.0–23.2 | [7, 8] |
| MSSA mortality | 19.2 | 10.0–41.0 | [7, 8, 16] |
| Cefazolin | |||
| MSSA cure | 85.0 | 80.0–88.3 | [7, 8, 16] |
| MSSA recurrence | 9.1 | 0.0–10.0 | [7] |
| MSSA mortality | 5.9 | 2.0–6.0 | [7, 16] |
| Alternativeb | |||
| MSSA cure | 86.4 | 36.0–90.0 | [7, 8, 16, 23] |
| MSSA recurrence | 13.2 | 0.0–23.2 | [7, 8, 23] |
| MSSA mortality | 0.4 | 0.0–41.0 | [7, 8, 16, 23] |
| Allergic reactions | |||
| Vanc | |||
| Allergic reaction to vancomycinc | 3.0 | 2.0–6.4 | [39, 40] |
| Allergic reaction to vancomycin considered major | 8.3 | 0.0–10.0 | [40] |
| Hx-Cefaz | |||
| Patients with PCN allergy whose reaction has anaphylactic features | 5.6 | 0.0–11.2 | PEARd |
| Patients without anaphylactic PCN allergy history who react to cefazoline | 2.2f | 0.2–5.6 | [17, 30, 32, 41–44] |
| ST-Cefaz | |||
| Patients not eligible for PCN skin testing | 9.1 | 0.0–11.0 | [20, 21] |
| Patients with a positive PCN skin test | 1.2 | 0.0–10.0 | [20, 21, 24, 25, 45] |
| Patients with a nondiagnostic PCN skin test | 3.2 | 0.0–10.4 | [20, 21, 24, 25, 45] |
| Allergic reaction to cefazolin or nafcillin given a negative skin test to penicilline | 1.6 | 0.1–1.8 | [20, 21, 24, 25, 45] |
| General | |||
| Allergic reaction to cefazolin considered major | 4.7 | 0.0–5.6 | [26, 36] |
| Death from allergic reaction given a major allergic reaction occurs | 0.2 | 0.0–3.8 | [46–49] |
| Adverse drug reactions | |||
| Vancomycin | 5.3 | 0.0–43.0 | [40, 50, 51] |
| Cefazolin | 4.4 | 0.0–7.0 | [40,51,52] |
| Description . | Baseline Value, % . | Range for Sensitivity Analyses, % . | Source . |
|---|---|---|---|
| Treatment efficacya | |||
| Vancomycin | |||
| MSSA cure | 62.0 | 36.0–90.0 | [7, 8, 16] |
| MSSA recurrence | 18.8 | 0.0–23.2 | [7, 8] |
| MSSA mortality | 19.2 | 10.0–41.0 | [7, 8, 16] |
| Cefazolin | |||
| MSSA cure | 85.0 | 80.0–88.3 | [7, 8, 16] |
| MSSA recurrence | 9.1 | 0.0–10.0 | [7] |
| MSSA mortality | 5.9 | 2.0–6.0 | [7, 16] |
| Alternativeb | |||
| MSSA cure | 86.4 | 36.0–90.0 | [7, 8, 16, 23] |
| MSSA recurrence | 13.2 | 0.0–23.2 | [7, 8, 23] |
| MSSA mortality | 0.4 | 0.0–41.0 | [7, 8, 16, 23] |
| Allergic reactions | |||
| Vanc | |||
| Allergic reaction to vancomycinc | 3.0 | 2.0–6.4 | [39, 40] |
| Allergic reaction to vancomycin considered major | 8.3 | 0.0–10.0 | [40] |
| Hx-Cefaz | |||
| Patients with PCN allergy whose reaction has anaphylactic features | 5.6 | 0.0–11.2 | PEARd |
| Patients without anaphylactic PCN allergy history who react to cefazoline | 2.2f | 0.2–5.6 | [17, 30, 32, 41–44] |
| ST-Cefaz | |||
| Patients not eligible for PCN skin testing | 9.1 | 0.0–11.0 | [20, 21] |
| Patients with a positive PCN skin test | 1.2 | 0.0–10.0 | [20, 21, 24, 25, 45] |
| Patients with a nondiagnostic PCN skin test | 3.2 | 0.0–10.4 | [20, 21, 24, 25, 45] |
| Allergic reaction to cefazolin or nafcillin given a negative skin test to penicilline | 1.6 | 0.1–1.8 | [20, 21, 24, 25, 45] |
| General | |||
| Allergic reaction to cefazolin considered major | 4.7 | 0.0–5.6 | [26, 36] |
| Death from allergic reaction given a major allergic reaction occurs | 0.2 | 0.0–3.8 | [46–49] |
| Adverse drug reactions | |||
| Vancomycin | 5.3 | 0.0–43.0 | [40, 50, 51] |
| Cefazolin | 4.4 | 0.0–7.0 | [40,51,52] |
Abbreviations: Hx-Cefaz, allergy history-guided treatment: if history excludes anaphylactic features, give cefazolin; MSSA, methicillin-sensitive Staphylococcus aureus; PCN, penicillin; PEAR, Partners Enterprise Allergy Repository; ST-Cefaz, complete allergy evaluation with penicillin skin testing, give cefazolin if negative; Vanc, no allergy evaluation, give vancomycin.
a Outcomes defined 12 weeks after initial blood culture.
b Base case outcomes based on 1 study [23]; examined range for outcomes from vancomycin therapy in sensitivity analyses.
c Excludes infusion reactions (eg, “red man syndrome”).
d Partners Enterprise Allergy Repository, accessed November 2013.
e The sum of these reactions were considered potentially iatrogenic allergic reactions.
f Mathematical computation = ∑ (patients who react to cefazolin who are skin test positive + patients who react to cefazolin who are skin test negative) = ∑ [{ proportion of patients skin test positive (0.49)} × {positive predictive value of PCN skin testing (0.4)} × {the cross-reactivity of PCN and cefazolin (0.029)} + {patients who react to cefazolin who are skin test negative (0.016)}] = (0.49 × 0.4 × 0.029) + 0.016 = 0.022 = 2.2%.
Within treatment efficacy, parameters include the 12-week MSSA cure with vancomycin (62.0%) and cefazolin (85.0%), and the 12-week probability of MSSA recurrence and mortality associated with vancomycin (18.8% and 19.2%, respectively) and cefazolin (9.1% and 5.9%, respectively) [7, 8, 16]. Because only 1 study reports MSSA recurrence (13.2%) and death (0.4%) with an alternative drug, we used vancomycin literature ranges to define cure, recurrence, and death with an alternative, non–β-lactam drug [23].
The chance of allergic reaction to vancomycin was 3.0%, of which 8.3% of reactions were considered major and 91.7% considered minor [39, 40]. In the Hx-Cefaz strategy, data from an internal database informed the proportion of patients whose reported reaction to PCN had anaphylactic features (5.6%), defined by reported reactions of bronchospasm, wheezing, anaphylaxis, hypotension, and angioedema [53]. The proportion of patients without an anaphylactic history who react to cefazolin was 2.2%, derived from cohort data and mathematical computation [17, 30, 32, 41–44]. In the ST-Cefaz strategy, we defined 9.1% of patients as ST ineligible [20, 21]; among those eligible, 95.6% were ST negative, 1.2% were ST positive, and 3.2% had a nondiagnostic ST [20, 21, 24, 25, 45]. After a negative ST, 1.6% of patients react to cefazolin [20–22, 45]. In both the Hx-Cefaz and ST-Cefaz strategies, once an allergic reaction occurs, 4.7% can be considered major (95.3% are minor); 0.2% of major reactions result in death [26, 36, 46–49, 54]. ADR rate was 5.3% with vancomycin and 4.4% with cefazolin [40, 50–52].
Sensitivity Analysis
We conducted sensitivity analyses using probability ranges from the primary literature (Table 1). We varied parameters individually (1-way sensitivity analysis) for each outcome and identified influential parameters. We then varied these key inputs simultaneously in a series of multiway sensitivity analyses. Finally, we used literature estimates to create distributions for the key input parameters to conduct PSA, whereby 100 000 patients travel through the decision tree sampling different probabilities from the defined distributions (Supplementary Table 1). We conducted a PSA for all 3 strategies on all outcomes. Because results for the Hx-Cefaz and ST-Cefaz groups were similar, we subsequently ran the PSA for Vanc compared with a sample allergy evaluation strategy (Hx-Cefaz).
RESULTS
Base Case
Individual Outcomes
The Vanc strategy results in 67.3% chance of cure, 14.8% chance of recurrence, and 17.9% chance of death (Table 2). Total allergic reactions are 3.0%, with 0.3% of patients experiencing a major reaction and 0.8% experiencing a potentially iatrogenic reaction. ADRs are present in 5.2% of vancomycin-treated patients. Hx-Cefaz results in better clinical outcomes than Vanc, including a much higher chance of cure (83.4%), as well as a substantially lower chance of recurrence (9.3%), and less than half the 12-week mortality (7.3%). Fewer patients in Hx-Cefaz have allergic reactions (2.4%) and ADRs (4.6%) than in Vanc (3.0% and 5.2%, respectively). While major allergic reactions (0.1%) are less frequent in Hx-Cefaz, more patients experience a reaction that could be considered iatrogenic (2.1%) than in the Vanc strategy.
Clinical Outcomes of 3 Strategies for Management of Methicillin-Sensitive Staphylococcus aureus Bacteremia and Reported Penicillin Allergy
| Outcome . | Vanc . | Hx-Cefaz . | ST-Cefaz . |
|---|---|---|---|
| Cure, % | 67.3 | 83.4 | 84.5 |
| Recurrence, % | 14.8 | 9.3 | 8.9 |
| Death, % | 17.9 | 7.3 | 6.6 |
| Allergic reactionsa, total, % | 3.0 | 2.4 | 1.7 |
| Major, % | 0.3 | 0.1 | 0.1 |
| Minor, % | 2.8 | 2.2 | 1.6 |
| Iatrogenic allergic reactionsb, % | 0.8 | 2.1 | 1.6 |
| Adverse drug reactions, % | 5.2 | 4.6 | 4.7 |
| Composite outcome valuec (strategy rank) | 21.3 (3) | 35.9 (2) | 36.8 (1) |
| Outcome . | Vanc . | Hx-Cefaz . | ST-Cefaz . |
|---|---|---|---|
| Cure, % | 67.3 | 83.4 | 84.5 |
| Recurrence, % | 14.8 | 9.3 | 8.9 |
| Death, % | 17.9 | 7.3 | 6.6 |
| Allergic reactionsa, total, % | 3.0 | 2.4 | 1.7 |
| Major, % | 0.3 | 0.1 | 0.1 |
| Minor, % | 2.8 | 2.2 | 1.6 |
| Iatrogenic allergic reactionsb, % | 0.8 | 2.1 | 1.6 |
| Adverse drug reactions, % | 5.2 | 4.6 | 4.7 |
| Composite outcome valuec (strategy rank) | 21.3 (3) | 35.9 (2) | 36.8 (1) |
Abbreviations: Hx-Cefaz, allergy history-guided treatment: if history excludes anaphylactic features, give cefazolin; PCN, penicillin; ST-Cefaz, complete allergy evaluation with PCN skin testing, give cefazolin if negative; Vanc, no allergy evaluation, give vancomycin.
a Major and minor reactions do not sum to total due to rounding.
b Reactions from cefazolin in a patient with reported PCN allergy.
c Composite outcome weights: cure, +50; recurrence of infection, −20; death, −50; major allergic reactions, −10; minor allergic reactions, −2; adverse drug reactions, −6.
Clinical Outcomes of 3 Strategies for Management of Methicillin-Sensitive Staphylococcus aureus Bacteremia and Reported Penicillin Allergy
| Outcome . | Vanc . | Hx-Cefaz . | ST-Cefaz . |
|---|---|---|---|
| Cure, % | 67.3 | 83.4 | 84.5 |
| Recurrence, % | 14.8 | 9.3 | 8.9 |
| Death, % | 17.9 | 7.3 | 6.6 |
| Allergic reactionsa, total, % | 3.0 | 2.4 | 1.7 |
| Major, % | 0.3 | 0.1 | 0.1 |
| Minor, % | 2.8 | 2.2 | 1.6 |
| Iatrogenic allergic reactionsb, % | 0.8 | 2.1 | 1.6 |
| Adverse drug reactions, % | 5.2 | 4.6 | 4.7 |
| Composite outcome valuec (strategy rank) | 21.3 (3) | 35.9 (2) | 36.8 (1) |
| Outcome . | Vanc . | Hx-Cefaz . | ST-Cefaz . |
|---|---|---|---|
| Cure, % | 67.3 | 83.4 | 84.5 |
| Recurrence, % | 14.8 | 9.3 | 8.9 |
| Death, % | 17.9 | 7.3 | 6.6 |
| Allergic reactionsa, total, % | 3.0 | 2.4 | 1.7 |
| Major, % | 0.3 | 0.1 | 0.1 |
| Minor, % | 2.8 | 2.2 | 1.6 |
| Iatrogenic allergic reactionsb, % | 0.8 | 2.1 | 1.6 |
| Adverse drug reactions, % | 5.2 | 4.6 | 4.7 |
| Composite outcome valuec (strategy rank) | 21.3 (3) | 35.9 (2) | 36.8 (1) |
Abbreviations: Hx-Cefaz, allergy history-guided treatment: if history excludes anaphylactic features, give cefazolin; PCN, penicillin; ST-Cefaz, complete allergy evaluation with PCN skin testing, give cefazolin if negative; Vanc, no allergy evaluation, give vancomycin.
a Major and minor reactions do not sum to total due to rounding.
b Reactions from cefazolin in a patient with reported PCN allergy.
c Composite outcome weights: cure, +50; recurrence of infection, −20; death, −50; major allergic reactions, −10; minor allergic reactions, −2; adverse drug reactions, −6.
Clinical outcomes for ST-Cefaz are marginally better than for Hx-Cefaz and markedly improved over Vanc. The probabilities of MSSA cure, recurrence, and death with ST-Cefaz are 84.5%, 8.9%, and 6.6%, respectively. ST-Cefaz projects the fewest allergic reactions of the strategies, with 1.7% having an allergic reaction, including 0.1% experiencing a major reaction and 1.6% of patients experiencing an allergic reaction that could be considered iatrogenic. ST-Cefaz projects similar ADRs to Hx-Cefaz (4.7% vs 4.6%).
Composite Outcome
Using the composite outcome, ST-Cefaz is the optimal strategy, with a value of 36.8, over Hx-Cefaz (35.9) and Vanc (21.3). Overall, the optimal strategy was insensitive to clinically reasonable alternative weighting of individual outcomes chosen for the composite score. Even when iatrogenicity was included in the composite with weight on par with death (−50), ST-Cefaz remains the optimal strategy (36.0) compared with Hx-Cefaz (34.9) and Vanc (20.9).
Sensitivity Analyses
Vanc Versus Allergy Evaluation
Because the allergy evaluation strategies offer similar outcomes, for simplicity, we focused sensitivity analyses on Vanc compared to a sample allergy strategy, Hx-Cefaz.
One-Way Sensitivity Analyses
With variation of input parameters within all reasonable ranges from reported literature, Vanc consistently has inferior clinical outcomes of MSSA cure, recurrence, death, and the composite outcome. The input parameters with the greatest effect on the value of the composite outcome are the probability of death and recurrence with vancomycin (Figure 2). However, varying these inputs within reported literature ranges still leads to Vanc giving the least desired outcomes.
![One-way sensitivity analyses for the composite outcome for patients with methicillin-sensitive Staphylococcus aureus (MSSA) bacteremia and reported penicillin (PCN) allergy, comparing Vanc to Hx-Cefaz (see Figure 1 for decision tree). This tornado diagram summarizes the results of all influential 1-way sensitivity analyses on the composite outcome (cure, +50; recurrence of infection, −20; death, −50; major allergic reactions, −10; minor allergic reactions, −2; adverse drug reactions [ADRs], −6) with the variables examined within the probabilities described on the vertical axis. Each horizontal bar represents the range of expected values generated by varying the related variable across its plausible range, as indicated at opposite ends of each bar. A wide bar indicates that the associated variable has a large potential effect on the composite outcome. The vertical dotted lines represent the expected value of the composite outcome for Vanc (21.3) and Hx-Cefaz (35.9). Because there is no overlap between bars in Vanc and Hx-Cefaz, there is no plausible input parameter change that could result in the Vanc strategy having a higher composite outcome than the Hx-Cefaz strategy. Abbreviations: Hx-Cefaz, allergy history–guided treatment: if history excludes anaphylactic features, give cefazolin; Vanc, no allergy evaluation, give vancomycin.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/61/5/10.1093_cid_civ394/1/m_civ39402.gif?Expires=1750298146&Signature=Il0Vabp9lahVyWdzl~yKtfPEJzAw~OTdU~HhC1UVN2D7dnOgYjcA9r38a35LPe5Ns2wYlhFxcN4t3wAoYL46pasazTuh9DNQF87ngs-r3xDsDMzRULm5DU47rQLeGhOOiGs1ZNgn6lwX9dVzJXYIhk3-jFEIdPfMRYzMRYH0-qyxgWK8UWsorcx1GbRoWhb8C9HHXFTGhgaG4URxSASH66k4Yq6OJRoVrwNjd3Y0h8~Qi0w2CRmtmgDI2zGftLtQPclxMT2ANCcG14tQL8UUu9oqLMg~MlvIUQrT3QnEmfFZCcXbkHAPAe0OHsNLA0oxfJVV0k020rKRWlpbyyMq9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
One-way sensitivity analyses for the composite outcome for patients with methicillin-sensitive Staphylococcus aureus (MSSA) bacteremia and reported penicillin (PCN) allergy, comparing Vanc to Hx-Cefaz (see Figure 1 for decision tree). This tornado diagram summarizes the results of all influential 1-way sensitivity analyses on the composite outcome (cure, +50; recurrence of infection, −20; death, −50; major allergic reactions, −10; minor allergic reactions, −2; adverse drug reactions [ADRs], −6) with the variables examined within the probabilities described on the vertical axis. Each horizontal bar represents the range of expected values generated by varying the related variable across its plausible range, as indicated at opposite ends of each bar. A wide bar indicates that the associated variable has a large potential effect on the composite outcome. The vertical dotted lines represent the expected value of the composite outcome for Vanc (21.3) and Hx-Cefaz (35.9). Because there is no overlap between bars in Vanc and Hx-Cefaz, there is no plausible input parameter change that could result in the Vanc strategy having a higher composite outcome than the Hx-Cefaz strategy. Abbreviations: Hx-Cefaz, allergy history–guided treatment: if history excludes anaphylactic features, give cefazolin; Vanc, no allergy evaluation, give vancomycin.
When examining individual outcomes, iatrogenic allergic reactions, although generally infrequent in all strategies (<2.1%), are always minimized with Vanc. If <1.7% of patients (base case 3.0%) have an allergic reaction to vancomycin, then Vanc results in the fewest total allergic reactions. If >5% of patients experience an ADR from cefazolin (base case 4.4%), then Vanc becomes optimal over allergy evaluation in terms of minimizing ADRs.
Multiway Sensitivity Analyses
We varied the most influential parameters determining the composite outcome (Figure 2); we also set all inputs to be the most attractive with regard to the Vanc strategy. In all situations, Vanc projects the least attractive infectious outcomes and composite score. As in the 1-way sensitivity analyses, within reasonable parameter variations from the literature-reported values, specific parameter variations lead to Vanc being optimal in terms of minimizing allergic reactions and ADRs (Supplementary Figures 1 and 2).
Probabilistic Sensitivity Analysis
Using distributions for influential input parameters (Supplementary Table 1), average results are very close to the base case values, and Vanc consistently provides the lowest composite score (Supplementary Table 2). In PSA, Hx-Cefaz is preferred over Vanc to maximize cure (99.8%), minimize recurrence (89.5%), minimize death (99.9%), minimize total allergic reaction (61.3%), minimize ADRs (76.8%), and maximize the composite outcome 99.9% of the time (Figure 3). Only if the most important goal is to minimize total potentially iatrogenic allergic reactions would Vanc be favored (93.8%).

Optimal strategy selection in Monte Carlo simulation of 100 000 patients with methicillin-sensitive Staphylococcus aureus bacteremia and reported penicillin allergy using probabilistic sensitivity analysis, comparing Vanc to Hx-Cefaz (see Figure 1 for decision tree). The bars (horizontal axis) display the frequency (vertical axis) with which a strategy is optimal for each clinical outcome defined, allowing variability in all input parameters simultaneously in probabilistic sensitivity analyses. Hx-Cefaz is the optimal choice for all outcomes, except minimizing iatrogenic allergic reactions where the Vanc strategy is preferred. Abbreviations: ADR, adverse drug reaction; Hx-Cefaz, allergy history–guided treatment: if history excludes anaphylactic features, give cefazolin; Vanc, no allergy evaluation, give vancomycin.
Optimal Allergy Evaluation Strategy
One-Way Sensitivity Analyses
Although outcomes between Hx-Cefaz and ST-Cefaz were quite close, variations of most parameters within literature-reported ranges continued to modestly favor ST-Cefaz. MSSA cure, recurrence, and death were sensitive to the proportion of patients with a positive ST, with Hx-Cefaz becoming optimal within the range examined. Considering total allergic reactions, the optimal strategy is most sensitive to the proportion of patients with a nonanaphylactic PCN allergy history who react to cefazolin. Specifically, Hx-Cefaz becomes optimal if <1.4% (base case, 2.2% [range, 0.02%–5.6%]) of patients with a nonanaphylactic PCN allergy history react to cefazolin. Considering ADRs, ST-Cefaz becomes optimal if the probability of an ADR from cefazolin is <2.9% (base case, 4.4% [range, 0%–7.0%]). The composite outcome favored ST-Cefaz, but was also sensitive to the probability of a positive ST; Hx-Cefaz becomes optimal when >8.5% (base case, 1.2% [range, 0%–10.0%]) of patients are ST positive.
Multiway Sensitivity Analyses
Variation of 2–3 input parameters at the same time favored ST-Cefaz. Parameters identified in 1-way sensitivity analyses lead to different conclusions about the optimal allergy strategy.
Probabilistic Sensitivity Analysis
Using distributions for influential input parameters (Supplementary Table 1), ST-Cefaz was preferred to Hx-Cefaz to maximize cure 99.5% of the time, minimize recurrence 90.4% of the time, minimize death 99.7% of the time, minimize total allergic reactions 83.8% of the time, and maximize the composite outcome 99.8% of the time. Hx-Cefaz was preferred to ST-Cefaz to minimize ADRs 96.1% of the time.
DISCUSSION
Using a decision analysis model, we project that patients with MSSA bacteremia and reported PCN allergy will have inferior outcomes if treated with vancomycin rather than having their PCN allergy addressed. Both the base case analysis and sensitivity analyses favored a full evaluation with PCN skin testing to optimize outcomes compared with basing therapy on PCN allergy history alone; however, differences were small and sensitive to uncertain input parameters.
Despite our finding that no allergy evaluation and treatment with vancomycin is inferior, patients with MSSA bacteremia and reported PCN allergy commonly receive vancomycin [11, 23]. This could reflect providers' concerns about iatrogenic allergic reactions and limited PCN allergy knowledge [18, 55]. Indeed, the only model outcome that suggested benefit to vancomycin was minimizing potential iatrogenic allergic reactions. Given our findings, the practice of giving vancomycin to patients with MSSA bacteremia and a reported PCN allergy without investigating the allergy should not be standard, regardless of the availability of allergy specialists or PCN skin testing. A brief bedside allergy history that excludes anaphylactic features can guide cefazolin use and result in better outcomes than choosing vancomycin.
Perhaps counterintuitively, our model projects that the use of vancomycin would result in more allergic reactions than the use of cefazolin through allergy history–guided therapy or skin testing–guided therapy. Allergy to vancomycin includes immediate reactions as well as delayed reactions (eg, morbilliform eruptions, drug rash with eosinophilia, and systemic symptoms syndrome) [17, 56–58]. Notably, our projection of increased allergic reactions with vancomycin excluded the common infusion reaction “red man syndrome” [17, 39, 59].
Our model projects that ST-Cefaz results in the most favorable outcomes. However, the differences were small, and literature-reported variations suggest that data may be inadequate to definitively determine which allergy evaluation strategy is optimal. Whereas much of the allergy skin testing and challenge literature is derived from outpatients, those with MSSA bacteremia are usually inpatients [24, 26, 36]. Because we may see different skin testing eligibility and results among inpatients, more robust inpatient data would be of value. A key parameter in determining the optimal allergy strategy is the proportion of patients without an anaphylactic PCN allergy history who react to cefazolin; this parameter was based on sparse literature and mathematical calculation. Last, because of the declining rate of reported ST positivity in the literature, we deliberately used recent allergy data to inform skin testing inputs [20, 21, 24, 26, 45, 51]. The optimal allergy strategy was sensitive to this parameter within reported ranges, and older data that used more skin-testing reagents reported higher rates of ST positivity [31].
While the model suggests that Hx-Cefaz may be as good as ST-Cefaz, ST-Cefaz is likely preferable for avoiding iatrogenic allergic reactions that can result in patient dissatisfaction. PCN skin testing is currently performed using Pre-Pen (penicilloyl-poly-lysine) and dilutions of PCN. The negative predictive value with these reagents is at least 95%; the procedure can safely be performed on general medical floors, and results are available within 1 hour [17, 21, 26, 45]. With proper training, PCN skin testing can be performed by registered nurses, which allows for reduced costs of providing the service [22].
Although data limitations and variability of input parameters in this analysis made it challenging to project which allergy strategy was optimal, either strategy is substantially better than Vanc. This conclusion is insensitive to parameters and despite evaluations of Vanc in a “best case for Vanc” scenario. We did not consider that patients with PCN allergy may have other drug allergies that could impact treatment. We justify exclusion of nafcillin because cefazolin is considered to be equally effective, better tolerated, and less expensive than nafcillin for MSSA bacteremia [16, 52, 60–62]. We additionally justify exclusion of other alternative agents (eg, daptomycin) as initial therapy because of limited comparative infectious outcome data in MSSA bacteremia [63–66]. However, given vancomycin's poor efficacy and tolerability in MSSA bacteremia, future comparisons of β-lactams to alternative agents will be important to consider. Finally, although we limited our outcome assessment to 12 weeks, we believe this strengthens our conclusion that the allergy must be addressed, as recurrences are more likely with vancomycin than cefazolin [7–11, 15, 16, 23].
Compared to treatment with vancomycin, we find that patients with MSSA bacteremia and reported PCN allergy have improved outcomes when treated with cefazolin, either by an allergy history–guided treatment or full allergy evaluation with skin testing. Although full allergy evaluation is likely preferred over history alone, more data from inpatients are needed.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the NIH (grant number T32 HL116275 to K. G. B.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




