-
PDF
- Split View
-
Views
-
Cite
Cite
Oliver Bader, Michael S. Weig, Uwe Gross, Michael P. Schön, Martin Mempel, Timo Buhl, A 32-Year-Old Man With Ulcerative Mucositis, Skin Lesions, and Nail Dystrophy, Clinical Infectious Diseases, Volume 54, Issue 7, 1 April 2012, Pages 1035–1036, https://doi.org/10.1093/cid/cir943
Close - Share Icon Share
(See page 972 for the Photo Quiz.)
Diagnosis: Chronic Mucocutaneous Candidiasis by Multidrug-Resistant Candida albicans
Microbiological analyses in our patient repeatedly revealed Candida albicans as the responsible oral pathogen (Figure 2). Due to unselected repetitive antifungal therapies, the colonizing yeast developed resistance to all ergosterol-targeting antimycotics as well as flucytosine, leaving echinocandins as the only remaining treatment. The current regimen with intermittent caspofungin treatments leads to a reduction in fungal burden but not total clearance, resulting in recolonization within a few weeks. A phylogenetic analysis showed that the patient harbors a set of closely related, but distinguishable, C. albicans strains with individual resistance mechanisms including increased efflux pump activity and alterations in the ergosterol biosynthesis pathway. Here, most prominent is an isolate with mutations in the ERG5 and ERG11 gene [1]. Due to the reduction of ergosterol in the plasma membrane, this leads to a rare phenotype of cross resistance to azoles and polyenes. Increasing antimycotic resistance is a major challenge in systemic Candida infections, especially in heavily pretreated patients [2].
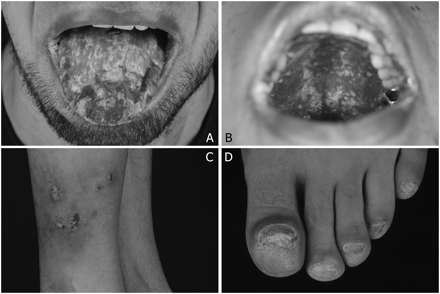
Ulcerative mucositis of the (A) tongue and (B) palate; (C) mycosis of normal skin (right lateral calf) and (D) onychomycosis of all nails.
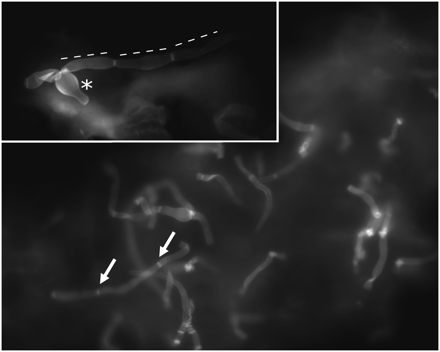
Calcofluor white mainly stains the chitin of the fungal cell wall. Morphologic characteristics are those of Candida albicans, including septated mycelium (white arrows), germinating yeast cells (asterisk), and pseudomycelium (dashed lines). The formation of the characteristic chlamydospores is not to be expected under these conditions (original magnification ×400, insert upper left: original magnification ×1000).
Chronic mucocutaneous candidiasis (CMC) represents a rare but prototypic condition for the development of refractory candidiasis [3]. Clinically, this disease seems very heterogeneous, appearing either alone, as in our patient (Figure 1), or in association with severe immunodeficiencies and/or autoimmune disorders such as autoimmune polyendocrinopathy candidiasis with ectodermal dystrophy syndrome [4, 5]. Over the last years, rare mutations in the signaling cascade for the initial recognition of Candida (Dectin-1/CARD9 pathway) were identified to result in increased susceptibility to fungal infections [6, 7]. Only recently, the key role of interleukin 17 (IL-17) in CMC was established by revelation of mutations in the IL-17 pathway and inhibitory auto-antibodies against IL-17 and interleukin 22 (IL-22) [8–11]. Furthermore, mutations in STAT1 have been linked to defective T-helper 1 and T-helper 17 responses in CMC patients, resulting in reduction or absence of the cytokines interferon-γ, IL-17, and/or IL-22 [12, 13]. In the presented case, the patient’s mother, but no other family members (including his child), shows signs of CMC. This points toward an autosomal-dominant inherited disease, and genetic analysis indeed revealed a novel heterozygous mutation in the IL17F gene. All other known mutations associated with CMC including Dectin-1, CARD9, IL-17A, STAT3, and STAT1 have been excluded in our patient. An association of the rhabdomyosarcoma of the mother with the CMC cannot be fully excluded. Although chronic inflammation is clearly recognized as a significant contributor in carcinogenesis, rhabdomyosarcomas have not been connected with such conditions in the past [14].
Note
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




