-
PDF
- Split View
-
Views
-
Cite
Cite
Matteo Zignol, Wayne van Gemert, Dennis Falzon, Ernesto Jaramillo, Léopold Blanc, Mario Raviglione, Modernizing Surveillance of Antituberculosis Drug Resistance: From Special Surveys to Routine Testing, Clinical Infectious Diseases, Volume 52, Issue 7, 1 April 2011, Pages 901–906, https://doi.org/10.1093/cid/cir081
Close - Share Icon Share
Abstract
Resistance to antituberculosis drugs has been documented since the 1940s, when the first medicines for tuberculosis were introduced. Since the initiation in 1994 of a global project to monitor the development of drug-resistant tuberculosis, nearly 60% of all countries in the world have implemented surveillance activities. In the past 15 years, special surveys have been the most common approach to investigate the frequency and patterns of drug-resistant tuberculosis. The major obstacle to the expansion of routine surveillance activities has been the lack of laboratory capacity needed to detect resistance. We are now in a new era for antituberculosis drug resistance surveillance due to the advent of new diagnostic tools and global commitment towards universal access to care for all patients with tuberculosis, including those with drug-resistant disease. Routine surveillance linked to patient care, which represents the best approach to monitor drug resistance, now has the possibility of becoming a reality even in resource-limited countries.
Development of resistance to anti-tuberculosis drugs was recognized shortly after the initial introduction of chemotherapy for the treatment of tuberculosis. The large majority of patients treated with streptomycin in the first Medical Research Council randomized clinical trial in the 1940s acquired resistance to that drug [1]. The spread of drug-resistant strains was soon recognized, and a survey of clinics in England in the 1950s found that >5% of patients with tuberculosis who had no history of previous treatment had strains resistant to at least 1 of the 3 major drugs in use at that time [2]. It is known today that at least 3 effective drugs used in combination are needed to treat tuberculosis while preventing development of drug resistance [3]. However, despite the introduction of combination regimens throughout the world many years ago, the presence of drug resistance has been progressively documented from an ever wider geographical area [4]. Recent estimates by the World Health Organization (WHO) suggest that nearly half million cases of multidrug-resistant tuberculosis (MDR-TB, defined as tuberculosis caused by strains of Mycobacterium tuberculosis that are resistant to at least isoniazid and rifampicin, the 2 most powerful first-line anti-TB drugs) emerged globally in 2008 [5].
Measuring the magnitude of drug resistance, particularly that of MDR-TB and extensively drug-resistant tuberculosis (XDR-TB, defined as MDR-TB with additional resistance to a fluoroquinolone and at least 1 second-line injectable agent: [amikacin, kanamycin, and/or capreomycin]), in a patient population and monitoring epidemiological trends is critical to assess the performance of any tuberculosis control program and design effective standardized treatment regimens.
Drug resistance may be transmitted (also called primary resistance) or acquired. Primary resistance, which occurs when the infecting strain is already resistant to ≥1 antituberculosis drug at the time of its first encounter with the subject, is an indicator of transmission in the community. Acquired resistance, defined when the patient's bacterial population acquires resistance during treatment consequent to exposure to inadequate therapy, is an indication of poor patient adherence to treatment, caregiver errors in prescribing and administering drugs, poor quality of drugs, and programmatic problems, including drug stockouts [6, 7].
In this article, we describe the history of drug resistance surveillance, detail the direction into which it is moving in the era of increased commitment towards universal access to care and greater availability of diagnostics tools, and discuss the continuing challenges it faces.
ESTABLISHMENT OF A GLOBAL ANTI-TUBERCULOSIS DRUG RESISTANCE SURVEILLANCE PROJECT
History of the Project
In 1994, the Global Project on Anti-Tuberculosis Drug Resistance Surveillance was initiated by the WHO and the International Union against Tuberculosis and Lung Diseases, aiming to measure the magnitude of drug-resistant tuberculosis and to monitor trends [8]. At this time, a first set of guidelines was developed to assist national tuberculosis control programs in conducting anti-tuberculosis drug resistance surveys [9]. The guidelines were based on 3 main principles that are still essential in today's drug resistance surveillance and are described in detail elsewhere [10]: (1) data should be representative of the patients with tuberculosis in the country/geographical setting under study; (2) patients' treatment histories should be carefully obtained and available medical records reviewed, to clearly determine whether patients have or have not previously received antituberculosis drugs; and (3) laboratory methods for antituberculosis drug susceptibility testing should be selected from among those recommended by WHO, and all laboratory processes should be quality-assured in cooperation with a partner Supranational Reference Laboratory [8, 11–13].
Since 1994, five global reports on anti-tuberculosis drug resistance surveillance have been published [5, 14–17]. Drug resistance data have been systematically collected and analyzed from 114 countries worldwide (59% of all countries of the world). Of these countries, only 42 can rely on continuous surveillance systems based on routine diagnostic drug susceptibility testing of all patients. The remaining 72 countries have relied on special surveys of representative samples of patients (Figure 1). Trends in drug resistance are available for only 59 countries or subnational settings in which >1 drug-resistance survey or surveillance procedure was conducted during 1994–2009. Given the limited availability of susceptibility testing to second-line antituberculosis drugs worldwide, a large number of resource-limited countries do not have yet the laboratory capacity to diagnose XDR-TB, which has been identified in 68 countries thus far (Figure 2).
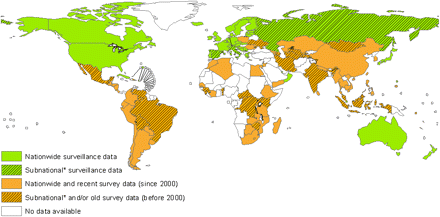
Characteristics of available first-line antituberculosis drug resistance data, 1994–2010.
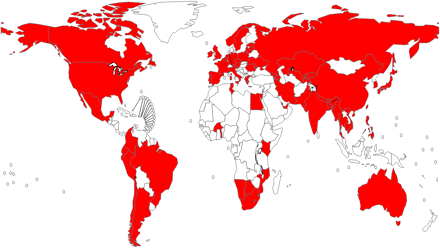
Countries that reported at least one case of extremely drug-resistant tuberculosis (XDR-TB) by end 2010.
Methods of the Project
Since the beginning of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance, 2 main mechanisms to measure drug resistance have been used: the organization of special surveys (surveys are defined as discrete studies measuring drug resistance among a specially-designed sample of tuberculosis cases representative of an entire population of TB cases) on selected samples of patients, and the establishment of a surveillance system based on routine drug susceptibility testing of all patients.
Over the past 15 years, special surveys have been the most popular approach to monitor drug resistance. Although surveys face several limitations as described below, in the absence of a feasible alternative that could provide an equivalent amount of information, they are still used in resource-constrained settings with limited laboratory capacity to routinely monitor drug resistance [6].
Limitations of Surveys.
Surveys face a number of limitations. First, they may be limited in their representativeness and may possibly underestimate the true magnitude of primary and acquired resistance, particularly in settings where concurrent tuberculosis and human immunodeficiency virus (HIV) infection is frequent or where patients with tuberculosis are commonly treated by private health providers [18]. Second, surveys are logistically complex and demanding, taking considerable amounts of human and financial resources of the national tuberculosis control program and reference laboratory during the planning, implementation, and analysis phases of a study [19]. Third, surveys often are not able to monitor epidemiological trends, because frequencies of drug resistance among patient populations usually do not change rapidly, and only small differences would be expected between surveys conducted a few years apart. The smaller the difference in frequencies, the larger the sample size would be needed to detect a statistically significant difference; in most circumstances, this means that excessively large sample sizes would be needed. Fourth, surveys are not designed to detect localized outbreaks, which could go completely unrecognized even during the course of a study if the outbreak site was not among those selected for patient enrollment. A different surveillance approach is needed to capture the heterogeneity of drug resistance at the local level and avoid over- or underestimating the magnitude of drug resistance by random inclusion or exclusion of outbreaks. Finally, the precision of survey results is usually suboptimal in countries with relatively low frequencies of MDR-TB. Sample sizes for surveys are calculated to achieve a targeted precision for a predefined estimated proportion of MDR-TB; the value of such a precision is generally recommended to be no more than 20% of the estimated proportion [6]. In settings with low frequencies of MDR-TB, a high precision can not be reached without capturing a sample that would be so large as to make the survey unfeasible.
Sentinel surveillance systems represent a useful interim approach for countries that intend to establish countrywide routine drug susceptibility testing but that lack necessary health care resources [6]. Under sentinel surveillance, specific laboratory or hospital sites are selected to routinely perform drug susceptibility testing on all notified tuberculosis cases. Ideally, sites are selected that provide geographical variability and minimize bias. As laboratory capacity is enhanced, more diagnostic sites can progressively join the sentinel system until routine surveillance in all sites is achieved countrywide.
The only option to reduce bias and to accurately measure the magnitude of drug resistance and monitor its trends is through the establishment of routine surveillance, which implies systematic ongoing collection, collation, and analysis of data for public health purposes, as reiterated by the World Health Assembly [20, 21].
MOVING TOWARDS ROUTINE ANTITUBERCULOSIS DRUG RESISTANCE SURVEILLANCE
In the past 15 years, surveys and surveillance have been largely relying on culture and drug susceptibility testing methods based on solid media, which are associated with a very long turn-around times for results (at least 3–4 months) and enormous workload for laboratory personnel. We are now in a new era for tuberculosis and MDR-TB diagnosis resulting from the advent of technological advances that make it possible to detect tuberculosis and rifampicin resistance much more rapidly. For example, line probe assays cut down the time of diagnosis of rifampicin resistance, which acts as a proxy for MDR-TB in most settings, to 2 days [22]. This technology can be directly applied on smear-positive sputum samples stored in ethanol, for which it performs similarly to phenotypic drug susceptibility testing to correctly identify resistance to rifampicin. It requires less laboratory infrastructure and workload and allows for safer transportation of specimens and simplified survey logistics [23, 24].
The time needed for diagnosis of tuberculosis and rifampicin resistance has been cut down even further to 2 h by a more recent diagnostic tool, Xpert MTB/RIF [25]. This technology is almost completely automated and very simple to use, and it requires little training and minimal biosafety measures. It has not yet been tested on specimens preserved in alcohol, which would further simplify specimens' transportation and logistics, but—very importantly—this technique has high sensitivity with smear-negative specimens. With this tool, it will be feasible to study frequencies of drug resistance in patients with smear-negative specimens, such as children, HIV-positive patients, and others with paucibacillary forms of tuberculosis. In the past 15 years, patients with smear-negative specimens have been excluded from surveys to avoid excessive workload in the laboratories and complex logistics, given that the culture yield in this group is relatively low, compared with that for smear-positive cases [26]. More experience should be gained to determine the best role of this new technology in the diagnostic algorithm for tuberculosis and MDR-TB as well as in surveillance activities.
The WHO and the Global Laboratory Initiative are committed to support the expansion of new and rapid tuberculosis diagnostic technologies in 27 countries over the next 5 years through EXPAND-TB, an extensive laboratory capacity–building initiative [27]. This initiative will greatly enhance the possibility for countries to diagnose MDR-TB and XDR-TB using the most up-to-date technologies. Although the aim is to improve diagnosis and clinical care by expanding access to drug susceptibility testing, a large amount of population-level drug resistance data will also be generated that, if properly collected and analyzed, could be used by national TB control programs for surveillance purposes.
Access to routine molecular drug susceptibility tests will be initially prioritized among patients at higher risk of carrying drug-resistant strains, such as persons with history of tuberculosis treatment [28]. For this reason, special surveys or sentinel surveillance will still have a role for several years to measure the magnitude of drug resistance in patients not at high risk of drug resistance, including those who have never previously been treated for tuberculosis.
The use of different surveillance approaches, such as Lot Quality Assurance Sampling techniques that are already used to investigate drug resistance in HIV [29], or the development of high-throughput single Nnucleotide Ppolymorphism–based surveillance technologies could represent alternatives to special surveys or sentinel surveillance to improve our understanding of drug resistance, particularly for persons who have never previously been treated for tuberculosis, until routine surveillance becomes available everywhere.
In addition to greater availability of diagnostic tests, we are in an era of enhanced commitment toward universal access to treatment for all patients with tuberculosis, including those who have been previously treated. These patients historically have received less attention by country programs and the international community, given that they usually have more serious forms of disease that often are more difficult and expensive to diagnose and cure [30]. Previously treated patients constitute a very heterogeneous group composed of patients who experience relapse after receiving successful treatment, those who return after default, and those who start receiving a re-treatment regimen after having experienced previous treatment failure, as well as other patients (ie, those who do not fit into one of the aforementioned categories), such as those who received unknown or nonstandardized treatment regimens [28]. Frequencies of MDR-TB vary substantially between the different categories of previously treated persons; overall, the frequency is >60% in some former Soviet Union settings. It is evident that no single standardized re-treatment regimen would be effective for all previously treated persons with tuberculosis, and understanding the magnitude and patterns of resistance in each of the categories mentioned above is crucial to guide the choice of treatment [28, 31].
Routine surveillance of drug resistance allows for proper treatment of all patients with tuberculosis and is critical for accurate planning, budgeting, and monitoring of tuberculosis and MDR-TB control activities. This is relevant for all countries, including those with particularly limited resources and where the management of MDR-TB has to compete with other pressing health needs in a context of increasing financial constraints. Even if the majority of patients with tuberculosis globally do not have drug-resistant infection, the epidemiological situation may change dramatically in a few years time if drug-resistant cases are not adequately managed [32]. The prospects of a successful outcome of treatment for patients with MDR-TB are much lower than for those with drug-susceptible disease, with only 60% of treatment success reached globally, compared with 86% in the group of new smear-positive patients [5, 33]. These facts make the surveillance of drug resistance today and in the coming years even more pertinent than it was when the Global Project on Anti-Tuberculosis Drug Resistance Surveillance was launched.
CONTINUING CHALLENGES IN DRUG RESISTANCE SURVEILLANCE AND POSSIBLE SOLUTIONS
Whether performing routine surveillance, sentinel surveillance, or special surveys to monitor antituberculosis drug resistance, 3 major challenges are encountered: the need to incorporate HIV testing, the need to expand surveillance efforts to all health care providers, and the need to assure appropriate care to all those found with drug-resistant disease.
Outbreaks of drug-resistant tuberculosis among people living with HIV infection have been widely documented in nosocomial and other congregate settings. If not rapidly diagnosed and treated, MDR-TB and XDR-TB can indeed lead to very high case-fatality rates among persons with concurrent HIV infection [34]. Unfortunately little information is available on the association of HIV infection and drug-resistant tuberculosis at the population level [35, 36]. In recent years, national tuberculosis control programs have experienced great difficulties in incorporating HIV testing in drug-resistant tuberculosis surveillance activities, because this requires strong collaboration and coordination between tuberculosis and HIV control programs. Knowing the relationship between HIV and MDR-TB epidemics at population level can help in the identification of high-risk groups and in the planning of effective public health control measures. Inclusion of HIV testing should therefore be encouraged during antituberculosis drug resistance surveillance activities.
In many regions of the world, patients with tuberculosis symptoms seek care from private health care providers before approaching the services of the national tuberculosis control program [37]. In these areas, private providers are often perceived to deliver better services and treatment options. In reality, this may not be the case, because it is known that the majority of patients seeking re-treatment in the public sector had been unsuccessfully treated in the first instance by private providers [38]. Drug-resistance surveys are usually conducted only in the public sector for logistic and organizational reasons. Therefore, in countries with a large private health care sector, these studies may not be able to accurately capture the real magnitude of the problem. In such settings, it has been suggested that drug-resistance surveys in the public sector should be complemented by small surveys in the private sector to determine the existence and direction of any bias introduced by excluding private providers from surveys [18]. Additionally, public-private mix initiatives can serve as platforms to gradually involve private laboratories and practitioners in surveillance activities.
In the early years of drug-resistance surveillance, second-line drugs for the treatment of MDR-TB generally were completely unavailable in resource-limited countries. Surveys were conducted to estimate the magnitude of the problem and helped to advocate for more resources to diagnose and treat patients with drug-resistant tuberculosis. The disparity between the number of patients with MDR-TB receiving second-line treatment and those awaiting diagnosis and/or treatment is still enormous, with only 12% of those in need estimated to have received treatment in 2009 [33]. Globally the number of centers capable of providing care to patients with MDR-TB according to international standards has increased, and presently, most countries have at least 1 referral center for treatment with second-line drugs [39]. In this changing environment, implementation of surveys and the scale-up of surveillance systems for drug resistance should proceed in parallel with the scale-up of MDR-TB treatment services. This will ensure appropriate treatment with second-line antituberculosis drugs for all persons in whom drug-resistance tuberculosis is detected [6, 40].
CONCLUSIONS
Surveillance efforts have been monitoring national and regional trends in drug-resistant tuberculosis since the mid-1990s. As a result of the recent commitment towards provision of universal access to care and the availability of new diagnostic tools, we are now in a new era for anti-tuberculosis drug resistance surveillance. For the first time in the history of tuberculosis control, technologies to rapidly detect tuberculosis and rifampicin resistance have become a reality, allowing for surveillance activities with less demanding laboratory infrastructure and capacity. Although neither rapid diagnosis nor treatment for MDR-TB is currently widely available, there is an unprecedented level of political commitment and resource mobilization to accelerate access in the coming few years, changing the way in which drug resistance is monitored. Routine surveillance linked to patient care will gradually replace special surveys that, until now, have been the main approach to monitor drug resistance in resource-limited countries. Because resource-limited programs are making efforts to establish routine drug susceptibility testing of all patients with histories of previous treatment, a new opportunity has been made available to obtain surveillance data for use in strengthening TB control program planning and performance.
M.Z., W.V.G., D.F., E.J., L.P., and M.R. are staff members of the World Health Organization (WHO). The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions or policies of WHO.
Financial support. World Health Organization.
Potential conflicts of interest. All authors: no conflicts.




