-
PDF
- Split View
-
Views
-
Cite
Cite
Allan R. Tunkel, Carol A. Glaser, Karen C. Bloch, James J. Sejvar, Christina M. Marra, Karen L. Roos, Barry J. Hartman, Sheldon L. Kaplan, W. Michael Scheld, Richard J. Whitley, The Management of Encephalitis: Clinical Practice Guidelines by the Infectious Diseases Society of America, Clinical Infectious Diseases, Volume 47, Issue 3, 1 August 2008, Pages 303–327, https://doi.org/10.1086/589747
Close - Share Icon Share
Abstract
Guidelines for the diagnosis and treatment of patients with encephalitis were prepared by an Expert Panel of the Infectious Diseases Society of America. The guidelines are intended for use by health care providers who care for patients with encephalitis. The guideline includes data on the epidemiology, clinical features, diagnosis, and treatment of many viral, bacterial, fungal, protozoal, and helminthic etiologies of encephalitis and provides information on when specific etiologic agents should be considered in individual patients with encephalitis.
Executive Summary
Encephalitis is defined by the presence of an inflammatory process of the brain in association with clinical evidence of neurologic dysfunction. Of the pathogens reported to cause encephalitis, the majority are viruses. However, despite extensive testing, the etiology of encephalitis remains unknown in most patients. Another major challenge for patients with encephalitis is to determine the relevance of an infectious agent identified outside of the CNS; these agents may play a role in the neurologic manifestations of illness but not necessarily by directly invading the CNS. In addition, it is important to distinguish between infectious encephalitis and postinfectious or postimmunization encephalitis or encephalomyelitis (e.g., acute disseminated encephalomyelitis [ADEM]), which may be mediated by an immunologic response to an antecedent antigenic stimulus from an infecting microorganism or immunization. Noninfectious CNS diseases (e.g., vasculitis, collagen vascular disorders, and paraneoplastic syndromes) can have clinical presentations similar to those of infectious causes of encephalitis and should also be considered in the differential diagnosis.
In the approach to the patient with encephalitis, an attempt should be made to establish an etiologic diagnosis. Although there are no definitive effective treatments in many cases of encephalitis, identification of a specific agent may be important for prognosis, potential prophylaxis, counseling of patients and family members, and public health interventions. Epidemiologic clues that may help in directing the investigation for an etiologic diagnosis include season of the year, geographic locale, prevalence of disease in the local community, travel history, recreational activities, occupational exposure, insect contact, animal contact, vaccination history, and immune status of the patient. Various clinical clues may also be helpful to physicians in considering specific etiologies.
The diagnostic evaluation of a patient who presents with encephalitis needs to be individualized and should be guided by epidemiologic and clinical clues and laboratory data; these generally include cultures and analysis (i.e., antigen detection and nucleic acid amplification tests, such as PCR) of body fluid specimens, biopsy of specific tissues (with culture, antigen detection, PCR, and histopathologic evaluation) outside the CNS, and serologic testing (for specific IgM and acute- and convalescent-phase IgG antibody titers). MRI of the brain should be performed in all patients, with CT used only if MRI is unavailable, unreliable, or cannot be performed; neuroimaging findings may also suggest disease caused by specific etiologic agents. CSF analysis is critical, unless contraindicated, and may be quite helpful in establishing an etiology. Detection of specific viral IgM antibodies in CSF specimens obtained from patients with encephalitis caused by numerous viruses is considered to be diagnostic of neuroinvasive disease. CSF cultures are generally of limited value in the determination of the viral causes of encephalitis but are very important in the diagnosis of bacterial and fungal infections. The utility of nucleic acid amplification testing (e.g., PCR) of CSF specimens has greatly increased the ability to diagnose infections of the CNS, especially viral infections caused by the herpesviruses. At present, brain biopsy is rarely performed to establish the etiology of encephalitis, but it may play a role in some patients with encephalitis of unknown etiology whose conditions deteriorate despite treatment with acyclovir.
Despite the wide range of viruses that have been reported to cause encephalitis, specific antiviral therapy is generally limited to infections caused by the herpesviruses—specifically, herpes simplex virus—and HIV. Acyclovir treatment should be initiated in all patients with suspected encephalitis, pending results of diagnostic studies. During the appropriate season, patients who present with clinical clues suggestive of rickettsial or ehrlichial infection should be treated empirically with doxycycline. Empirical therapy for acute bacterial meningitis should also be initiated if clinically indicated. In patients with acute disseminated encephalomyelitis, corticosteroids are recommended; plasma exchange should be considered in patients who do not respond to this treatment.
Recommendation categories are shown in table 1.
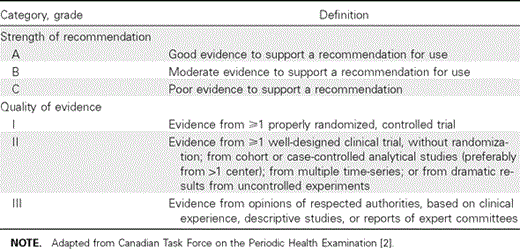
Infectious Diseases Society of America–US Public Health Service Grading System for ranking recommendations in clinical guidelines.
Etiology
1. Epidemiologic clues and assessment of risk factors to identify potential etiologic agents should be sought in all patients with encephalitis (table 2) (A-III).
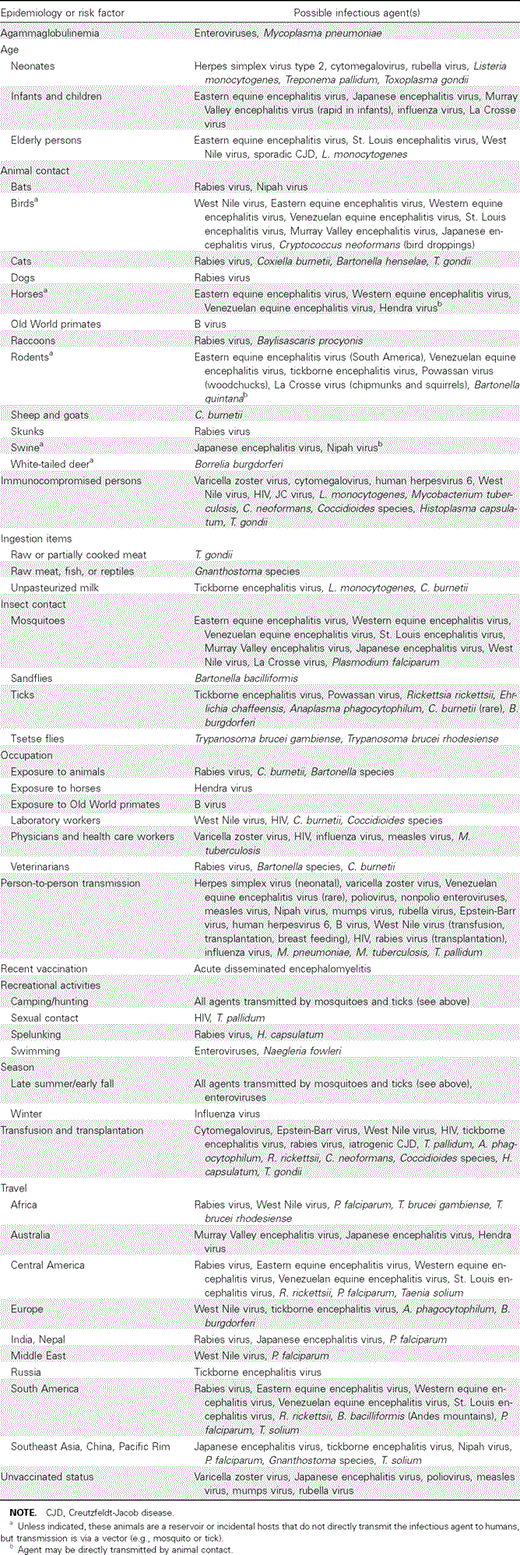
Possible etiologic agents of encephalitis based on epidemiology and risk factors.
2. Clinical clues (general and specific neurologic findings) may be helpful in suggesting certain causative agents in patients with encephalitis (table 3) (B-III).
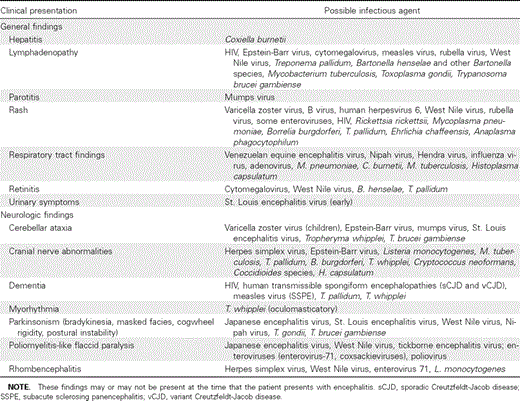
Possible etiologic agents of encephalitis based on clinical findings.
3. In patients with encephalitis and a history of recent infectious illness or vaccination, the diagnosis of ADEM should be considered (B-III).
Diagnosis
4. Specific diagnostic studies should be performed for the majority of patients who present with encephalitis (table 4) (A-III).
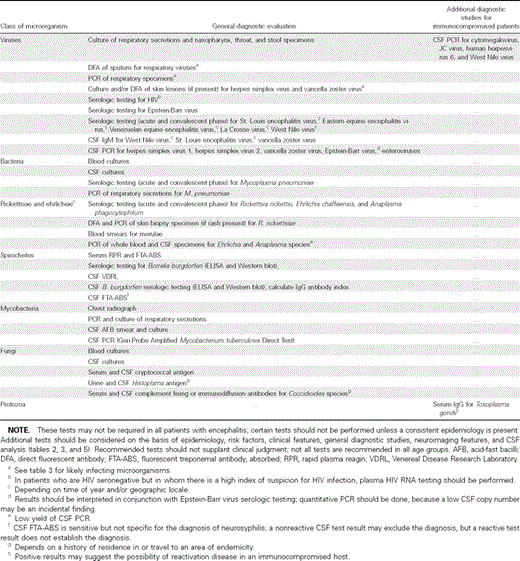
Diagnostic evaluation to consider in determining the microbial etiology in patients with encephalitis (A-III).
5. Additional diagnostic studies should be performed for patients with encephalitis on the basis of specific epidemiologic and clinical clues (tables table 2, 3, and 5) (A-III).
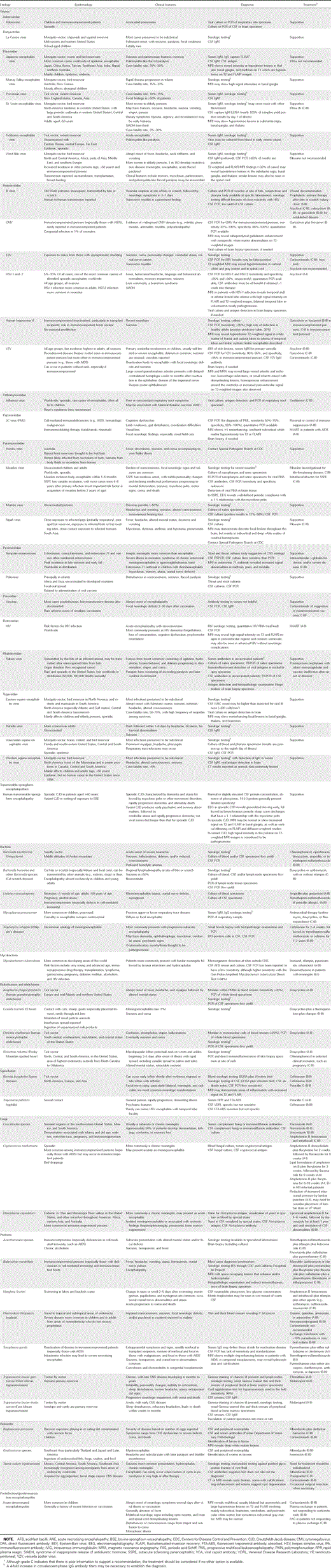
Epidemiology, clinical features, diagnosis, and treatment of selected causes of encephalitis.
Diagnostic Studies outside the CNS
6. Cultures of body fluid specimens (e.g., from blood, stool, nasopharynx, or sputum), if clinical and epidemiologic clues are suggestive, should be performed in an attempt to identify various viral, bacterial, and fungal etiologies of encephalitis (table 5) (B-III); positive results do not necessarily indicate that the isolated microorganism is the etiology of encephalitis and must be interpreted in the context of the appropriate epidemiologic findings, clinical findings, and other diagnostic study results.
7. Biopsy of specific tissues for culture, antigen detection, nucleic acid amplification tests (such as PCR), and histopathologic examination should be performed in an attempt to establish an etiologic diagnosis of encephalitis (table 5) (A-III).
8. Certain causes of encephalitis may be diagnosed by detection of IgM antibodies in serum (table 5) (A-III).
9. Although acute- and convalescent-phase serum samples are generally not useful in establishing the etiology during the acute presentation in a patient with encephalitis, they may be useful for the retrospective diagnosis of an infectious agent (table 5) (B-III).
10. Nucleic acid amplification tests (such as PCR) of body fluids outside of the CNS may be helpful in establishing the etiology in some patients with encephalitis (table 5) (B-III).
11. MRI is the most sensitive neuroimaging test to evaluate patients with encephalitis (A-I).
12. CT, with and without contrast enhancement, should be used to evaluate patients with encephalitis if MRI is unavailable, impractical, or cannot be performed (B-III).
13. Fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET) scanning is not routinely recommended for patients with encephalitis.
14. Electroencephalography (EEG) is rarely helpful in establishing an etiology in patients with encephalitis, but it has a role in identifying patients with nonconvulsive seizure activity who are confused, obtunded, or comatose and should be performed in all patients with encephalitis (A-III).
15. CSF analysis is essential (unless contraindicated) in all patients with encephalitis (A-III).
Diagnostic Studies in the CNS
16. For certain viral agents, the presence of virus-specific IgM in CSF specimens may be indicative of CNS disease caused by that pathogen (table 5) (A-III).
17. Nucleic acid amplification tests (such as PCR) should be performed on CSF specimens to identify certain etiologic agents in patients with encephalitis (table 5) (A-III). Although a positive test result is helpful in diagnosing infection caused by a specific pathogen, a negative result cannot be used as definitive evidence against the diagnosis.
18. Herpes simplex PCR should be performed on all CSF specimens in patients with encephalitis (A-III). In patients with encephalitis who have a negative herpes simplex PCR result, consideration should be given to repeating the test 3–7 days later in those with a compatible clinical syndrome or temporal lobe localization on neuroimaging (B-III).
19. Viral cultures of CSF specimens are of limited value in patients with encephalitis and are not routinely recommended.
20. Brain biopsy should not be routinely used in patients with encephalitis but should be considered in patients with encephalitis of unknown etiology whose condition deteriorates despite treatment with acyclovir (B-III).
Treatment
Empirical Therapy
21. Acyclovir should be initiated in all patients with suspected encephalitis, pending results of diagnostic studies (A-III).
22. Other empirical antimicrobial agents should be initiated on the basis of specific epidemiologic or clinical factors (tables 2 and 3), including appropriate therapy for presumed bacterial meningitis, if clinically indicated (A-III).
23. In patients with clinical clues suggestive of rickettsial or ehrlichial infection during the appropriate season, doxycycline should be added to empirical treatment regimens (A-III).
Specific Therapy
Viruses
24. Herpes simplex virus: acyclovir is recommended (A-I).
25. Varicella-zoster virus: acyclovir is recommended (B-III); ganciclovir can be considered an alternative (C-III); adjunctive corticosteroids can be considered (C-III).
26. Cytomegalovirus: the combination of ganciclovir plus foscarnet is recommended (B-III); cidofovir is not recommended, because its ability to penetrate the blood-brain barrier has been poorly studied.
27. Epstein-Barr virus: acyclovir is not recommended; the use of corticosteroids may be beneficial (C-III), but the potential risks must be weighed against the benefits.
28. Human herpesvirus 6: ganciclovir or foscarnet should be used in immunocompromised patients (B-III); use of these agents in immunocompetent patients can be considered (C-III), but there are not good data on their effectiveness.
29. B virus: valacyclovir is recommended (B-III); alternative agents are ganciclovir (B-III) and acyclovir (C-III).
30. Influenza virus: oseltamivir can be considered (C-III).
31. Measles virus: ribavirin can be considered (C-III); intrathecal ribavirin can be considered in patients with subacute sclerosing panencephalitis (C-III).
32. Nipah virus: ribavirin can be considered (C-III).
33. West Nile virus: ribavirin is not recommended.
34. Japanese encephalitis virus: IFN-α is not recommended.
35. St. Louis encephalitis virus: IFN-2α can be considered (C-III).
36. HIV: HAART is recommended (A-II).
37. JC virus: reversal of immunosuppression (A-III)—or HAART in HIV-infected patients (A-II)—is recommended.
Bacteria
38. Bartonella bacilliformis: chloramphenicol, ciprofloxacin, doxycycline, ampicillin, or trimethoprim-sulfamethoxazole is recommended (B-III).
39. Bartonella henselae: doxycycline or azithromycin, with or without rifampin, can be considered (C-III).
40. Listeria monocytogenes: ampicillin plus gentamicin is recommended (A-III); trimethoprim-sulfamethoxazole is an alternative in the penicillin-allergic patient (A-III).
41. Mycoplasma pneumoniae: antimicrobial therapy (azithromycin, doxycycline, or a fluoroquinolone) can be considered (C-III).
42. Tropheryma whipplei: ceftriaxone, followed by either trimethoprim-sulfamethoxazole or cefixime, is recommended (B-III).
Mycobacteria
43. Mycobacterium tuberculosis: 4-drug antituberculous therapy should be initiated (A-III); adjunctive dexamethasone should be added in patients with meningitis (B-I).
Rickettsioses and ehrlichioses
44. Anaplasma phagocytophilum: doxycycline is recommended (A-III).
45. Ehrlichia chaffeensis: doxycycline is recommended (A-II).
46. Rickettsia rickettsii: doxycycline is recommended (A-II); chloramphenicol can be considered an alternative in selected clinical scenarios, such as pregnancy (C-III).
47. Coxiella burnetii: doxycycline plus a fluoroquinolone plus rifampin is recommended (B-III).
Spirochetes
48. Borrelia burgdorferi: ceftriaxone, cefotaxime, or penicillin G is recommended (B-II).
49. Treponema pallidum: penicillin G is recommended (A-II); ceftriaxone is an alternative (B-III).
Fungi
50. Coccidioides species: fluconazole is recommended (A-II); alternatives are itraconazole (B-II), voriconazole (B-III), and amphotericin B (intravenous and intrathecal) (C-III).
51. Cryptococcus neoformans: initial treatment with amphotericin B deoxycholate plus flucytosine (A-I) or a lipid formulation of amphotericin B plus flucytosine (A-II) is recommended.
52. Histoplasma capsulatum: liposomal amphotericin B followed by itraconazole is recommended (B-III).
Protozoa
53. Acanthamoeba: trimethoprim-sulfamethoxazole plus rifampin plus ketoconazole (C-III) or fluconazole plus sulfadiazine plus pyrimethamine (C-III) can be considered.
54. Balamuthia mandrillaris: pentamidine, combined with a macrolide (azithromycin or clarithromycin), fluconazole, sulfadiazine, flucytosine, and a phenothiazine can be considered (C-III).
55. Naegleria fowleri: amphotericin B (intravenous and intrathecal) and rifampin, combined with other agents, can be considered (C-III).
56. Plasmodium falciparum: quinine, quinidine, or artemether is recommended (A-III); atovaquone-proguanil is an alternative (B-III); exchange transfusion is recommended for patients with >10% parasitemia or cerebral malaria (B-III); corticosteroids are not recommended.
57. Toxoplasma gondii: pyrimethamine plus either sulfadiazine or clindamycin is recommended (A-I); trimethoprim-sulfamethoxazole alone (B-I) and pyrimethamine plus either atovaquone, clarithromycin, azithromycin, or dapsone (B-III) are alternatives.
58. Trypanosoma brucei gambiense: eflornithine is recommended (A-II); melarsoprol is an alternative (A-II).
59. Trypanosoma brucei rhodesiense: melarsoprol is recommended (A-II).
Helminths
60. Baylisascaris procyonis: albendazole plus diethycarbamazine can be considered (C-III); adjunctive corticosteroids should also be considered (B-III).
61. Gnathostoma species: albendazole (B-III) or ivermectin (B-III) is recommended.
62. Taenia solium: need for treatment should be individualized; albendazole and corticosteroids are recommended (B-III); praziquantel can be considered as an alternative (C-II).
Postinfectious/postvaccination status
63. Acute disseminated encephalomyelitis: high-dose corticosteroids are recommended (B-III); alternatives include plasma exchange (B-III) and intravenous immunoglobulin (C-III).
Introduction
Encephalitis is defined by the presence of an inflammatory process of the brain in association with clinical evidence of neurologic dysfunction [1]. The syndrome of acute encephalitis shares many clinical features with acute meningitis, such that patients with either syndrome may present with fever, headache, and altered level of consciousness. Although mental status changes early in the disease course are generally more common in patients with encephalitis, this finding does not reliably differentiate patients with encephalitis from those with bacterial meningitis, and it is important to consider both diagnoses at presentation. Other findings in patients with encephalitis include acute cognitive dysfunction, behavioral changes, focal neurologic signs, and seizures. In most cases, there is some concomitant meningeal inflammation, in addition to the encephalitic component—a condition commonly referred to as “meningoencephalitis.”
It is important to try to distinguish between infectious encephalitis and postinfectious or postimmunization encephalitis or encephalomyelitis; these latter syndromes are presumed to be mediated by an immunologic response to an antecedent antigenic stimulus provided by the infecting microorganism or immunization or to other antigens revealed as part of the initial infection or vaccination. One example of this condition is termed “ADEM” and is more commonly seen in children and adolescents. Distinction between acute infectious encephalitis and ADEM is important, because the management approach is different. Encephalitis should also be distinguished from encephalopathy (e.g., secondary to metabolic disturbances, hypoxia, ischemia, drugs, intoxications, organ dysfunction, or systemic infections), which is defined by a disruption of brain function in the absence of a direct inflammatory process in the brain parenchyma.
The objective of this guideline is to provide clinicians with evidence-based recommendations in the approach to patients with encephalitis. Recommendation categories are shown in table 1 [2]. The initial treatment approach to the patient with suspected encephalitis includes early recognition of the clinical syndrome, appropriate diagnostic evaluation (including neuroimaging, serologic testing, and CSF analysis, which often includes serologic and molecular studies), and emergent administration of certain antimicrobial agents (see below). Unfortunately, despite extensive testing to identify an etiologic agent, most cases of presumed infectious encephalitis remain unexplained. Another major challenge in patients with encephalitis is to determine the significance of an infectious agent found outside the CNS, usually identified by serologic testing or culture of a non-CNS site in the clinical context of encephalitis; these agents (e.g., hepatitis C virus, rotavirus, M. pneumoniae, Chlamydia species, and respiratory syncytial virus) may play a role in the CNS manifestations of illness, but not necessarily by directly invading the CNS, or they may be present and unrelated to the encephalitis.
The Guidelines Panel addressed the following clinical questions in patients with suspected or proven encephalitis:
1. What are the epidemiologic and clinical clues that suggest a specific etiology of encephalitis?
2. What general diagnostic studies outside the CNS should be performed in patients with suspected encephalitis?
3. What neurodiagnostic tests should be performed in patients with encephalitis?
4. What tests of CSF and brain tissue specimens can help in establishing the etiology of encephalitis?
5. What specific empirical antimicrobial agents(s) should be used in patients with suspected encephalitis?
6. Once the etiology of encephalitis is determined, what specific treatment regimen should be administered?
Methodology
Panel composition. A panel of experts comprising infectious diseases specialists (pediatric and adult) and neurologists from North America who are experts in encephalitis was convened. The panelists had both laboratory and clinical experience with patients with encephalitis and other CNS infections.
Literature review and analysis. For the guideline, the panel reviewed the literature on the diagnosis and management of encephalitis through articles obtained via searches of the Medline database that were published since 1996, as well as articles in published reviews and authoritative book chapters. Computerized searches included only review of articles published in the English language and were limited to human-only studies. In evaluating the evidence in regard to diagnosis and management of encephalitis, the panel followed the process used in the development of other guidelines published by the Infectious Diseases Society of America (table 1). Given the lack of randomized, controlled data on the diagnosis and management of encephalitis, many recommendations were developed from case reports and small series, combined with the opinion of expert panel members.
Consensus development based on evidence. The development of the guideline was done initially via drafts prepared by the panel Chair, followed by intensive review by panel members and comment via e-mail on specific aspects of the guideline. The panel met via teleconference on 2 occasions and in person at the 2006 Annual Meeting of the Infectious Diseases Society of America. Additional work on the guideline was performed via e-mail and telephone between the Chair and specific panel members. All members of the panel participated in the preparation and review of the guideline. Feedback from external peer reviewers was obtained. The guideline was reviewed and approved by the Standards and Practice Guidelines Committee and Board of Directors prior to dissemination.
Guidelines and conflicts of interest. All members of the panel complied with the Infectious Diseases Society of America policy on conflicts of interest, which requires disclosure of any financial or other interest that might by construed as constituting an actual, potential, or apparent conflict. Members of the panel were provided with the Infectious Diseases Society of America conflict of interest disclosure statement and were asked to identify ties to companies developing products that might be affected by promulgation of the guideline. Information was requested regarding employment, consultancies, stock ownership, honoraria, research funding, expert testimony, and membership in company advisory committees. The panel made decisions on a case-by-case basis as to whether an individual's role should be limited as a result of conflict. No limiting conflicts were identified.
Etiology
A wide variety of pathogens have been reported to cause encephalitis (table 5), most of which are viruses [1, 3–10]. The epidemiology of various causes of encephalitis has changed in recent years in the United States, primarily as a result of the decrease in vaccine-preventable conditions, such as measles, mumps, rubella, and varicella. In general, the most commonly identified etiologies in the United States are herpes simplex virus, West Nile virus, and the enteroviruses, followed by other herpesviruses. Although M. pneumoniae is the most common agent identified in some studies in patients with encephalitis, the significance is unclear; M. pneumoniae is generally diagnosed by serologic methods (which are inherently problematic), is not neurotropic, and is rarely detected within the CNS. In many cases of encephalitis (32%–75%), however, the etiology remains unknown, despite extensive diagnostic evaluation. In the California Encephalitis Project, an underlying cause of encephalitis was not identified in 208 (62%) of 334 patients during 1998–2000, despite extensive testing and evaluation [11]; of note, ∼10% of patients initially thought to have an infectious cause of their encephalitis ultimately received a diagnosis of a noninfectious condition. In a follow-up report of 1570 cases over a 7-year period [12], a confirmed or probable etiologic agent was identified for only 16% of cases of encephalitis, and an additional 13% of cases had a possible etiology identified. Of the confirmed or probable cases, 69% were viral, 20% were bacterial, 7% were prion related, 3% were parasitic, and 1% were fungal. However, it is important to note that the failure to identify an etiologic agent in many of these cases may be related to referral bias towards diagnostically challenging cases, as well as lack of access to appropriate specimens and suboptimal specimen handling. In another study from Finland, the etiology of encephalitis remained undefined in as many as 64% of patients, despite extensive laboratory evaluation [13].
Although many cases of encephalitis go without an identified etiology, attempts at identification of a specific etiologic agent are important for prognosis, potential prophylaxis, counseling of patients and families, and public health interventions. For the majority of patients who present with encephalitis, we recommend that specific diagnostic studies be performed (table 4). Additional diagnostic studies should be performed on the basis of specific epidemiologic and clinical clues (tables 2 and 3).
It is beyond the scope of this guideline to review, in detail, every specific infectious etiology of encephalitis. Instead, the text of the guideline will concentrate on those etiologies that are most common and those with particular public health significance that should be considered in patients who present with a clinical syndrome consistent with encephalitis. The tables also contain the specifics of other etiologic agents that clinicians should consider in individual patients who present with encephalitis.
What Are the Epidemiologic and Clinical Clues that Suggest a Specific Etiology of Encephalitis?
Evidence summary. Epidemiologic clues that may help in establishing the etiologic diagnosis of encephalitis include season of the year, geographic locale, prevalence of disease in the local community, travel history, recreational activities, occupational exposures, insect or animal contacts, vaccination history, and the immune status of the patient (table 2) [1, 3–10, 14–36]. Although the clinical features of many of these infectious agents overlap, certain pathogens may be associated with distinct neurologic features based on tropism for specific areas in the CNS. In addition, other systemic physical examination findings (e.g., rash or upper respiratory or pulmonary findings) may also suggest certain etiologic agents (table 3). Epidemiologic and clinical clues that may suggest a particular etiologic diagnosis often cannot be elicited from the patient, who may be confused, disoriented, obtunded, or comatose; therefore, relevant information may need to be provided by relatives or friends.
In patients with encephalitis and a history of recent infectious illness or vaccination, the diagnosis of ADEM should be considered [1, 37–39]. ADEM is a monophasic illness thought to be an autoimmune response to a preceding antigenic challenge; a febrile illness or immunization often precedes the neurologic syndrome and varies according to the precipitant (e.g., it typically occurs 1–14 days after vaccination or ⩽1 week after the appearance of a rash in an exanthematous illness). A number of different viral infections have been associated with ADEM, including measles, mumps, rubella, varicella zoster, Epstein-Barr virus infection, cytomegalovirus infection, herpes simplex, hepatitis A, influenza, and enterovirus infections. Immunizations temporally associated with this syndrome include vaccines against anthrax and against Japanese encephalitis, yellow fever, measles, influenza, smallpox, and rabies viruses; however, a causal association in the context of these immunizations is difficult to establish. Fever is usually absent at the onset of neurologic illness, and patients present with multifocal neurologic signs affecting the optic nerves, brain, and spinal cord [37, 38]. The disturbance of consciousness ranges from stupor and confusion to coma.
Diagnosis
Certain diagnostic studies should be performed or considered in patients who present with encephalitis (table 4), in hopes of identifying treatable infectious etiologies; additional studies are based on specific epidemiologic and clinical findings (tables 2 and 3). The diagnostic evaluation in patients with encephalitis should include a complete blood cell count, tests of renal and hepatic function, coagulation studies, and chest radiography, although results of these studies are generally nonspecific. Neuroimaging studies and CSF analyses are critical to document CNS pathology (table 5). Although there may be no definitive treatment for many causes of encephalitis, establishing the diagnosis may still affect management (e.g., discontinuation of therapy that is not necessary). More details, with discussion of available evidence based on specific etiologic agents, are shown in table 5 and discussed in the text below [1, 3–10, 14–36, 40–45].
What General Diagnostic Studies Outside the CNS Should Be Performed in Patients with Suspected Encephalitis?Evidence Summary
Cultures. Cultures of specimens of body fluids other than CSF may be useful in establishing the etiologic diagnosis in selected patients with encephalitis. All patients with encephalitis should undergo blood culturing to identify potential bacterial and fungal etiologies, although positive culture results may be indicative of encephalopathy secondary to systemic infection rather than encephalitis. Specific clinical findings should also direct other sites for culture (e.g., stool, nasopharynx, and sputum). In patients with varicella or herpes zoster, the etiology may be determined by scrapings from the base of active vesicles and testing by direct fluorescent antibody to identify viral antigen. However, a positive result for a vesicular fluid sample does not necessarily indicate that this is the etiology of encephalitis, because varicella zoster virus may be reactivated in the context of CNS disease caused by other agents; furthermore, a negative result cannot be used to exclude the diagnosis. For most arboviral infections in humans, viremia is of such low magnitude and brief duration that virus is generally undetectable by the time the patient seeks medical attention.
Biopsy. Biopsy of specific tissues with culture, antigen detection, nucleic acid amplification testing (e.g., PCR), and histopathologic examination of specimens may aid in the etiologic diagnosis. Biopsy of skin lesions should be performed; for example, biopsy with direct fluorescent antibody of maculopapular or petechial lesions may identify R. rickettsii, the etiologic agent of Rocky Mountain spotted fever. In patients with rabies, full-thickness skin biopsy from the nape of the neck with staining of sensory neurons using immunofluorescent antibodies has a sensitivity of 50%–94% and a specificity that approaches 100%; the diagnosis of rabies can also be established by identification of rabies virus in the brain (via immunofluorescence or histopathologic examination) of the infecting animal if the animal is available for testing.
Serologic testing. Some causes of encephalitis may be diagnosed by detection of IgM antibodies in serum (e.g., primary varicella virus and many arboviruses). In recent years, IgM and IgG capture ELISAs have become the most useful and widely used tests for the diagnosis of arboviral encephalitis, although there may be cross-reactivity, particularly among the flaviviruses (e.g., Japanese encephalitis, St. Louis encephalitis, and West Nile viruses). Plaque-reduction neutralization testing is recommended in areas where multiple flaviviridae cocirculate or in patients who have received previous vaccination against a related arbovirus (e.g., prior Japanese encephalitis or yellow fever immunization in the setting of suspected flavivirus encephalitis). Antibodies (ELISA and confirmatory Western blot) to B. burgdorferi and serologic testing for Rickettsia, Ehrlichia, and Anaplasma species should be performed in all patients with encephalitis who reside in or have traveled to an area of endemicity, given that positive results would identify a treatable etiology; however, empirical therapy directed towards these latter microorganisms should never be withheld, because acute-phase serologic test results may be negative.
Because of the amount of time involved, assessment of acute- and convalescent-phase serum samples to show seroconversion to a specific pathogen is generally not useful in the decision to institute specific therapy, but this does remain a helpful tool for the retrospective diagnosis of infection with a specific agent. At the time of initial presentation, we recommend that serum specimens be stored and tested at a later time with convalescent-phase serum samples. In other diseases in which encephalitis may be a result of reactivation of previously acquired infection (e.g., toxoplasmic encephalitis in patients with AIDS), detection of serum IgG antibodies may identify persons at risk for encephalitis with a specific agent.
Nucleic acid amplification tests. PCR of biologic specimens for amplification of microbial nucleic acid from outside the CNS has been used to establish the etiology of some cases of encephalitis and should be performed in the proper clinical setting. For example, molecular testing of saliva samples may establish the diagnosis of rabies [46, 47]. In patients with human monocytotrophic ehrlichiosis (caused by E. chaffeensis) and in those with human granulocytotrophic ehrlichiosis (caused by A. phagocytophilum), the diagnostic sensitivity of PCR with whole blood samples ranges from 56% to 100% and from 54% to 86%, respectively [48–50]. PCR of lymph node tissue specimens is also useful in establishing the diagnosis of B. henselae infection [51].
What Neurodiagnostic Tests Should Be Performed in Patients with Suspected Encephalitis?Evidence Summary
Neuroimaging. CT and MRI are most frequently used to evaluate patients with encephalitis, with MRI being more sensitive and specific [52]. These studies may also be useful in excluding other conditions with a clinical presentation similar to that of encephalitis. CT (with and without intravenous contrast administration) should only be used if MRI is unavailable, impractical, or cannot be performed. Although MRI is useful for detection of early changes in encephalitis, it does not necessarily assist in differentiation of a specific etiology of encephalitis, and the findings may initially be normal or remain normal during the course of illness. Diffusion-weighted imaging is superior to conventional MRI for the detection of early signal abnormalities in viral encephalitis caused by herpes simplex virus, enterovirus 71, and West Nile virus [52].
Some characteristic neuroimaging patterns have been observed in patients with encephalitis caused by specific agents (table 5). In patients with herpes simplex encephalitis, there may be significant edema and hemorrhage in the temporal lobes, as well as hypodense areas on T1-weighted images and nonhomogeneous contrast enhancement; bilateral temporal lobe involvement is nearly pathognomonic for herpes simplex encephalitis, but this is a late development. More than 90% of patients with herpes simplex encephalitis documented by CSF PCR will have abnormalities seen on MRI [53]. In patients with encephalitis caused by flaviviruses and Eastern equine encephalitis virus, MRI may display a characteristic pattern of mixed intensity or hypodense lesions on T1-weighted images in the thalamus, basal ganglia, and midbrain; these lesions are hyperintense on T2 and fluid-attenuated inversion recovery (FLAIR) images. In patients with enterovirus 71 encephalitis, MRI may demonstrate hyperintense T2 and FLAIR lesions localized to the midbrain, pons, and medulla. Follow-up MRI may also be useful for evaluation of evolving necrosis or demyelination.
In cases of suspected ADEM, MRI is the diagnostic neuroimaging procedure of choice, generally revealing multiple focal or confluent areas of signal abnormality in the subcortical white matter and, sometimes, subcortical gray matter on T2 and FLAIR sequences [37–39]. Lesions are generally enhancing and display similar stages of evolution, with most or all lesions either enhancing or nonenhancing, a feature that helps distinguish these lesions from the subcortical white matter lesions of multiple sclerosis. This also differs from MRI findings for patients with progressive multifocal leukoencephalopathy, in which the lesions are similar but rarely enhance and uncommonly affect the gray matter. Because MRI findings may be negative early in the clinical course in patients with ADEM, scanning should be repeated if clinically indicated.
FDG-PET scanning has been studied in patients with encephalitis, with findings published in case reports and small case series. FDG-PET scanning usually shows hypermetabolism, although there can also be areas of hypometabolism [54], indicating that there are no clear characteristics that distinguish encephalitis from other conditions. Therefore, FDG-PET is not routinely recommended for patients with encephalitis, although it could be used as an adjunct in patients with other compatible clinical and diagnostic findings.
EEG. EEG is a sensitive indicator of cerebral dysfunction and may demonstrate cerebral involvement during the early stage of encephalitis [55]. The results of EEG are generally nonspecific but can be helpful in suggesting a specific etiologic diagnosis of encephalitis. In >80% of patients with herpes simplex encephalitis, there is a temporal focus demonstrating periodic lateralizing epileptiform discharges [56]. These stereotypical sharp and slow wave complexes occur at intervals of 2–3 s and are typically seen on days 2-14 after symptom onset. The EEG abnormalities in brainstem encephalitis may be disproportionately mild, compared with the clinical state of the patient; diffuse slow wave activity and intermittent rhythmic δ activity have been described in these patients. Although EEG is rarely useful in identifying a pathogen, it has a role in identifying patients with nonconvulsive seizure activity who are confused, obtunded, or comatose. The severity of abnormal EEG findings does not usually correlate with the extent of disease in the acute phase of illness, but rapidly improving EEG findings often indicate a good prognosis.
CSF analysis. Evaluation of CSF samples is essential (unless contraindicated) for all patients with encephalitis. In instances in which CSF specimens are not available, the likely etiology of the encephalitis should be based on epidemiology, clinical features, and results of other diagnostic studies (tables 2, 3 and 5). In patients with viral encephalitis, CSF analysis typically reveals a mild mononuclear pleocytosis, although a polymorphonuclear cell predominance may initially be seen if the sample is obtained early in the course of illness; persistent neutrophilic pleocytosis has been observed in patients with West Nile virus encephalitis. CSF protein concentration is generally mildly or moderately elevated. Patients may have significant numbers of RBCs in the CSF, as a result of the development of hemorrhagic encephalitis. The presence of CSF eosinophils may suggest certain etiologic agents (i.e., highest with the helminths, but this may be seen with T. pallidum, M. pneumoniae, R. rickettsii, C. immitis, and T. gondii), and accurate laboratory identification of these cells is important. Eosinophils can be mistaken for neutrophils if CSF cell counts are done in an automated cell counter; eosinophils can also be easily distorted or destroyed during CSF processing, and the cytologic features of eosinophils are not easily discernible without Wright or Giemsa staining. A decreased CSF glucose concentration is unusual in viral encephalitis and suggests disease caused by bacteria (e.g., L. monocytogenes and M. tuberculosis), fungi, or protozoae (e.g., Naegleria species). Up to 10% of patients with viral encephalitis can have completely normal CSF findings. Despite clues provided by conventional CSF analysis, additional CSF studies (see below) (table 5) are needed to establish the specific cause of encephalitis.
The CSF findings in patients with ADEM are generally similar to those seen in patients with viral encephalitis—that is, lymphocytic pleocytosis, elevated protein concentration, and normal glucose concentration. Pleocytosis in ADEM tends to be less marked than in acute infectious encephalitis, and it may be absent. Markers of intrathecal immunoglobulin synthesis, including oligoclonal bands and elevated IgG index and synthesis rate, may be present, although less frequently than in multiple sclerosis.
What Tests of CSF and Brain Tissue Specimens Can Help in Establishing the Etiology of Encephalitis?Evidence Summary
Antibody. Detection of CSF antibody is a helpful diagnostic tool in some patients with encephalitis. New diagnostic assays have simplified the diagnosis of certain viral CNS infections. The presence of virus-specific IgM in CSF is usually indicative of CNS disease, because IgM antibodies do not readily diffuse across the blood-brain barrier. For example, the detection of IgM antibodies by an ELISA assay in CSF specimens obtained from patients with presumed flavivirus encephalitis is considered to be diagnostic of neuroinvasive disease. CSF varicella zoster virus IgM antibodies may also be present in patients with a negative CSF varicella zoster virus PCR result (see “Nucleic acid amplification tests,” below).
Nucleic acid amplification tests. The development of PCR for amplification of microbial nucleic acids has greatly increased the ability to diagnose infections of the CNS, especially viral infections that are caused by herpesviruses and enteroviruses [57–60]. The utility of PCR assays for the diagnosis of herpes simplex encephalitis (usually caused by herpes simplex virus type 1 in adults) has been reliably demonstrated, with reported sensitivities and specificities of 96%–98% and 95–99%, respectively, in adults [61]; CSF PCR results are positive early in the disease course and remain positive during the first week of therapy, although false-negative results may occur if hemoglobin or other inhibitors are present in CSF. The sensitivity and specificity of CSF PCR for herpes simplex encephalitis in neonates and infants is more variable, with a reported sensitivity of 75%–100% [62]. An initially negative CSF PCR result for herpes simplex virus may become positive if the test is repeated 1–3 days after the initiation of treatment [63, 64], and the presence of <10 WBCs/mm3 in CSF has been associated with a higher likelihood of a negative CSF PCR result [65]. Therefore, in undiagnosed cases in which patients have clinical features of herpes simplex encephalitis or temporal lobe lesions on neuroimages, consideration should be given to repeating the PCR for herpes simplex virus 3–7 days later on a second CSF specimen. In this instance, a negative CSF PCR result may allow discontinuation of acyclovir therapy.
PCR can detect varicella zoster virus DNA, although a negative test result does not exclude the diagnosis of varicella encephalitis. PCR is also of value for detection of cytomegalovirus, with a high sensitivity and specificity for CNS involvement. Epstein-Barr virus can be detected by PCR, although a positive test result does not necessarily denote CNS infection, because latently infected mononuclear cells can cause a false-positive result and should be correlated with clinical findings and a compatible serologic test result. Results of CSF PCR for West Nile virus are positive in <60% of serologically confirmed cases. Measurement of JC virus DNA concentrations in CSF samples may be a useful virologic marker of disease activity for progressive multifocal leukoencephalopathy in HIV-infected patients receiving HAART [66], because it may indicate response to therapy. CSF PCR may detect evidence of M. pneumoniae in children with acute encephalitis [67], but the yield of this test was very low (2%) in one recent review [29], indicating that evidence of suspected infection with this microorganism should be determined by serologic testing or PCR of respiratory specimens. Although a positive CSF PCR result is very helpful for documentation of infection caused by a specific pathogen, a negative PCR result cannot be used as definitive evidence against the diagnosis.
Culture. CSF cultures are of limited value in the isolation of viral causes of encephalitis. In a review of 22,394 viral cultures of CSF samples, viruses were recovered from only 5.7% of specimens, the majority of which were enteroviruses (98.4%) and herpes simplex viruses (1.3%) [68]. Because viruses can be detected more rapidly and with greater sensitivity through nucleic acid amplification, viral culture generally offers no benefit and is, therefore, not routinely recommended. CSF cultures remain important in the diagnosis of nonviral causes of encephalitis, especially encephalitis caused by bacteria (e.g., L. monocytogenes) and fungi, although a number of the bacterial causes (e.g., Mycoplasma, Bartonella, Ehrlichia, and Rickettsiae species and T. pallidum) cannot be isolated in culture.
Brain biopsy. Brain biopsy to establish the etiology of encephalitis is rarely used today and is not routinely recommended, given the availability of diagnostic PCR and antibody assays. However, biopsy will continue to play a limited role in the diagnosis of some cases of encephalitis and should be considered in patients with encephalitis of unknown etiology who deteriorate neurologically despite treatment with acyclovir. For example, in patients with diffuse toxoplasmic encephalitis without focal lesions, definitive diagnosis can only be established by brain biopsy. The importance of this approach was reflected by a study from the National Institutes of Allergy and Infectious Diseases and Collaborative Antiviral Study Group that revealed that 43% of patients with suspected herpes simplex encephalitis had an alternative diagnosis [69], although this study was conducted prior to routine use of MRI; this high percentage of alternative diagnoses identified through brain biopsy is now unlikely. Neuroimaging should be used to guide the neurosurgeon to a specific area of the brain for biopsy; a biopsy specimen is obtained from an area of abnormality, in a non-eloquent area of the brain, with a recommendation that at least 1 cm3 of tissue be removed. Once tissue is obtained, a portion of the sample should be sent for pathogen isolation, PCR, immunofluorescence, and electron microscopy; a second portion should be placed in formalin and sent for routine histopathologic examination, with appropriate staining for infectious agents. If considering brain biopsy in a patient with encephalitis, the biopsy should be performed earlier—rather than later—in the clinical course, in hopes of identifying a potentially treatable infectious etiology.
Treatment
What Specific Empirical Antimicrobial Agent(s) Should Be Used in Patients with Suspected Encephalitis?
Evidence summary. Although a wide range of viruses have been reported to cause encephalitis, specific antiviral therapy for viral encephalitis is generally limited to disease caused by the herpesviruses, especially herpes simplex virus. Because the earlier that treatment is started for herpes simplex encephalitis, the less likely that death or serious sequelae will result, acyclovir (10 mg/kg intravenously every 8 h in children and adults with normal renal function; 20 mg/kg intravenously every 8 h in neonates) should be initiated in all patients with suspected encephalitis as soon as possible, pending results of diagnostic studies. Other empirical antimicrobial agents should be initiated on the basis of specific epidemiologic or clinical factors (tables 2 and 3), including appropriate therapy for presumed bacterial meningitis if clinically indicated [70]. In patients with clinical clues suggestive of rickettsial or ehrlichial infection during the appropriate season, doxycycline should be added to empirical treatment regimens [71].
Once the Etiology of Encephalitis Is Determined, What Specific Treatment Regimen Should Be Administered?
Evidence summary. Following the identification of a particular microorganism (by antibody studies, molecular methods, or culture) in a patient with encephalitis, appropriate antimicrobial therapy or management should be initiated (table 5) (or continued, if herpes simplex virus is identified as the etiology). In the following sections, we review some of the specific data for our recommendations for treatment of encephalitis caused by selected treatable viruses and ADEM. Although other therapies may be appropriate if some of these infectious agents cause disease outside the CNS, only therapy specific for encephalitis is included. The rationale for recommendations for therapy of other microorganisms that are not discussed be-low can be found in other reviews, book chapters, and published guidelines (table 5) (http://www.idsociety.org) [3, 29–39, 70–86].
Herpes simplex virus. Acyclovir is the treatment of choice for patients with herpes simplex encephalitis [3, 4, 61, 87], but morbidity and mortality remain high (mortality at 18 months after treatment, 28%). In patients with herpes simplex encephalitis, predictors of an adverse outcome include age of the patient (>30 years), level of consciousness (Glasgow coma score, <6), and duration of symptoms prior to starting acyclovir therapy (>4 days) [88]; mortality decreased to 8% if therapy was initiated <4 days after onset of clinical symptoms. In a retrospective, multicenter trial of 93 adult patients, multivariate analysis also identified a Simplified Acute Physiology Score <27 at hospital admission and a delay of >2 days between hospital admission and administration of acyclovir therapy as independent predictors of poor outcome [89]. The dosage of acyclovir in patients with normal renal function is 10 mg/kg intravenously every 8 h for 14–21 days. Recently, the use of higher-dose acyclovir (20 mg/kg intravenously every 8 h for 21 days) in neonates with herpes simplex encephalitis has decreased mortality to 5%, with ∼40% of survivors developing normally [90, 91]. Relapse of herpes simplex encephalitis has been reported after completion of acyclovir therapy [92]. In studies of neonates, ∼8% had a documented relapse if treated with acyclovir for 10 days at a dosage of 10 mg/kg intravenously every 8 h, although relapse has not been documented when higher doses (20 mg/kg intravenously every 8 h) were administered for 21 days. A negative CSF PCR result at the end of therapy was associated with a better outcome, suggesting that another CSF specimen should be subjected to PCR for herpes simplex virus at the end of therapy in patients who have not had the appropriate clinical response; if the result is positive, antiviral therapy should be continued [93]. It is not clear how often relapse occurs in children or adults, but relapse rates as high as 5% have been reported.
Use of adjunctive corticosteroids was assessed in one nonrandomized, retrospective study of 45 patients with herpes simplex encephalitis treated with acyclovir [94]. Although a worse outcome was observed in patients who were not treated with corticosteroids, these results need to be confirmed before this adjunctive treatment can be recommended.
Varicella zoster virus. Although no clinical trial has established the efficacy of antiviral therapy for varicella zoster virus-associated encephalitis, on the basis of case reports and small series, acyclovir (10–15 mg/kg intravenously every 8 h for 10–14 days) is the drug of choice [95]. Ganciclovir has shown efficacy in some patients with varicella zoster virus meningoencephalitis [96] and can be considered as an alternative agent for treatment.
Corticosteroids have been proposed for primary varicella zoster virus encephalitis and in immunocompetent patients with severe varicella zoster virus encephalitis and vasculopathy [41, 95], but there are no reliable data to support their use.
Cytomegalovirus. The optimal approach to the antiviral treatment of cytomegalovirus encephalitis is not clearly defined [97]. Ganciclovir (5 mg/kg intravenously every 12 h for 2–3 weeks) has been used, although therapeutic failures are common. Response to treatment in patients who have cytomegalovirus encephalitis with ganciclovir or foscarnet alone has not improved survival; even prophylaxis with ganciclovir or foscarnet, at doses used for maintenance in cases of cytomegalovirus retinitis, does not guarantee protection against development of cytomegalovirus encephalitis [16]. A combination of ganciclovir (5 mg/kg intravenously every 12 h) and foscarnet (60 mg/kg intravenously every 8 h or 90 mg/kg intravenously every 12 h) for 3 weeks, followed by maintenance therapy, is recommended and has led to success in HIV-infected patients, with improvement or stabilization in 74% of 31 patients with either cytomegalovirus encephalitis or myelitis [98]. However, cytomegalovirus ventriculoencephalitis was reported in a severely immunocompromised bone marrow transplant recipient receiving combination ganciclovir and foscarnet for treatment of cytomegalovirus viremia and retinitis, as a result of development of drug resistance [17]. Effective concentrations of ganciclovir and foscarnet may be difficult to achieve in the CSF. In HIV-infected infants with cytomegalovirus encephalitis, combination ganciclovir and foscarnet, along with HAART, has shown efficacy in some patients [99]. Cidofovir is not an alternative, because its ability to penetrate the blood-brain barrier is poorly studied. Because CMV encephalitis almost always develops in the context of profound suppression of cell-mediated immunity, the treatment approach should include attempts to decrease immunosuppression whenever possible.
Epstein-Barr virus. Acyclovir inhibits replication of Epstein-Barr virus in vitro, but a meta-analysis of 5 clinical trials did not show benefit in the treatment of infectious mononucleosis [100]. Although acyclovir has been used in some cases of CNS disease [101], it probably provides little or no benefit and is not recommended.
Corticosteroids were reported to be helpful in several anecdotal reports of neurologic complications of infection with Epstein-Barr virus (including encephalomyelitis) and have been used in patients with increased intracranial pressure [3]; these data suggest that they may be beneficial in selected patients, but their potential benefits must be weighed against potential risk of perpetuating viral infection or of delaying the diagnosis of or partially treating AIDS-related CNS lymphoma.
Human herpesvirus 6. Randomized, controlled clinical trials to evaluate the usefulness of antiviral agents in patients with primary or viral reactivation caused by human herpesvirus 6 are lacking. Case reports have described the successful treatment of human herpesvirus 6-associated encephalitis in bone marrow transplant recipients with ganciclovir or foscarnet [102–104], but reactivation and neurologic symptoms have also developed while patients were receiving antiviral prophylaxis with these medications. Despite these contradictions, treatment with one of these agents, alone or in combination, in immunocompromised patients with human herpesvirus 6 encephalitis may be reasonable, because no other therapies are currently available. The data are less clear for benefit of treatment in immunocompetent patients [105], but therapy with one of these agents can be considered.
B Virus. Prophylactic antiviral therapy is recommended for individuals who have a high risk exposure to B virus [106]; use of valacyclovir (1 g orally every 8 h for 14 days) is recommended, because it leads to higher serum concentrations. Acyclovir (12.5–15 mg/kg intravenously every 8 h) has been suggested for acute infection; case reports in patients with B virus encephalitis have demonstrated full recovery, although the efficacy of this approach has not been systematically examined. Valacyclovir (1 g orally every 8 h) is recommended because higher serum concentrations can be achieved. Some experts recommend ganciclovir (5 mg/kg intravenously every 12 h for a minimum of 14 days or until all CNS symptoms have resolved) if the CNS is involved; subsequent administration of valacyclovir for suppression of latent infection may also be considered.
Other viruses. The use of antiviral therapy is less well defined in patients with encephalitis caused by other viruses (table 5). Although no controlled trials are available for the treatment of measles virus encephalitis, ribavirin may decrease the severity and duration of measles in normal adults and immunocompromised children with life-threatening disease [107]. Although ribavirin therapy is not currently recommended for treatment of measles virus encephalitis, if administered, it should be continued for 2–3 weeks. In 5 patients with subacute sclerosing panencephalitis, use of intraventricular ribavirin was associated with clinical improvement in 4 patients [108], suggesting that this mode of therapy should be further studied for its efficacy in this condition. Ribavirin has been used in an open-label trial in patients with Nipah virus encephalitis [109]; the results suggested that the drug was able to reduce the mortality of acute Nipah encephalitis with no associated serious side effects, but more data are needed. One study that examined the efficacy of oral ribavirin in patients during an outbreak of West Nile virus infection in Israel found no significant benefit, but there was a potentially deleterious effect in 11% of patients who survived and 45.4% of patients who died [110]; although multivariate analysis suggested that use of ribavirin may have been a surrogate marker for use in sicker patients [111], its use is not recommended. Oseltamivir has been used in children with influenza B-associated encephalitis [26], although it is unclear that therapy aided in the recovery of the patients; oseltamivir and its metabolite, oseltamivir carboxylate, were not detected in the CSF in another report of a patient with influenza B-associated encephalitis [112].
Various adjunctive agents have been studied for their use in patients with viral encephalitis. IFN-α has been used in the context of nonrandomized, nonblinded assessments of patients with West Nile virus encephalitis, but the results are inconclusive [113], and a randomized, placebo-controlled trial among children infected with the closely related Japanese encephalitis virus demonstrated no benefit [114]. A recently concluded study sponsored by the National Institutes of Allergy and Infectious Diseases and Collaborative Antiviral Study Group assessed the efficacy of intravenous immunoglobulin containing high anti-West Nile virus antibody titers in patients with West Nile virus neuroinvasive disease in a randomized, placebo-c ontrolled trial; results of this trial are still pending. Early initiation of therapy with IFN-α-2b was shown, in a limited series, to reduce the severity and duration of complications of St. Louis encephalitis virus meningoencephalitis [115]; however, a prospective, randomized trial is needed before this approach is warranted. In children with x-linked agammaglobulinemia and enteroviral encephalitis, intraventricular γ-globulin therapy (0.2 mL/kg) via an Ommaya reservoir could be considered, although benefits are unproven [116].
The recommended treatment for exposure to rabies virus is postexposure prophylaxis with rabies immunoglobulin and vaccination [117–119]. One recent case involving a 15-year-old girl who survived without postexposure vaccination was reported after she was treated with ribavirin and drug-induced coma [120]. Despite the recovery of this patient, the protocol has been unsuccessful in other cases [121, 122], and no proven therapy for clinical rabies has been established.
ADEM. Although not fully assessed in randomized, placebo-controlled trials, high-dose intravenous corticosteroids (methylprednisolone, 1 g intravenously daily for at least 3–5 days) are generally recommended for ADEM [39]. Reports of successful treatment with plasma exchange have also been documented, although no data from randomized trials are available. Plasma exchange should be considered in patients who respond poorly to corticosteroids [123]; responses in ADEM have been reported with plasma exchange, although the coadministration of corticosteroids and cyclophosphamide is frequent and makes interpretation of these results difficult. There are limited data on the use of intravenous immunoglobulin for the treatment of ADEM, but this approach may be considered in patients who have not responded to corticosteroids or plasma exchange [124–126].
Performance Measures
1. The diagnostic approach to patients with encephalitis must include neuroimaging—either MRI or CT. If neuroimaging is not used, the medical record should include documentation of the specific reasons.
2. Empirical antimicrobial therapy for patients with suspected encephalitis should include rapid administration of intravenous acyclovir at appropriate dosages; if appropriate, treatment for bacterial meningitis and rickettsial or ehrlichial infection should be included.
3. Once an etiologic agent of encephalitis is identified, antimicrobial therapy should be targeted to that infectious agent, or therapy should be discontinued if treatment directed against the etiologic agent is not available.
Acknowledgments
Financial support. The Infectious Diseases Society of America.
Potential conflicts of interest. S.L.K. received investigator-initiated grants from Pfizer, Wyeth, and Sanofi-Pasteur and served on the advisory board for Pfizer. All other authors: no conflicts.
References
It is important to realize that guidelines cannot always account for individual variation among patients. They are not intended to supplant physician judgment with respect to particular patients or special clinical situations. The Infectious Diseases Society of America considers adherence to these guidelines to be voluntary, with the ultimate determination regarding their application to be made by the physician in the light of each patient's individual circumstances.




