-
PDF
- Split View
-
Views
-
Cite
Cite
Aaron T. Fleischauer, James C. Kile, Molly Davidson, Marc Fischer, Kevin L. Karem, Robert Teclaw, Hans Messersmith, Pamela Pontones, Bradley A. Beard, Zachary H. Braden, Joanne Cono, James J. Sejvar, Ali S. Khan, Inger Damon, Matthew J. Kuehnert, Evaluation of Human-to-Human Transmission of Monkeypox from Infected Patients to Health Care Workers, Clinical Infectious Diseases, Volume 40, Issue 5, 1 March 2005, Pages 689–694, https://doi.org/10.1086/427805
Close - Share Icon Share
Abstract
Background. In 2003, human monkeypox was first identified in the United States. The outbreak was associated with exposure to infected prairie dogs, but the potential for person-to-person transmission was a concern. This study examines health care worker (HCW) exposure to 3 patients with confirmed monkeypox.
Methods. Exposed HCWs, defined as HCWs who entered a 2-m radius surrounding case patients with confirmed monkeypox, were identified by infection-control practitioners. A self-administered questionnaire and analysis of paired serum specimens determined exposure status, immune response, and postexposure signs and symptoms of monkeypox.
Results. Of 81 exposed HCWs, 57 (70%) participated in the study. Among 57 participants, 40 (70%) had ⩾1 unprotected exposure; none reported signs or symptoms consistent with monkeypox illness. One exposed HCW (2%), who had been vaccinated for smallpox within the past year, had serological evidence of recent orthopoxvirus infection; acute- and convalescent-phase serum specimens tested positive for anti-orthopoxvirus IgM. No exposed HCWs had signs and symptoms consistent with monkeypox.
Conclusion. More than three-quarters of exposed HCWs reported at least 1 unprotected encounter with a patient who had monkeypox. One asymptomatic HCW showed laboratory evidence of recent orthopoxvirus infection, which was possibly attributable to either recent infection or smallpox vaccination. Transmission of monkeypox likely is a rare event in the health care setting.
In 2003, monkeypox virus, a member of the orthopoxvirus genus previously endemic only to central Africa [1–4], was first identified as a human pathogen in North America [5, 6]. The outbreak was traced to exposure to infected prairie dogs [6], but data from investigations of monkeypox virus in Africa and other related orthopoxviruses suggested that person-to-person transmission could occur [2, 7]. Although person-to-person transmission of monkeypox has been documented in previous outbreaks, transmission has never been assessed in a health care setting [8]. Studies of variola virus, a related orthopoxvirus, suggest that health care workers (HCWs) are at increased risk for acquiring smallpox [8, 9]. To investigate transmission of monkeypox in the health care setting, we examined the rate of HCW transmission, based on the frequency of clinical illness and changes in immune status (i.e., IgM and IgG seroconversion), among HCWs exposed to 3 case patients with confirmed monkeypox treated at 2 hospitals (A and B) and a physician's office (clinic A) in Indiana.
Subjects and Methods
Index case patients. The 3 index case patients—a 33-year-old man, a 30 year-old woman, and their 6-year-old daughter—were exposed to monkeypox virus—infected prairie dogs and subsequently developed illness (figure 1). Detailed clinical findings and illness courses are described elsewhere [10]. In brief, the father had mild systemic illness and mild rash (e.g., fever, headache, and 2 nonpruritic vesicles). The mother had more pronounced systemic illness and severe rash (e.g., fever, headache, lymphadenopathy, and ∼200 disseminated lesions). The daughter had severe systemic illness and rash, with neurologic complications (e.g., fever, headache, lymphadenopathy, and encephalitis) requiring hospitalization in an intensive care unit [10].
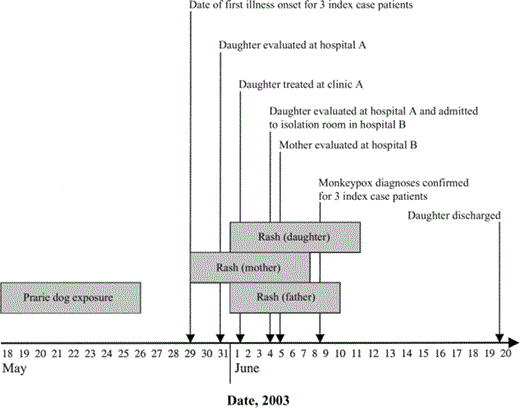
Duration of prairie dog exposure, time of illness onset, duration of rash, and times of health care interaction for 3 index case patients with confirmed monkeypox who were treated at 3 facilities in Indiana (clinic A and hospitals A and B), 2003. Grey boxes, durations of prairie dog exposure and rash (i.e., rash onset to scab sloughing).
Exposure status. HCWs exposed to 3 case patients with confirmed monkeypox (as defined by Centers for Disease Control and Prevention [CDC; Atlanta, GA] criteria [11]) at 3 health care facilities (clinic A and hospitals A and B) were identified by infection-control professionals at each facility. HCW exposure was defined as entrance into the immediate-care area of a case patient with confirmed monkeypox. The immediate-care area was defined as the 2-m radius surrounding case patients either in their rooms or in common-care areas, such as the emergency department. Protected exposure was defined as use of personal protective equipment recommended for droplet precautions, including wearing of gloves, gown, and a surgical mask or N95 respirator during each episode of exposure [12].
Data collection. This study was part of a public health investigation and was exempted from institutional review board review. A self-administered questionnaire was used to collect data about the day and time of first and last exposure to case patients with monkeypox, median duration of exposure, frequency of personal protective equipment use during exposure (i.e., never, sometimes, usually, or always) for each component (i.e., gloves, gown, and surgical mask or N95 respirator), and smallpox vaccination history. The questionnaire was administered 1–10 days after the last exposure. An acute-phase serum sample was obtained at the time of questionnaire completion. HCWs also were encouraged to record their daily temperature in a fever log and to document any other notable signs or symptoms (e.g., rash) for 14 days after exposure. Six weeks after study enrollment, the clinical follow-up section of the questionnaire was administered to gather data on signs and symptoms that occurred ⩽21 days after the first exposure that were consistent with monkeypox illness. Signs and symptoms consistent with monkeypox illness were defined on the basis of the following case definition: presence of fever or rash with at least 2 other symptoms, including chills, headache, backache, lymphadenopathy, sore throat, cough, and shortness of breath [11]. A convalescent-phase serum sample was collected during the clinical follow-up visit.
Laboratory analysis. Laboratory testing for monkeypox infection was performed on acute- and convalescent-phase serum specimens obtained from HCWs using an IgG ELISA and an IgM-capture ELISA for immune response to orthopoxvirus antigen [10]. Cutoff values for these assays were determined based on serological tests of 7 nonexposed human control specimens. Data were presented as adjusted optical density minus the cutoff value; a value >0 was considered to be positive.
Results
Between 4 June and 20 June 2003, a total of 3 case patients with confirmed monkeypox were treated at clinic A and hospitals A and B. The extent of health care evaluation and management varied between family members (figure 1). After visits to clinic A on 31 May and a physician's clinic office on 1 June, the daughter was evaluated at the emergency department of hospital A and admitted to a pediatric intensive care unit at hospital B on 4 June. Isolation precautions for varicella (e.g., contact and airborne precautions for susceptible individuals) were implemented at the time of admission. On 8 June, monkeypox was confirmed by the Indiana State Department of Health, and the patient was placed in contact and airborne isolation until 11 June, when all scabbed lesions had sloughed and scarring had developed. The patient's mother underwent a health care evaluation in the emergency department of hospital B on 5 June. Both parents visited their daughter nearly continuously during her hospitalization at hospital B, and efforts were made by the hospital to limit contact with others outside of the isolation room after monkeypox was diagnosed.
A total of 81 HCWs were identified by infection-control professionals as having met the definition for exposure. Fifty-seven (70%) of 81 exposed HCWs consented to participate. Three (75%) of 4 HCWs at the physician's office were enrolled, 25 (96%) of 26 at hospital A were enrolled, and 29 (57%) of 51 at hospital B were enrolled. Convalescent-phase serum samples were later obtained from 47 (82%) of these 57 participants.
The median age of the 57 participating HCWs was 39 years (range, 19–61 years) (table 1), and the majority (74%) were female. Thirty-five percent of the HCWs were members of the nursing staff, 28% were respiratory or radiology technicians, 18% were physicians, 10% were emergency medical service professionals, and 9% were ancillary staff. No differences in the distribution of job specifications were observed between participating HCWs and nonparticipating HCWs.
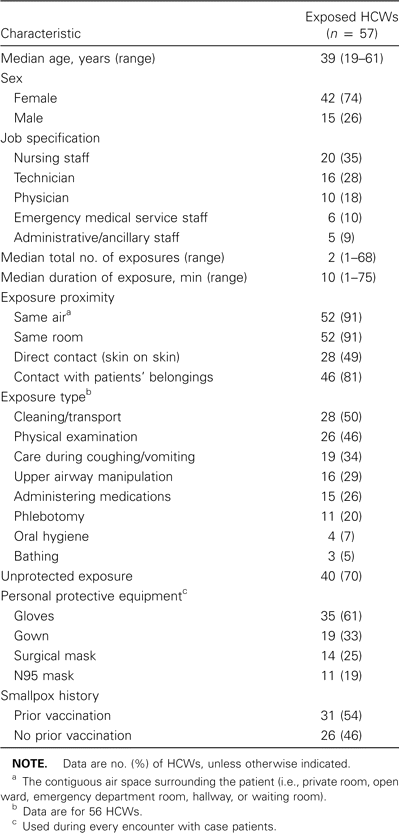
Demographic and exposure-related characteristics of health care workers (HCWs) at 3 health care facilities in Indiana who were exposed to index case patients with monkeypox in 2003.
There were a total of 270 separate encounters with case patients with monkeypox. The median number of separate exposures per HCW was 2 (range, 1–68 exposures), and the median duration of each exposure episode was estimated to be 10 min (range, 1–75 min). Although the median number of case patient encounters was highest for nurses (9), compared with physicians and technicians (4 and 2, respectively), the median time spent per encounter was slightly longer for physicians (19 min) than for nurses (12 min) and technicians (12 min). However, these differences were not statistically significant.
Among the 57 exposed HCWs, only 17 (29%) reported using gloves, a gown, and either a surgical mask or an N95 respirator during all encounters with case patients with monkeypox. Conversely, 40 (70%) had ⩾1 unprotected exposure. Specifically, 35 HCWs (61%) used gloves during every case patient encounter. Gown use (33% of HCWs), surgical mask use (25%), or N95 respirator use (19%) during every case patient encounter was less frequent. A comparison of HCWs who reported all of their patient exposures before monkeypox was diagnosed in the hospitalized case patient with those who reported all of their exposures after monkeypox was diagnosed (i.e., before vs. after day 4 of hospital admission) revealed that 25% and 63%, respectively, reported use of gloves, gown, and either surgical mask or N95 respirator during all encounters with case patients with monkeypox.
No signs or symptoms consistent with monkeypox illness (as defined by CDC criteria) were reported. Two HCWs (4%) reported having had a low-grade fever (temperature, 38.2°C) for <2 days without other signs or symptoms. No exposed HCWs reported having had a rash.
HCWs were evaluated for monkeypox virus infection by ELISA of paired acute- and convalescent-phase serum specimens for anti-orthopoxvirus IgM and IgG reactivity. Thirty-one (54%) of 57 HCWs reported a past history of smallpox vaccination; 4 of these vaccinations were ⩽6 months before exposure to a case patient with monkeypox. Of these 4 recent vaccinees, 3 had received vaccinations during childhood (before 1985), and 1 was a primary vaccinee.
One HCW had anti-orthopoxvirus IgM detected in acute- and convalescent-phase serum specimens. This HCW was also the only primary vaccinee. Known exposure occurred during a single patient encounter, in which the vital signs of a case patient were measured and physical examination was performed while the HCW was wearing gloves; the HCW recalled no direct skin contact. No clinical signs or symptoms during the 21-day period after this exposure were reported.
A total of 29 (94%) of 31 previously vaccinated, exposed HCWs tested positive for anti-orthopoxvirus IgG antibodies (figure 2). Of the 26 HCWs tested who had no known history of smallpox vaccination, 3 had evidence of IgG antibodies in both acute- and convalescent-phase serum specimens. All 3 of these HCWs were born before 1970, during the era of routine smallpox vaccination. No individuals appear to have had a significant change in anti-orthopoxvirus IgG levels suggestive of effects due to recent booster exposure.
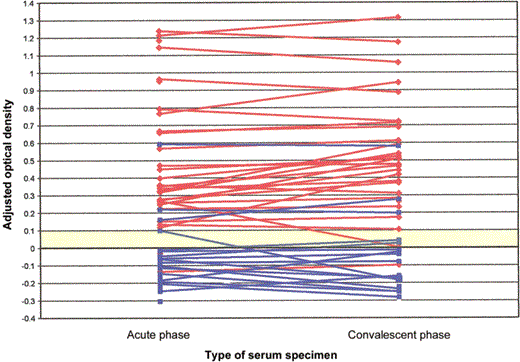
Results of ELISA for detection of monkeypox virus—specific IgG in acute- and convalescent-phase serum specimens obtained from health care workers (HCWs) from 3 facilities in Indiana (clinic A and hospitals A and B) in 2003, according to smallpox vaccination status. The adjusted optical density is defined in Subjects and Methods. Blue squares, patients who did not receive smallpox vaccination; red diamonds, patients who received smallpox vaccination; yellow region, equivocal.
Discussion
Reports of person-to-person transmission of monkeypox during outbreaks of this disease in Africa and long-standing evidence of smallpox transmission in hospitals raised concern about transmission of monkeypox in health care settings [3, 7, 8, 9]. In this study, nearly three-quarters of exposed HCWs reported at least 1 unprotected exposure to 1 of the 3 case patients with monkeypox. On the basis of postexposure surveillance data, no HCWs reported symptoms consistent with the case definition of monkeypox illness. One HCW, who had a single encounter with a case patient with monkeypox but also received a smallpox vaccination 4 months before exposure, had evidence of recent orthopoxvirus infection, as determined by acute- and convalescent-phase IgM reactivity. It is uncertain whether the presence of IgM reflects monkeypox transmission or recent smallpox vaccination.
Secondary attack rates (SARs) among household and close contacts of persons with monkeypox have been documented in Africa [2, 3, 13, 14]. The first investigations of human monkeypox during the smallpox eradication campaign between 1970 and 1977 in western and central Africa revealed infrequent and limited person-to-person transmission [13]. Investigations during 1981–1985 of 2278 close contacts of 203 primary and 42 coprimary monkeypox cases revealed a SAR of 3.7% among household contacts and an overall SAR of 3.0% among all contacts. Specifically, the SAR among unvaccinated household contacts was 9.3% (7.5% among all unvaccinated contacts), whereas the SAR among vaccinated household contacts was 1.3% (0.9% among all vaccinated contacts) [3]. Investigations of the reemergence of monkeypox in the Democratic Republic of Congo between 1996 and 1997 reported that, 7–21 days before the onset of illness, 73% of case patients were exposed to another patient infected with monkeypox virus, with estimates of a SAR in the household of 8.3 per 100 population [2, 14].
There were a number of potential limitations in this study that may have affected our results. First, the participation rate at hospital B was lower than that at the other 2 facilities, raising concerns about potential selection bias. However, job specifications (e.g., nursing staff, technician, and physician) and unit assignments (e.g., pediatric intensive care unit and emergency department) were well represented by enrolled HCWs at both hospital A and hospital B. Some items in the questionnaire may have introduced a reporter bias, particularly self-report of the use of personal protective equipment and signs or symptoms suggestive of monkeypox. An additional limitation was that absence of the opportunity to study an unexposed cohort of individuals precluded assessment of the accuracy of the IgG and IgM ELISA results; thus, false-positive or -negative results may have been observed. The use of a cutoff value for the ELISAs, determined to be 3 SDs above the mean value determined for 7 randomly selected control patients without exposure to orthopoxvirus, makes false-positive results less likely.
Of the 3 HCWs who did not report a history of smallpox vaccination and who had IgG antibodies in both acute- and convalescent-phase serum specimens, all were born prior to 1970 and were presumably vaccinated for smallpox but may have been misclassified because of recall bias. Known smallpox vaccination history can help interpret anti-orthopoxvirus serology results, because serologies of anti-vaccinia virus (due to smallpox vaccination) and anti—monkeypox virus are cross-reactive. It is also unclear whether recent vaccination or infection produced the single positive IgM result; the average duration of IgM persistence after smallpox vaccination is unknown. Anecdotal experience with CDC vaccines suggests that an IgM response can be detected in some primary vaccinees for as long as 6 months (unpublished data).
We found that surveillance of HCWs for exposure to infectious agents can be rapidly implemented to monitor health care facility—associated transmission. Overall, despite a variety of unprotected exposures to case patients with monkeypox (i.e., exposures in which various personal protective equipment was not used), no symptomatic illnesses consistent with monkeypox were reported in HCWs. The majority of HCWs most likely observed standard precautions (i.e., use of gloves, gown, and mask when indicated), which may have prevented transmission [12]. By analogy with variola transmission, spread of monkeypox virus most likely occurs via large respiratory droplets or contact, although airborne transmission has been rarely reported in hospital settings during smallpox outbreaks. Use of both contact and airborne precautions are recommended for any generalized vesicular rash of unknown etiology in which monkeypox and smallpox are included in the differential diagnosis [15]. In our investigation, transmission of monkeypox to HCWs likely was a rare event in the health care setting.
Acknowledgments
We thank the numerous professionals who contributed to our article, including the Centers for Disease Control and Prevention (CDC) Monkeypox Investigative Team, the Indiana Department of Health, and the staff at participating hospitals. We are especially grateful to Richard Kanwal, Mary Chamberland, and Mary Reynolds of the CDC Monkeypox Investigative Team for their assistance.
Potential conflicts of interest. All authors: no conflicts.




