-
Views
-
Cite
Cite
Robina Aerts, Alienor Xhaard, Christine Robin, Andreas H Groll, Catherine Cordonnier, Katrien Lagrou, Stéphane Bretagne, Toxoplasma qPCR Kinetics to Guide Preemptive Treatment of Toxoplasmosis After Allogeneic Hematopoietic Stem Cell Transplantation, Clinical Infectious Diseases, 2024;, ciae488, https://doi.org/10.1093/cid/ciae488
Close - Share Icon Share
Abstract
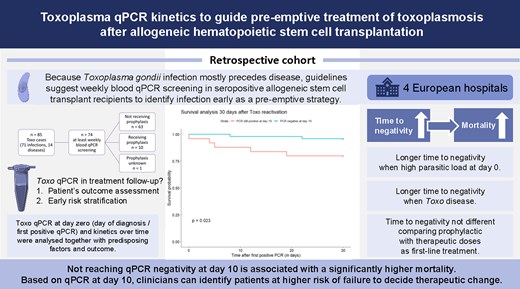
Recent European Conference on Infections in Leukemia (ECIL) guidelines recommend a quantitative polymerase chain reaction (qPCR) guided preemptive treatment approach to toxoplasmosis in seropositive recipients of allogeneic hematopoietic cell transplantation (allo-HCT). Although qPCR might serve as a sensitive tool for early Toxoplasma detection, its role in treatment follow-up remains unknown.
We analyzed the qPCR kinetics of allo-HCT recipients experiencing either Toxoplasma infection (TI, n = 71) or disease (TD, n = 14) in relation to different parameters. We included 85 patients with available qPCR values expressed as quantitative cycle (Cq) from 4 large hematological centers from 2009 to 2023, and kinetic analysis was performed in a selection of 74 patients screened at least weekly with blood qPCR. Day 0 (D0) was the day of anti-Toxoplasma treatment start or (when untreated) day of diagnosis.
Time to qPCR negativity was inversely proportional to the Cq value at D0 (P = .0063). Not reaching negativity at D10 was associated with a significantly higher mortality at D30 (P = .023). Patients with a high D0-parasitic load and patients with TD showed slower clearance (P < .001, P = .032). Time to negativity was not significantly different for patients started on prophylactic versus curative doses as first-line treatment regimen (P = .16).
This study underscores the predictive value of qPCR kinetics monitoring in allo-HCT patients with toxoplasmosis. With the aforementioned risk factors, clinicians can identify patients at high risk for worse outcome. Our results support to consider a therapeutic change or reinforcement if the parasitic load does not decrease after 10 days, supplementing existing clinical guidelines.