-
PDF
- Split View
-
Views
-
Cite
Cite
Steven O’Reilly, Senescence in diffuse systemic sclerosis is elevated and may play a role in fibrosis, Clinical and Experimental Immunology, Volume 219, Issue 1, 2025, uxad077, https://doi.org/10.1093/cei/uxad077
Close - Share Icon Share
Dear Editor,
Systemic sclerosis (SSc) is an autoimmune connectives tissue disease in which there is inflammation, vascular disruption, and fibrosis [1]. The fibrosis primarily affects the skin and can affect the lungs. Although much progress has been made in recent times there is currently no specific fibrosis modifying treatment. Most recently, SSc has been demonstrated to show specific features of an increased ageing phenotype these include reductions in telomeres, epigenetic alterations, alterations in the functions of stem cells and increased reactive oxygen species (ROS) and mitochondrial alterations [2]. Cellular senescence is also a hallmark of ageing and is associated with a multitude of age-related diseases [3]. Cell senescence is defined as an irreversible arrest of cell proliferation and which is a mechanism to restrain uncontrolled proliferation and tumour development [4].
It is known that in idiopathic pulmonary fibrosis (IPF) there is a high level of senescence and telomere attrition. The senescent cell marker p16INK4a is elevated in IPF cells and tissue. P16ink4s is a tumour suppressor that prevents cell cycle progression by inhibiting cell cycle-dependant kinases [5, 6]. Further evidence in IPF comes from specific mutations in the enzyme telomerase associating with lung fibrosis. As such IPF is seen as an age-related disease. Various senescent features are associated with IPF [6]. Given the role of senescence in IPF recent investigations in SSc have also found alterations in telomere levels in lymphocytes but not granulocytes, and this is associated with interstitial lung disease in SSc [7]. Furthermore, in a small subset of SSc patients autoantibodies were identified against telomerase and shelterin complex proteins and this was associated with patients with severe lung disease and reduced telomere length in lymphocytes [8, 9]. In IPF lungs elevated transcriptomic senescence signatures have been found and also senolytic therapy reduced experimentally induced lung fibrosis in a pre-clinical animal model of lung fibrosis [10], suggesting that senloytics could be useful in fibrosis. In SSc, it has been demonstrated that fibroblasts have increased levels of genomic instability that could provoke senescence [11] as genomic instability is a hallmark of chronological ageing [3]. Most recently open label clinical trial in a subset of SSc patients a senescence gene signature was found in whole skin biopsies and this was reduced with the senolytic agent dasatinib [12]. Also, a recent clinical trial of senolytics in IPF also demonstrated overall benefit in physical function, although lung fibrosis was unchanged [13]. In this concise report, we determine senescence and the role of senescence in SSc.
Six patients with early diffuse SSc defined as less than 2 years since the onset of first non-Raynaud’s symptom were included; full informed consent was given. The study has full ethical approval with the local research ethics committee (REC) with approval no REC/13/NE/0089. Skin biopsies were taken from affected skin area. Cells were cultured in standard culture medium. Heathy controls were taken from donors (age and gender matched) undergoing fat reduction surgery and all cells were used at very early passage (<3 passages).
Standard qPCR was performed.
ELISA was performed using specific ELISAs for TGF-β1, IL-1β, IL-6, TIMP-1, and VEGF (R&D Systems, UK) using conditioned media after senescence induction.
Induction of senescence was performed by culturing healthy dermal fibroblasts in standard media and exposing these cultures to X-ray irradiation with 9 Gy using a PXI C-Rad225 as previously described [14]. Immediately after irradiation media was changed and replaced. After irradiation, cells were left for 9 days post irradiation with a media change at day 4. After 9 days, the cells are senescent and the media was taken and transferred to normal healthy dermal fibroblasts and this was incubated with these cells for a further 24 h after which the supernatant was removed and quantified for secreted collagen using the Sircoll assay. Control cells received no irradiation, but were cultured identically other than that. RNA was isolated and gene expression was quantified using the specific primers listed in Supplementary data. In some experiments, the multi-kinase inhibitor nintedanib (1 μM; Sigma-Aldrich) was incubated for 2 h prior to the addition of the SASP conditioned media and importantly this was also incubated at the same time with the SASP conditioned media (1 μM) for a further 24 h before the media was analysed for collagen protein and ACTA2 (for α-Smooth muscle actin) by qPCR. Untreated media is the media transferred from none-senescent cells contained vehicle for nintedanib dimethyl sulfoxide (DMSO) at final concentration of 0.1%. Fibroblasts were subjected to irradiation as above but immediately after were then incubated with nintedanib 1 μM or vehicle control subsequently VEGF was quantified by ELISA at day 9.
The highest senescent SSc fibroblast cultures were chosen (highest expression of p16 and IL-6) and incubated with Dasatanib 20 μM and Quercetin (Sigma, UK) at 15 μM or DMSO vehicle control (0.1%) and left for 48 h in culture. Concentrations are from Zhu et al. [15] After which conditioned medium was taken and IL-6 was analysed by ELISA (R&D Systems), and collagen by Sircol assay and cell viability was quantified using MTT assay using the manufacturer’s instructions (Promega, USA). Vehicle control was set as 100% viability. Three donors were used and triplicates ran.
We used isolated SSc fibroblasts from 6 patients with early diffuse SSc (66 female, autoantibody positive for Scl-70, mean mRSS = 18, mean age 48.9 SD = 6.9, range 39–58) and compared these to healthy non diseased control cells (female, age 49.3 SD = 5.2) for P16INKa and found significantly elevated levels in SSc fibroblasts (Fig. 1A; P = 0.0054; Student’s t test, n = 6). P16INK4A is a validated marker of senescence [3]. We also detected the expression of Cyclin Dependant Kinase Inhibitor 1A (CDKN1A) also called p21, another classic maker of senescent cells, and found that this was significantly elevated in SSc dermal fibroblasts compared to healthy control fibroblasts (Fig. 1B; P = 0.0081; Student’s t test, n = 6). We also confirmed elevated expression in SSc fibroblasts of collagen1A1 expression (Fig. 1C; 1 HC versus 2.24 SSc fold change, P = 0.0001; Student’s t test, n = 6) and α-Smooth muscle actin ACTA2 gene (Fig. 1D; 1 HC versus 2.9 SSc fold change P = 0.0009 Student’s t test, n = 6) and the ECM promoter TIMP-1 protein expression (Fig. 1E; 111.1 HC versus 23.3 SSc ng/mL; P = 0.0211; Student’s t test, n = 6). This work corroborates a recent gene expression analysis of senescent genes in whole skin biopsies [16].
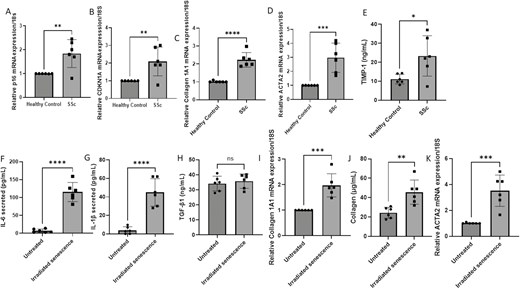
Senescence in SSc dermal fibroblasts. (A) P16INK4a expression was quantified in SSc dermal fibroblasts and compared to healthy controls (HC). Data are the mean and SD, **P = 0.0054, n = 6. (B) CDKN1A gene expression normalized to 18S. Data are the mean and SD, **P = 0.081; Student’s t test, n = 6. (C) Collagen gene expression data are normalized to 18S and shown as fold change to healthy controls. Data are the mean and SD; ****P = 0.0001, n = 6. (D) ACTA2 gene expression is normalized to 18S. Data are the mean and SD; ***P = 0.0009; Student’s t test, n = 6. (E). TIMP-1 levels were quantified in healthy control and SSc dermal fibroblasts by specific ELISA. Data are the mean and SD; ***P = 0.0.213; Student’s t test, n = 6. (F) IL-6 was measured after 9 days after irradiation of healthy dermal fibroblasts or untreated in the media by specific ELISA. Data are the mean and SD; ****P = <0.0001; Student’s t test, n = 6. (G) IL-1β was measured after 9 days after irradiation of healthy dermal fibroblasts or untreated in the media by specific ELISA. Data are the mean and SD; ****P < 0.0001; Student’s t test, n = 6. (H) TGF-β1 was measured after 9 days after irradiation of healthy dermal fibroblasts or untreated in the media by specific ELISA. Data are the mean and ns = nonsignificant, Student’s t test, n = 6. (I) Collagen1A1 expression was quantified 24 h after receiving the conditioned media from irradiated or untreated fibroblasts after 9 days post irradiation. Data are the mean and SD; ***P = 0.0004; Student’s t test, n = 6. (J) Collagen was quantified in the supernatant after 24 h post exposure to senescent cell media or untreated media after 9 days post irradiation. Data are the mean and SD; **P = 0.0035; Student’s t test, n = 6. (K) ACTA2 gene expression was quantified 24 h post exposure to senescent cell media or untreated media 9 days post irradiation. Data are the mean and SD; ***P = 0.0005; Student’s t test, n = 6.
While senescent cells have exited the cell cycle, they are metabolically active and secrete of a panoply of bioactive mediators termed the Senescent Associated Secretory Phenotype (SASP). The SASP is the release of a surfeit of inflammatory mediators including cytokines IL-6 and IL-1β, chemokines, growth factors and microRNAs. This secreted SASP may propagate or initiate fibrosis and has found to be associated with lung fibrosis [6]. We induced fibrosis in healthy dermal fibroblasts by irradiation of healthy dermal fibroblasts with 9 Gy and then they were left for 9 days post irradiation. We confirmed upregulation of P16INK4a mRNA in the irradiated cells (3-fold increase) and secretion of key SASP factors IL-6 (Fig. 1F; 6.9 versus 115.3 pg/mL, P = <0.0001; Student’s t test, n = 6) and IL-1β (Fig. 1G 3.8 versus 44.8 pg/mL P = <0.0001; Student’s t test, n = 6) also. There is an exhaustive and cell and context dependant list of SASP factors that depending on cell type and induction agent of senescence used can be heterogenous. Transforming growth factor-β1 (TGF-β1) has also been described to be a cytokine as part of the SASP and this is also one of the most potent pro-fibrotic molecules known to be involved in SSc and we predicted this would be elevated in the SASP from the senescent cells. Surprisingly, we saw no significant increase in TGF-β1 release by the senescent cells (Fig. 1H, non-significant P = 0.58; Student’s t test, n = 6). The reason that the predicted TGF-β1 from the SASP was not elevated maybe that longer time in senescence is necessary for its induction and release.
Given that senescence may play a role in SSc, we hypothesised that the SASP from senescent cells may induce fibrosis in bystander cells that are none-senescent and thus act in an autocrine and paracrine manner upon neighbouring cells. Hence after 9 days post-irradiation we transferred conditioned media from non-senescent and senescent cells to healthy dermal fibroblasts (passage < 3) and they were cultured in this media for a further 24 hours. After 24 hours post transfer of media, we examined the gene expression of collagen1A1 and collagen protein release. Figure 1I demonstrates that conditioned medium from senescent cells significantly upregulated collagen mRNA expression (P = 0.0004; Student’s t test, n = 6) and collagen protein release by the fibroblasts (Fig. 1j; P = 0.0035, Student’s t test, n = 6). Analysis of ACTA2, encoding α-Sma, demonstrated significant elevation after incubation with senescent cell conditioned media (Fig. 1K; HC 1 versus SSc 3.5-fold change; P = 0.0005; Student’s t test, n = 6). Taken together this demonstrates that senescent cells secrete the hallmark SASP that can mediate an increased fibrotic response in none-senescent fibroblasts.
Even though IL-6 is a prominent member of the SASP and is elevated it is known that dermal fibroblasts do not express the receptor for IL-6 and thus cannot signal in dermal fibroblasts without the use of a soluble receptor so called trans-signalling [17]. We therefore excluded this as a factor mediating fibrosis. Also because there was no significant elevation of the prime candidate TGF-β1 in the conditioned media we excluded this as a candidate. It is known as well as cytokines growth factors are also released as part of the SASP and many of these signals through receptor tyrosine kinases (RTKs). To this end we incubated fibroblasts with the multi-kinase inhibitor nintedanib (1 μM), now licensed for SSc associated pulmonary disease and could see significant reductions in collagen release and ACTA2 expression (Supplementary Fig. A). This suggests that the effect on collagen and α-Smooth muscle actin are mediated via a growth factor-dependant activation of RTK. Nintedanib is a global pan kinase inhibitor and shows no real specificity, but upstream activators include vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and fibroblast growth factor. We therefore chose to measure the levels of VEGF in the conditioned media as this is both pro-fibrotic and is defined as a SASP constituent Supplementary Fig. B demonstrates that the conditioned media has significantly elevated levels of VEGF compared to none-senescent cells (Supplementary Fig. B; P = <0.0001; Student’s t test, n = 6). To determine whether nintedanib regulates the release (rather than block) VEGF signalling, we treated cells with nintedanib immediately after irradiation induced senescence and measured VEGF after nine days. No significant difference in VEGF was demonstrated between cells treated with nintedanib and senescence and senescence alone or in the presence of vehicle (n = 6), suggesting it does not regulate release of SASP. The senolytic agent dasitinib and quercetin combined have shown clinical benefits in age-related diseases [18] including lung fibrosis [10] through selective elimination of cells. Further navitoclax, a different senolytic, is effective in lung fibrosis models [19]. We used the SSc cells with highest senescent markers and incubated with D&Q and compared to vehicle treated cells there was a significant reduction in cells (Supplementary Fig. C). This was coincident with SASP factor IL-6 reduction, P = 0.027 (n = 3) Supplementary Fig. D and collagen (Supplementary Fig. E).
This suggests that the senescent cell transfer of SASP that leads to increased ECM is mediated by VEGF as this is increased and the pro-fibrotic transition can be blocked by nintedanib, a kinase inhibitor. VEGF is known to be pro-fibrotic and is known to be perturbed in SSc. It is possible that other growth factors related to senescence are also released. Given that a clinical trial of senolytic therapy in a subset of patients showed some clinical benefit and was associated with reduction in a senescent gene signature [12] and we found senolytics led to cell reduction and SASP suppression with collagen reduced also, we suggest that senolytic therapy could be a therapy in a senescent subset of SSc patients.
Abbreviations
- CDKN1A
: cyclin-dependant kinase inhibitor 1A;
- DMSO
: dimethyl sulphoxide;
- IPF
: idiopathic pulmonary fibrosis;
- PDGF
: platelet-derived growth factor;
- REC
: research ethics committee;
- ROS
: reactive oxygen species;
- RTKs
: receptor tyrosine kinases;
- SASP
: senescent-associated secretory phenotype;
- SSc
systemic sclerosis
- VEGF
: vascular endothelial growth factor.
Ethical approval
The study has full ethical approval with the local research ethics committee (REC) with approval no REC/13/NE/0089.
Conflict of interests
The author declares no conflict of interest.



