-
Views
-
Cite
Cite
S M McLachlan, H A Aliesky, B Rapoport, To reflect human autoimmune thyroiditis, thyroid peroxidase (not thyroglobulin) antibodies should be measured in female (not sex-independent) NOD.H2h4 mice, Clinical and Experimental Immunology, Volume 196, Issue 1, April 2019, Pages 52–58, https://doi.org/10.1111/cei.13249
Close - Share Icon Share
Summary
NOD.H2h4 mice are the most commonly used model for human autoimmune thyroiditis. Because thyroid autoimmunity develops slowly (over months), NOD.H2h4 mice are usually exposed to excess dietary iodide to accelerate and amplify the process. However, unlike the female bias in human thyroid autoimmunity, autoantibodies to thyroglobulin (TgAb) are reported to be similar in male and female NOD.H2h4. We sought evidence for sexual dimorphism in other parameters in this strain maintained on regular or iodized water. Without iodide, TgAb levels are higher in males than in females, the reverse of human disease. In humans, autoantibodies to thyroid peroxidase (TPOAb) are a better marker of disease than TgAb. In NOD.H2h4 mice TPOAb develop more slowly than TgAb, being detectable at 6 months of age versus 4 months for the latter. Remarkably, unlike TgAb, TPOAb levels are higher in female than male NOD.H2h4 mice on both regular and iodized water. As previously observed, serum T4 levels are similar in both sexes. However, thyroid-stimulating hormone (TSH) levels are significantly higher in males than females with or without iodide exposure. TSH levels correlate with TgAb levels in male NOD.H2h4 mice, suggesting a possible role for TSH in TgAb development. However, there is no correlation between TSH and TPOAb levels, the latter more important than TgAb in human disease. In conclusion, if the goal of an animal model is to closely reflect human disease, TPOAb rather than TgAb should be measured in older female NOD.H2h4 mice, an approach requiring patience and the use of mouse TPO protein.
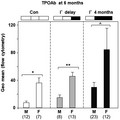
Hashimoto's thyroiditis in humans is associated with sexual dimorphism (female bias) and with autoantibodies to thyroid peroxidase (TPOAb) being a more sensitive marker of disease than autoantibodies to thyroglobulin (TgAb). Studies on NOD.H2h4 mice, the most commonly used animal model for human disease, are generally performed without regard to sex and by assaying TgAb. In the present study, we demonstrate a female bias in the development of TPOAb, not TgAb, the former more difficult to measure and requiring longer term studies than the latter. Therefore, despite these experimental handicaps, assaying TPOAb development in NOD.H2h4 mice more faithfully reflects human disease.





