-
Views
-
Cite
Cite
Jie Zhang, Wei Dong, Qin Yang, Li-Na Liu, Xi-Lun Cai, Dan Wang, Guo-Ji Yan, Yan-Bin Xiyang, Tao Hu, Jie Zhang, Dysregulation of G6PD by HPV E6 exacerbates cervical cancer by activating the STAT3/PLOD2 pathway, Carcinogenesis, Volume 46, Issue 2, February 2025, bgaf005, https://doi.org/10.1093/carcin/bgaf005
Close - Share Icon Share
Abstract
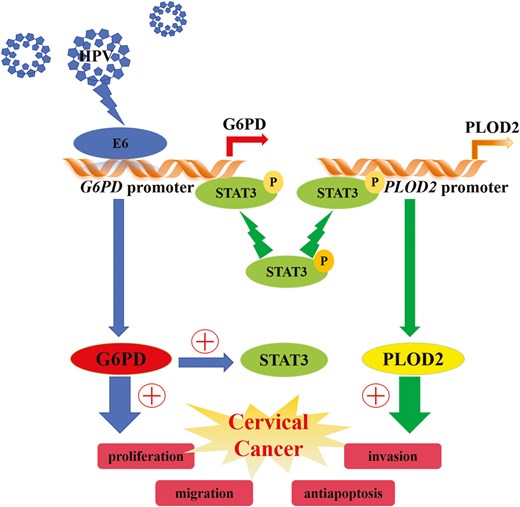
High-risk human papillomavirus (HPV) infection is strongly linked to the initiation and progression of cervical cancer (CC), yet the precise molecular mechanisms involved remain partially understood. This investigation examined differential protein expression profiles in various cohorts, including healthy controls and HPV-positive CC patients with different expression levels of glucose-6-phosphate dehydrogenase (G6PD), shedding light on the dysregulation of oncogenic proteins by HPV. Proteomic analysis of cervical tissues revealed specific protein signatures, indicating significant upregulation of HPV E6, G6PD, STAT3, phosphorylated STAT3, and procollagen-lysine 2-oxoglutarate 5-dioxygenase 2 (PLOD2) in HPV-infected CC tissues and cell lines. Functional experiments, involving the manipulation of G6PD and STAT3 activities in CC cells with HPV E6 modulation, demonstrated that dysregulated G6PD enhanced cell proliferation, migration, and invasion while suppressing apoptosis, primarily through the STAT3/PLOD2 pathway. Integrating these findings with the existing literature underscores the role of G6PD as an oncogene, potentially under STAT3 regulation, and highlights the role of PLOD2 as a pivotal factor in CC progression. This study also proposed a mechanism in which HPV E6-induced dysregulation of G6PD activates the STAT3-PLOD2 axis to promote CC progression. Understanding the intricate interplay between HPV E6, G6PD, STAT3, and PLOD2 offers valuable insights into the molecular landscape of CC. These findings may pave the way for targeted therapeutic approaches aimed at disrupting this axis to mitigate the progression of CC.